Abstract
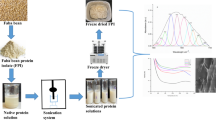
In recent years, protein nanoparticles have been well considered in the production of Pickering emulsions and low-calorie foods, but few of them have been introduced. Therefore, finding new sources of nanoparticles and modifying their physical and thermal properties is very important. In this paper, the effect of various levels of microbial transglutaminase (mTG) (0–30 μg/g of sample) and ultrasound time (0–20 min) on properties of nanoparticles prepared with Amaranth Protein Isolate (API) were investigated and optimal levels were achieved by Response surface methodology. Based on the data analysis, ultrasound had a significant effect on tested properties, so that with an increase in ultrasound time, the particle size became smaller. Excluding Zeta potential, all nanoparticles properties were influenced by the interaction of ultrasound and mTG. The enzyme had a great impact (P < 0.001) on Nanoparticles characteristics except for Glass Transition Temperature. However, depending on enzyme content, changeable behaviors in particle size, particle size distribution and denaturation temperature were detected which is generally associated with the changes in the structure and formation of covalent bonds with the function of mTG. Scanning electron microscopy of FE-SEM showed spherically synthesized nanoparticles with a size between 53 to 155 nm. This seems to facilitate the use of ultrasound reaction between nanoparticles and biopolymer matrix. Consequently, the optimized levels of 16.7 min for ultrasound and 9.47 micg/g for mTG resulted in generating a product with the considered characteristics. This novel and edible amaranth-based Nanoparticles could be suggested for Pickering emulsion preparation.
Graphical abstract









Similar content being viewed by others
Data Availability
All data supporting the findings of this study has been included within the paper and its supplementary material.
References
G.E. Inglett, D. Chen, S.X. Liu, Physical properties of gluten-free sugar cookies made from amaranth–oat composites. LWT 63, 214–220 (2015). https://doi.org/10.1016/j.lwt.2015.03.056
A. Dhiman, K. Thakur, V. Parmar, S. Sharma, R. Sharma, G. Kaur, B. Singh, R. Suhag, New insights into tailoring physicochemical and techno-functional properties of plant proteins using conventional and emerging technologies. J. Food Meas. Charact. 17, 3845–3873 (2023). https://doi.org/10.1007/s11694-023-01919-3
E. Dickinson, Biopolymer-based particles as stabilizing agents for emulsions and foams. Food Hydrocoll. 68, 219–231 (2017). https://doi.org/10.1016/j.foodhyd.2016.06.024
J.W. De Folter, M.W. Van Ruijven, K.P. Velikov, Oil-in-water Pickering emulsions stabilized by colloidal particles from the water-insoluble protein zein. Soft Matter 8, 6807–6815 (2012). https://doi.org/10.1039/C2SM07417F
M. Destribats, M. Rouvet, C. Gehin-Delval, C. Schmitt, B.P. Binks, Emulsions stabilised by whey protein microgel particles: towards food-grade Pickering emulsions. Soft Matter 10, 6941–6954 (2014). https://doi.org/10.1039/C4SM00179F
H.-N. Liang, C.-H. Tang, Pea protein exhibits a novel Pickering stabilization for oil-in-water emulsions at pH 3.0. LWT 58, 463–469 (2014). https://doi.org/10.1016/j.lwt.2014.03.023
F. Liu, C.-H. Tang, Soy glycinin as food-grade Pickering stabilizers: Part I. Structural characteristics, emulsifying properties and adsorption/arrangement at interface. Food Hydrocoll. 60, 606–619 (2016). https://doi.org/10.1016/j.foodhyd.2015.04.025
X.-S. Qin, Q.-Q. Sun, Y.-Y. Zhao, X.-Y. Zhong, D.-D. Mu, S.-T. Jiang, S.-Z. Luo, Z. Zheng, Transglutaminase-set colloidal properties of wheat gluten with ultrasound pretreatments. Ultrason. Sonochem. 39, 137–143 (2017). https://doi.org/10.1016/j.ultsonch.2017.04.027
F. Liu, S.-Y. Ou, C.-H. Tang, Ca2+-induced soy protein nanoparticles as pickering stabilizers: fabrication and characterization. Food Hydrocoll. 65, 175–186 (2017). https://doi.org/10.1016/j.foodhyd.2016.11.011
R. Sharma, M. Zakora, K.B. Qvist, Characteristics of oil–water emulsions stabilised by an industrial α-lactalbumin concentrate, cross-linked before and after emulsification, by a microbial transglutaminase. Food Chem. 79, 493–500 (2002). https://doi.org/10.1016/S0308-8146(02)00225-X
