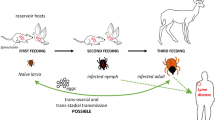
Abstract
Background
Ticks are important medical arthropods that can transmit hundreds of pathogens, such as parasites, bacteria, and viruses, leading to serious public health burdens worldwide. Unexplained fever is the most common clinical manifestation of tick-borne diseases. Since the emergence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the surge of coronavirus disease 2019 (COVID-19) cases led to the hospital overload and fewer laboratory tests for tick-borne diseases. Therefore, it is essential to review the tick-borne pathogens and further understand tick-borne diseases.
Purpose
The geographic distribution and population of ticks in the Northern hemisphere have expanded while emerging tick-borne pathogens have been introduced to China continuously. This paper focused on the tick-borne pathogens that are threatening public health in the world. Their medical significant tick vectors, as well as the epidemiology, clinical manifestations, diagnosis, treatment, prevention, and control measures, are emphasized in this document.
Methods
In this study, all required data were collected from articles indexed in English databases, including Scopus, PubMed, Web of Science, Science Direct, and Google Scholar.
Results
Ticks presented a great threat to the economy and public health. Although both infections by tick-borne pathogens and SARS-CoV-2 have fever symptoms, the history of tick bite and its associated symptoms such as encephalitis or eschar could be helpful for the differential diagnosis. Additionally, as a carrier of vector ticks, migratory birds may play a potential role in the geographical expansion of ticks and tick-borne pathogens during seasonal migration.
Conclusion
China should assess the risk score of vector ticks and clarify the potential role of migratory birds in transmitting ticks. Additionally, the individual and collective protection, vector control, comprehensive surveillance, accurate diagnosis, and symptomatic treatment should be carried out, to meet the challenge.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ticks (Acarina Ixodoidea) are obligate hematophagous arthropod ectoparasites, which can be distinguished by their morphology and divided into 3 families: Ixodidae (hard ticks), Argasidae (soft ticks) and Nuttalliellidae. At present, a total of 9 genera, and 124 species of ticks have been identified in China [1]. They can not only bite their hosts and suck their blood but also spread pathogens via their saliva. Tick-borne infections are zoonoses. Humans are usually considered the occasional hosts for ticks and have no role in maintaining tick-borne pathogens in the natural cycles [2]. The pathogens, spread by ticks, are called tick-borne pathogens. The diseases, caused by tick-borne pathogens, are called tick-borne diseases.
Ticks and tick-borne pathogens are one of the biggest public health threats and veterinary problems in the world. First, tick-borne pathogens, such as Babesia, Theileria, and Anaplasma phagocytophilum, are threatening over 80% of the cattle population. The reproduction of meat, milk, and leather and the death of infected animals have brought significant economic loss. According to the statistics of the Food and Agriculture Organization of the United Nations (FAO), the gross economic loss in the world is annually USD 13.9–18.7 billion [3], with the average loss being USD 7.3 for each animal per year. Moreover, the cost of the prevention and the control of tick-borne diseases are high. For example, the reported cost for cattle alone, which are in areas at risk of being affected by ticks, has reached USD 380 million [4]. Second, tick-borne pathogens have also significantly threatened public health. On the one hand, the expense of medical treatment is high and has led to a considerable burden on society. According to the statistics of the National Institutes of Health (NIH), the expense for tick-borne diseases totaled USD 56 million in the United States in 2020 [5]. The reported cost for Lyme disease reached USD 40 million in Germany and 20 million in Holland in 2017, respectively [6]. On the other hand, a multitude of pathogens is usually harbored by one tick. Given this, it is unsurprising to observe the co-infection with genetically distinct pathogens in human beings. A plethora of studies reported seropositivity against multiple pathogen combinations [7,8,9]. However, our understanding of the interactions of these agents remains poor. Various pathogen combinations might behave synergistically, indifferently, or antagonistically within hosts, and thus modulate disease severity [10]. Challenges have been presented during diagnosis and treatment.
In recent years, reported tick-borne pathogens have shown a resurgence trend while emerging tick-borne pathogens have been continuously identified from the tick population, which have attracted extensive attention from medical and veterinary personnel. The purpose of this review is to summarize the research progress of tick-borne pathogens, including their epidemiology, species of vector ticks, clinical manifestations, diagnosis, treatment, prevention, and control dynamics. Additionally, tick load has been detected in a variety of migratory birds [11]. They can easily cross the mountains, glaciers, deserts, and oceans, and transport ticks and tick-borne pathogens from epidemic countries to China. Therefore, further understanding of ticks and tick-borne pathogens will help to detect, monitor, prevent, and control these public health threats and challenges in China comprehensively.
