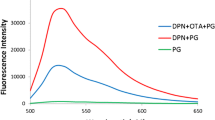
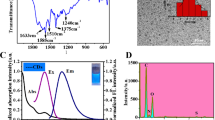
Abstract
Histamine (HA) plays an important role in many pathological processes including asthma, diarrhea, and anaphylactic shock. Therefore, accurate and rapid detecting HA is of great significance. In this work, we report on a novel turn-on fluorescent aptasensor for HA detection based on a competitive combination process performed by both HA-specific and HA-analogs of aptamers. The analogue of HA was environmental-friendly obtained on the polymer chains of carbonized polymer dots (CPDs) without complex functionalization, in which the histidine was used as the polymer precursors for the first time (His-CPDs). After modification with aptamer, the analogue of HA on the His-CPDs would combine with the aptamer, leading to the agglomeration of His-CPDs and the fluorescence quenching. In the presence of HA, the HA would compete with the analogue of HA to react with the aptamer, leading to the dissociation of the agglomerates and the recovery of fluorescence. Under optimized conditions, the proposed fluorescent aptasensor can sensitively detect HA in 7 min ranging from 50 ng/mL to 40 µg/mL with the limit of detection (LOD) of 30 ng/mL. More importantly, this proposed fluorescent aptasensor can sensitively detect histamine in human serum and real sardine samples without complex pre-processing, showing great potential in the medical diagnosis of histamine intoxication and anaphylactic shock.





Similar content being viewed by others
References
H.H. Dale, P.P. Laidlaw, The physiological action of beta-iminazolylethylamine. J. Physiol. 41, 318–344 (1910)
J.F. Huang, R.L. Thurmond, The new biology of histamine receptors. Curr. Allergy Asthma Rep. 8, 21–27 (2008)
D. Doeun, M. Davaatseren, M.S. Chung, Biogenic amines in foods. Food Sci. Biotechnol. 26, 1463–1474 (2017)
in The Food Safety Hazard Guidebook. (2008), pp. 136–171
R.Y. Lin et al, Histamine and tryptase levels in patients with acute allergic reactions: an emergency department-based study. J. Allergy Clin. Immunol. 106, 65–71 (2000)
S. Ichimata, Y. Hata, N. Nishida, An autopsy case of sudden unexpected death with loxoprofen sodium-induced allergic eosinophilic coronary periarteritis. Cardiovasc. Pathol. 44, 107154 (2020)
I.G. Fazekas, K. V. J. M. é. i. o. szemle, [Free histamine content as vital reaction in various injuries]. 11, 299–306 (1971)
G.I.J.T.T. i., A.C. Mohammed, A critical overview on the chemistry, clean-up and recent advances in analysis of biogenic amines in foodstuffs. 47, 84–94 (2016)
S. Gone et al., Validation study of MaxSignal((R)) histamine enzymatic assay for the detection of histamine in Fish/Seafood. J. AOAC Int. 101, 783–792 (2018)
S. Ghayyem, F. Faridbod, A fluorescent aptamer/carbon dots based assay for cytochrome c protein detection as a biomarker of cell apoptosis. Methods Appl. Fluoresc 7, 015005 (2018)
Z. Saberi, B. Rezaei, A.A. Ensafi, Fluorometric label-free aptasensor for detection of the pesticide acetamiprid by using cationic carbon dots prepared with cetrimonium bromide. Mikrochim Acta 186, 273 (2019)
A. Khalil, D.N. Mohammad, R. Mohammad, T.S. Mohammad, S.E.J.A.M. Ahmad, A novel amplified double-quenching aptasensor for cocaine detection based on split aptamer and silica nanoparticles. 10.1039.C1038AY00755A- (2018)
K. Mao et al., G-quadruplex–hemin DNAzyme molecular beacon probe for the detection of methamphetamine. 6, (2016)
W. Yao et al., SiC-functionalized fluorescent aptasensor for determination of Proteus mirabilis. Mikrochim Acta 187, 406 (2020). https://doi.org/10.1007/s00604-020-04378-5
M. Famulok, G. Mayer, Aptamer modules as sensors and detectors. Acc. Chem. Res. 44, 1349–1358 (2011)
Y. Du, B. Li, E. Wang, “Fitting” makes “sensing” simple: label-free detection strategies based on nucleic acid aptamers. Acc. Chem. Res. 46, 203–213 (2013)
C. Wang, Q. Zhao, A competitive thrombin-linked aptamer assay for small molecule: aflatoxin B(1). Anal. Bioanal Chem. 411, 6637–6644 (2019)