M.C. Condés, A.A. Scilingo, M.C. Añón, Characterization of amaranth proteins modified by trypsin proteolysis. Structural and functional changes. LWT 42, 963–970 (2009). https://doi.org/10.1016/j.lwt.2008.12.008
T. Zhang, Y. Zhao, X. Tian, J. Liu, H. Ye, X. Shen, Effect of ultrasound pretreatment on structural, physicochemical, rheological and gelation properties of transglutaminase cross-linked whey protein soluble aggregates. Ultrason. Sonochem. 74, 105553 (2021). https://doi.org/10.1016/j.ultsonch.2021.105553
M. Marsanasco, A.L. Márquez, J.R. Wagner, S.D.V. Alonso, N.S. Chiaramoni, Liposomes as vehicles for vitamins E and C: an alternative to fortify orange juice and offer vitamin C protection after heat treatment. Food Res. Int. 44, 3039–3046 (2011). https://doi.org/10.1016/j.foodres.2011.07.025
C. Cano-Sarmiento, D. Téllez-Medina, R. Viveros-Contreras, M. Cornejo-Mazón, C. Figueroa-Hernández, E. García-Armenta, L. Alamilla-Beltrán, H. García, G. Gutiérrez-López, Zeta potential of food matrices. Food Eng. Rev. 10, 113–138 (2018). https://doi.org/10.1007/s12393-018-9176-z
K.-O. Choi, N. Aditya, S. Ko, Effect of aqueous pH and electrolyte concentration on structure, stability and flow behavior of non-ionic surfactant based solid lipid nanoparticles. Food chem. 147, 239–244 (2014). https://doi.org/10.1016/j.foodchem.2013.09.095
L. Behlau, G. Widmann, Collection applications thermal analysis. Food Mettler Tolledo, 15–16 (2008)
B. Dery, L. Zaixiang, Scanning electron microscopy (SEM) as an effective tool for determining the morphology and mechanism of action of functional ingredients. Food Rev. Int. 39, 2007–2026 (2023). https://doi.org/10.1080/87559129.2021.1939368
M. Raei, G. Rajabzadeh, S. Zibaei, S.M. Jafari, A.M. Sani, Nano-encapsulation of isolated lactoferrin from camel milk by calcium alginate and evaluation of its release. Int. J. Biol. Macromol. 79, 669–673 (2015). https://doi.org/10.1016/j.ijbiomac.2015.05.048
K. Sarabandi, S.M. Jafari, Effect of chitosan coating on the properties of nanoliposomes loaded with flaxseed-peptide fractions: stability during spray-drying. Food Chem. 310, 125951 (2020). https://doi.org/10.1016/j.foodchem.2019.125951
R.H. Myers, D.C. Montgomery, C.M. Anderson-Cook, Response Surface Methodology: Process and Product Optimization Using Designed Experiments (Wiley, New York, 2016)
M. Yolmeh, S.M. Jafari, Applications of response surface methodology in the food industry processes. Food Bioprocess Technol. 10, 413–433 (2017). https://doi.org/10.1007/s11947-016-1855-2
J.S. Mounsey, B.T. O’Kennedy, P.M. Kelly, Influence of transglutaminase treatment on properties of micellar casein and products made therefrom. Lait 85, 405–418 (2005). https://doi.org/10.1051/lait:2005028
M.E. Steffolani, P.D. Ribotta, G.T. Pérez, A.E. León, Effect of glucose oxidase, transglutaminase, and pentosanase on wheat proteins: relationship with dough properties and bread-making quality. J. Cereal Sci. 51, 366–373 (2010). https://doi.org/10.1016/j.jcs.2010.01.010
D.J. Burgess, E. Duffy, F. Etzler, A.J. Hickey, Particle size analysis: AAPS workshop report, cosponsored by the Food and Drug Administration and the United States Pharmacopeia. AAPS J. 6, 23–34 (2004). https://doi.org/10.1208/aapsj060320
A.R. Jambrak, T.J. Mason, V. Lelas, L. Paniwnyk, Z. Herceg, Effect of ultrasound treatment on particle size and molecular weight of whey proteins. J. Food Eng. 121, 15–23 (2014). https://doi.org/10.1016/j.jfoodeng.2013.08.012
Y. Luo, B. Zhang, M. Whent, L.L. Yu, Q. Wang, Preparation and characterization of zein/chitosan complex for encapsulation of α-tocopherol, and its in vitro controlled release study. Colloids Surf. B 85, 145–152 (2011). https://doi.org/10.1016/j.colsurfb.2011.02.020
X. Shen, T. Fang, F. Gao, M. Guo, Effects of ultrasound treatment on physicochemical and emulsifying properties of whey proteins pre-and post-thermal aggregation. Food Hydrocoll. 63, 668–676 (2017). https://doi.org/10.1016/j.foodhyd.2016.10.003
Z. Teng, Y. Li, Q. Wang, Insight into curcumin-loaded β-lactoglobulin nanoparticles: incorporation, particle disintegration, and releasing profiles. J. Agric. Food Chem. 62, 8837–8847 (2014). https://doi.org/10.1021/jf503199g
S. Tamnak, H. Mirhosseini, C.P. Tan, H.M. Ghazali, K. Muhammad, Physicochemical properties, rheological behavior and morphology of pectin-pea protein isolate mixtures and conjugates in aqueous system and oil in water emulsion. Food Hydrocoll. 56, 405–416 (2016). https://doi.org/10.1016/j.foodhyd.2015.12.033