Tick-Borne Parasite
Piroplasma
Piroplasma (class: Apicomplexa, order: Piroplasmida) is the causative agent of piroplasmosis, which mainly consists of parasites in the family Babesiidae and Theileriidae. Ixodes ricinus is predominantly responsible for the transmission of Piroplasma in Europe, while Ixodes persulcatus is predominant in China. Through trans-ovarial and transstadial transmission, their larval, nymphal, and adult ticks can transmit the parasite to mammals (including human beings) during a blood meal. Additionally, other tick species have been proven to be vectors of Piroplasma: Rhipicephalus microplus, Rhipicephalus decoloratus, Rhipicephalus australis, Rhipicephalus sanguineus, Rhipicephalus annulatus, Dermacentor reticulatus, Haemaphysalis elliptica, and Haemaphysalis longicornis [12]. It should be noted that the distribution of tick vectors is far more extensive than that of the relevant piroplasmosis. The threat of this agent may be underestimated.
Babesiosis is a tick-borne parasitic disease endangering the world, which is caused by protozoan parasites of the genus Babesia. So far, more than 100 species of Babesia have been identified in domestic and wild animals, among which Babesia bigemina, Babesia Bovis, and Babesia divergens mainly infect livestock animals, while Babesia microti, B. divergens, Babesia Duncani, and Babesia venatorum are the causative agents of human babesiosis [13]. Bovine babesiosis could lead to severe economic loss, which is associated with apocleisis, low feed conversion efficiency, reduced production, abortion, and death of cattle. The clinical symptoms of human babesiosis are similar to malaria, including but not limited to fever, chills, and sleepiness. Additionally, the incidence of hemolytic anemia can be observed in severe cases. However, the severity of babesiosis depends on the species of Babesia and the immune status of the patients [14].
In China, the distribution of Babesia is shown in Fig. 1. It was previously thought that the responsible species of human infections were B. microti and B. venatorum in Eastern and Northeastern China. However, two emerging Babesia species, Babesia crassa, and Babesia sp. XXB/HangZhou, have been identified in Heilongjiang province and Zhejiang province respectively [15]. Additionally, an initial report of human infection with B. divergens in Gansu province has raised medical awareness [16]. It was the first report of Babesia infections in Gansu province. Interestingly, as the etiological agent of bovine babesiosis, B. divergens has not been identified in cattle in China. In the United States, the number of human infection cases has rapidly increased in the past 20 years. Therefore, the US government has included babesiosis as a nationally notifiable consideration [17]. This suggested that China should pay increased attention to this emerging threat and carry out systematic epidemiological surveys. In the prevalent regions, local physicians should be aware of the differential diagnoses for babesiosis and the risk for transfusion-transmitted babesiosis.
Theileria is an intracellular protozoan parasite, which could lead to bovine theileriosis and constrain cattle production in develo** countries, whereby ticks are their natural vectors. The cattle infected with Theileria exhibit fever, anemia, jaundice, and superficial lymphadenopathy. In China, Theileria infection is common (Fig. 1). The causative agents are Theileria orientalis, Theileria sinensis, and Theileria annulata. T. orientalis is a blood protozoan transmitted by Haemaphysalis ticks and clustered into the benign Theileria spp. group. This group has low pathogenicity to cattle and buffalo, leading to only inconspicuous clinical symptoms. However, the T. orientalis Ikeda genotype is a newly identified genotype with strong virulence. It can lead to erythrocyte lysis, causing cause anemia and hypoxia in cattle [18]. Additionally, even if the pregnant cows are cured, they are still at risk of abortion. T. sinensis, transmitted by H. qinghaiensis, was initially isolated from cattle in Gansu province in China. It showed solid host specificity and can only infect cattle, yaks, and buffalo. However, due to its low pathogenicity, farmers usually pay insufficient attention to this agent, leading to constant invisible losses to the cattle industry. T. annulata is the causative agent of tropical theileriosis and has brought a significant impact on the cattle industry. It is transmitted by Hyalomma ticks and is prevalent in the arid and semi-arid areas in Northern China. The diagnosis is based on clinical symptoms (such as jaundice, severe anemia, anterior shoulder, and posterior bone lymphadenopathy) and the results of blood and lymph node smear. However, in the case of chronic or subclinical infection, the parasitemia level is extremely low and the parasites might not be detected via these methods [19].