R. Chinnappan, R. AlZabn, K.M. Abu-Salah, M. Zourob, An aptamer based fluorometric microcystin-LR assay using DNA strand-based competitive displacement. Mikrochim Acta 186, 435 (2019)
Z. Cheng, J. Ling, W. Zhang, Y. Ding, Rapid detection of 17beta-estradiol based on shaddock peel derived fluorescent aptasensor for forensic examination. Forensic Sci. Int. 331, 111153 (2022)
N. Rattanachueskul, A. Saning, S. Kaowphong, N. Chumha, L. Chuenchom, Magnetic carbon composites with a hierarchical structure for adsorption of tetracycline, prepared from sugarcane bagasse via hydrothermal carbonization coupled with simple heat treatment process. Bioresour Technol. 226, 164–172 (2017)
Q. Zeng, T. Feng, S. Tao, S. Zhu, B. Yang, Precursor-dependent structural diversity in luminescent carbonized polymer dots (CPDs): the nomenclature. Light Sci. Appl. 10, 142 (2021)
T. Mairal Lerga et al., High Affinity Aptamer for the detection of the Biogenic Amine Histamine. Anal. Chem. 91, 7104–7111 (2019)
Y. Ji, J. Zhao, L. Zhao, Fabrication and characterization of magnetic molecularly imprinted polymer based on deep eutectic solvent for specific recognition and quantification of vanillin in infant complementary food. Food Chem. 374, 131720 (2022)
X. Wang et al., Bandgap-like strong fluorescence in functionalized carbon nanoparticles. Angew Chem. Int. Ed. Engl. 49, 5310–5314 (2010). https://doi.org/10.1002/anie.201000982
F. Rigodanza et al, Snapshots into carbon dots formation through a combined spectroscopic approach. Nat. Commun. 12, 2640 (2021)
J. **a, S. Chen, G.Y. Zou, Y.L. Yu, J.H. Wang, Synthesis of highly stable red-emissive carbon polymer dots by modulated polymerization: from the mechanism to application in intracellular pH imaging. Nanoscale 10, 22484–22492 (2018)
H. Ding, S.B. Yu, J.S. Wei, H.M. **ong, Full-color light-emitting Carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 10, 484–491 (2016)
J. Liu et al., One-step Hydrothermal Synthesis of Nitrogen-Doped Conjugated Carbonized Polymer Dots with 31% efficient Red Emission for in vivo imaging. Small 14, e1703919 (2018)
C. **a, X. Hai, X.W. Chen, J.H. Wang, Simultaneously fabrication of free and solidified N, S-doped graphene quantum dots via a facile solvent-free synthesis route for fluorescent detection. Talanta 168, 269–278 (2017)
Y. Zhang et al., Development and validation of a sample stabilization strategy and a UPLC-MS/MS method for the simultaneous quantitation of acetylcholine (ACh), histamine (HA), and its metabolites in rat cerebrospinal fluid (CSF). J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 879, 2023–2033 (2011)
O. Comas-Baste, M.L. Latorre-Moratalla, R. Bernacchia, M.T. Veciana-Nogues, M. C. Vidal-Carou, New approach for the diagnosis of histamine intolerance based on the determination of histamine and methylhistamine in urine. J. Pharm. Biomed. Anal. 145, 379–385 (2017)
A. Yadav, Y. Upadhyay, R.K. Bera, S.K. Sahoo, Vitamin B(6) cofactors guided highly selective fluorescent turn-on sensing of histamine using beta-cyclodextrin stabilized ZnO quantum dots. Food Chem. 320, 126611 (2020)
M. Dwidar, Y. Yokobayashi, Development of a histamine aptasensor for food safety monitoring. Sci. Rep. 9, 16659 (2019)
L.S. John Ho, R. Fogel, J.L. Limson, Generation and screening of histamine-specific aptamers for application in a novel impedimetric aptamer-based sensor. Talanta 208, 120474 (2020)
T.M. Lerga et al, Gold nanoparticle aptamer assay for the determination of histamine in foodstuffs. Mikrochim Acta 187, 452 (2020)
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81772025).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, W., Zhang, Z., Zhang, Z. et al. Rapid and sensitive determination of histamine based on a fluorescent aptamer probe with analogue on carbonized polymer dots. Food Measure 17, 4695–4704 (2023). https://doi.org/10.1007/s11694-023-01912-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-01912-w