A.B. Khatkar, A. Kaur, S.K. Khatkar, N. Mehta, Characterization of heat-stable whey protein: impact of ultrasound on rheological, thermal, structural and morphological properties. Ultrason. Sonochem. 49, 333–342 (2018). https://doi.org/10.1016/j.ultsonch.2018.08.026
J. Chandrapala, B. Zisu, M. Palmer, S. Kentish, M. Ashokkumar, Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason. Sonochem. 18, 951–957 (2011). https://doi.org/10.1016/j.ultsonch.2010.12.016
C.-H. Tang, Z. Chen, L. Li, X.-Q. Yang, Effects of transglutaminase treatment on the thermal properties of soy protein isolates. Food Res. Int. 39, 704–711 (2006). https://doi.org/10.1016/j.foodres.2006.01.012
A. Mizuno, M. Mitsuiki, M. Motoki, Effect of transglutaminase treatment on the glass transition of soy protein. J. Agric. Food Chem. 48, 3286–3291 (2000). https://doi.org/10.1021/jf9911500
A. Farahnaky, F. Badii, I.A. Farhat, J.R. Mitchell, S.E. Hill, Enthalpy relaxation of bovine serum albumin and implications for its storage in the glassy state. Biopolym.: Orig. Res. Biomol. 78, 69–77 (2005). https://doi.org/10.1002/bip.20265
A. Ameri Shahrabi, F. Badii, M. Ehsani, N. Maftoonazad, D. Sarmadizadeh, Functional and thermal properties of chickpea and soy-protein concentrates and isolates. Iran. J. Nutr. Sci Food Tech. 6, 49–58 (2011). http://nsft.sbmu.ac.ir/article-1-547-en.html
A. Quiroga, E.N. Martínez, H. Rogniaux, A. Geairon, M.C. Añón, Globulin-p and 11S-globulin from amaranthus hypochondriacus: are two isoforms of the 11S-globulin. Protein J. 28, 457–467 (2009). https://doi.org/10.1007/s10930-009-9214-z
M.F. Marcone, R.Y. Yada, Structural analysis of globulins isolated from genetically different Amaranthus hybrid lines. Food Chem. 61, 319–326 (1998). https://doi.org/10.1016/S0308-8146(97)00057-5
L.E. Abugoch, E.N. Martínez, M.C. Añón, Influence of the extracting solvent upon the structural properties of amaranth (Amaranthus hypochondriacus) glutelin. J. Agric. Food chem. 51, 4060–4065 (2003). https://doi.org/10.1021/jf025949e
P. Zhang, T. Hu, S. Feng, Q. Xu, T. Zheng, M. Zhou, X. Chu, X. Huang, X. Lu, S. Pan, Effect of high intensity ultrasound on transglutaminase-catalyzed soy protein isolate cold set gel. Ultrason. Sonochem. 29, 380–387 (2016). https://doi.org/10.1016/j.ultsonch.2015.10.014
E. Choi, S. Kwok, C.Y. Ma, Conformational study of SPI treated with ultrasound, in IFT Annual Meeting & Food Expo (2009)
D.J. McClements, Recent developments in encapsulation and release of functional food ingredients: delivery by design. Curr. Opin. Food Sci. 23, 80–84 (2018). https://doi.org/10.1016/j.cofs.2018.06.008
A. Vera, C. Tapia, L. Abugoch, Effect of high-intensity ultrasound treatment in combination with transglutaminase and nanoparticles on structural, mechanical, and physicochemical properties of quinoa proteins/chitosan edible films. Int. J. Biol. Macromol. 144, 536–543 (2020). https://doi.org/10.1016/j.ijbiomac.2019.12.120
L. Zhang, Z. Pan, K. Shen, X. Cai, B. Zheng, S. Miao, Influence of ultrasound-assisted alkali treatment on the structural properties and functionalities of rice protein. J. Cereal Sci. 79, 204–209 (2018). https://doi.org/10.1016/j.jcs.2017.10.013
M. Cortez-Trejo, S. Mendoza, G. Loarca-Piña, J. Figueroa-Cárdenas, Physicochemical characterization of protein isolates of amaranth and common bean and a study of their compatibility with xanthan gum. Int. J. Biol. Macromol. 166, 861–868 (2021). https://doi.org/10.1016/j.ijbiomac.2020.10.242
F.J. Barba, N. Nikmaram, S. Roohinejad, A. Khelfa, Z. Zhu, M. Koubaa, Bioavailability of glucosinolates and their breakdown products: impact of processing. Front. Nutr. 3, 1–12 (2016). https://doi.org/10.3389/fnut.2016.00024
Acknowledgements
The authors are grateful to Islamic Azad University, Azadshahr Branch, Iran for laboratorial support. This work is part of a research project, which was approved by scientific committee of mentioned university, with research code: 162269755.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alimi, S., Fadavi, A., Sayyed-Alangi, S.Z. et al. Physical and thermal characteristics of amaranth (Amaranthus hypochondriacus) protein nanoparticles affected by ultrasound time and microbial transglutaminase. Food Measure 18, 3391–3404 (2024). https://doi.org/10.1007/s11694-024-02412-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-024-02412-1