Toxoplasma gondii
T. gondii, in the family Apicomplexa, is an obligate intracellular parasite. The majority of warm-blooded animals (from birds to mammals, including human beings) can be infected as intermediate hosts, while only cats can be the definitive hosts. It is generally believed that T. gondii is acquired from the ingestion of water, soil, and/or food that is contaminated indirectly by feline feces. However, the oral infection route cannot explain the infection of herbivores, birds, and wild rodents. These animals would not actively eat raw meat nor come into contact with water and food contaminated from feline feces. Thus, some scholars have suggested that arthropods may be alternative vectors for the transmission of T. gondii. So far, T. gondii has been detected in Dermacentor variabilis, Dermacentor Andersoni, D. reticulatus, I. ricinus, H. Iongicornis, Amblyomma americanum, Amblyomma Cajennense, and Ornithodoros moubata [20]. It has been proved that T. gondii could survive and remain infective in H. Iongicornis for more than 15 days. Nevertheless, it could not be transmitted to hosts during a blood meal [21]. Overall, the presupposition needs more experimental data to confirm.
Toxoplasmosis, a neglected cousin of malaria, is a parasitic zoonosis with approximately a 30% infection rate in the world’s human population. However, the seroprevalence has obviously regional characteristics, which can nearly reach 90% in African populations, whereas 60% in Europe [22]. It is generally believed that acute infections of immunocompetent adults are asymptomatic or subclinical, manifesting as influenza-like symptoms. As for immunocompromised demographic groups, such as patients diagnosed with AIDS or cancer, their brains will be damaged [23]. Epilepsy, insanity, ataxia, schizophrenia, and cranial nerve palsy are some common symptoms. Pregnant women can transmit T. gondii to the fetus vertically, which leads to obstetric diseases for the fetus and newborns, including abortion and serious congenital anomalies such as hydrocephalus, microcephaly, and calcifications [24]. According to the latest sero-epidemiological investigations in China, the seropositivity of T. gondii antibodies in the general population is 5.1% [25]. The distribution of toxoplasmosis is nationwide (Fig. 1), whereby most of the infected people were in contact with cats. Therefore, eating well-cooked meat, drinking boiled water, and managing feline feces are key interventions for reducing the incidence of toxoplasmosis [26].
Tick-Borne Bacteria
Borrelia Burgdorferi Sensu Lato
B. burgdorferi sensu lato species complex, of order Spirochaetaceae and family Spirochaetales, is distributed throughout the Northern hemisphere. At present, a total of 21 species have been identified, of which 3 are associated with different clinical manifestations in afflicted human beings: B. burgdorferi sensu stricto, Borrelia garini, and Borrelia afzelii. Relying on the basic metabolic function of Ixodes ticks, B. burgdorferi could interact with the intestinal and salivary proteins of vector ticks to colonize, survive, and finally exit the ticks successfully [27]. This may be due to evolving stiffer peptidoglycan, as an adaptation to obligate parasitization of vector ticks [28]. Ixodes ticks are primarily responsible for the transmission of B. burgdorferi: I. scapularis mainly transmits the agent on the Eastern coast of the United States, while I. ricinus in Europe, and I. persuletus in Asia [17]. In Northern China, B. burgdorferi is transmitted by I. persuletus. As for Southern China, it is transmitted by Ixodes sinensis and Ixodes granulolatus. However, ticks can only transmit B. burgdorferi, B. garini, and B. afzelii transstadially as opposed to trans-ovarially [29].
Lyme disease is the most common zoonosis caused by B. burgdorferi, of which the incidence peak is in late spring and summer every year (because nymphal ticks are most abundant and active during that time). At present, more than 30 provinces in China have reported cases of Lyme disease (Fig. 2). Acute infection in dogs is characterized by fever, claudication, lymphadenopathy, and multiple arthritis. In addition to the above symptoms, weight loss and laminitis could also be observed in infected horses and cattle. Due to the high invasion capability of B. burgdorferi, the number of human cases is far more than that of any other tick-borne bacteria in Europe, North America, and Asia. Human infection is often characterized by a complicated multisystem illness, including erythema migrans (localized skin inflammation) [30], Bannwarth syndrome, and typical acrodermatitis chronica atrophicans. The skin, joints, heart, and nervous system are often damaged. The diagnosis is based on the standard two-tier testing (STTT) that consists of an enzyme immunoassay (EIA) or a chemiluminescence immunoassay (CIA) as an initial test, followed by Western blotting if the result is positive or equivocal. However, if a patient is in the early phase of Lyme disease, the EIA result may be negative or equivocal. Hence, an alternative test should be considered [31].
As for infected patients, antibiotics, such as penicillin, amoxicillin, ceftriaxone, doxycycline, and azithromycin, should be given as soon as possible. Otherwise, the lesion advanced to an early disseminated stage, and finally reaching the late stage with myocarditis, neuritis, or arthritis. Additionally, Hygromycin A, a compound produced by Streptomyces hygroscopicus, could be selectively taken up by B. burgdorferi and clear the infection in mice [32]. This compound holds the promise of providing a more selective antibiotic for Lyme disease. As for the vaccine, VLA15 is the only Lyme disease vaccine candidate that is reported to undergo phase-3 clinical trials [33]. It targets the outer surface protein A (OspA) of B. burgdorferi, which is one of the most important surface proteins, and induces the bacterium’s ability to leave the tick and infect human beings. Blocking OspA means that the transmission of B. burgdorferi is blocked. Additionally, OspA-based animal vaccines can effectively reduce nymphal infection in white-footed mice (Peromyscus leucopus), which are reservoirs of I. scapularis [33]. Interestingly, a new vaccine targeting the vector ticks instead of the pathogen has also been under development [34]. The vaccine contains 19 distinct mRNA snippets, which can order host cells to produce important tick salivary proteins. In turn, when faced with a tick bite, the immune system will rapidly react causing redness, inflammation, and itchiness around the bite for people to notice and pull the tick off. Within 36 h after the ticks attach, pulling the ticks off could effectively block the transmission of B. burgdorferi.
Spotted Fever Group Rickettsiae
Rickettsia (order: Rickettsiales, family: Rickettsiaceae) is an obligate intracellular Gram-negative bacterium, which is transmitted by arthropods such as ticks, mites, and fleas. At present, a total of 25 pathogenic species have been identified and can be phylogenetically divided into three groups: spotted fever, typhus, and transition groups. The spotted fever group Rickettsiae (SFGR) is one of the emerging tick-borne pathogens identified in mainland China. Through both trans-ovarial and transstadial transmission, Ixodidae ticks could reserve and transmit SFGR, which is distributed geographically, possibly expanding their distribution range through vector ticks [35].
Spotted fever is the general name for clinical diseases caused by SFGR. Different Rickettsia species will lead to various endemic diseases (Table 1). In the United States, reported Rocky Mountain spotted fever (RMSF) cases have dramatically increased tenfold over the past 20 years [36]. Emerging SFGR subspecies have also been discovered. Additionally, several Rickettsia species, which were previously considered non-pathogenic for decades, have been definitively reported to associate with human disease in Italy [37].
In recent years, an increasing number of emerging SFGRs have been reported in China, including Rickettsia parkeri [38], Rickettsia Japonica [39], Rickettsia sp. XY99 [40], Candidatus Rickettsia xinyangensis [77]. Following binding, DVD-121-801, a bispecific antibody, can provide therapeutic protection and become a strong candidate for therapeutic antibody cocktails [78].
In China, **-Ill virus paraphyletic taxa. Mol Phylogenet Evol 169:107411. https://doi.org/10.1016/j.ympev.2022.107411 " href="/article/10.1007/s11686-023-00658-1#ref-CR83" id="ref-link-section-d277691574e2061">83]. In recent years, two novel subtypes of TBEV have been isolated, named Himalayan (Him-TBEV) [84] and Baikalian (Bkl-TBEV) [85] respectively. At present, it has been reported that Eu-TBEV is mainly transmitted by I. ricinus, while Sib-TBEV and FE-TBEV are both transmitted by I. persulcatus. Additionally, D. reticulatus could also transmit the virus [86].
Most TBEV infections in human beings are asymptomatic. However, neurological symptoms, including encephalitis, meningitis, and meningoencephalitis can be observed in one-third of infection cases. The disease caused by TBEV is called tick-borne encephalitis (TBE), which is the most serious tick-borne neurological disease prevalent across the Eurasian continent from Japan to France, even in the United Kingdom [87]. The incidence and mortality rate are dependent on the subtype of the virus. The mortality of Eu-TBEV and Sib-TBEV is about 2%, associated with neurological sequelae and long-term infection. However, the mortality of FE-TBEV is up to 30% [88]. At present, there is no specific drug for TBEV. Therefore, active vaccination is a practical measure to prevent TBEV infection. Two safe and effective vaccines have been approved: FSME-IMMUN® (Pfizer, USA) and Encepur® (GlaxoSmithKline) [89]. The vaccines are generally safe with rare serious adverse events [90].
In China, a total of 3364 TBE cases have been reported from 2007 to 2018 [91]. It is mainly endemic in Northern and Western China (Fig. 3). Most cases involved people living or working in forests. Recently, evidence of TBEV in 2 areas of the United Kingdom attracted our attention [92]. According to the climatic forecasts, TBEV was thought to be incapable of establishing itself in the United Kingdom, as their vector ticks could not overcome the temperature limit. However, they challenged the climate, which was speculated to be carried by migratory birds [93]. This suggested that the contribution of migratory birds for TBE is substantial, particularly for medium- and long-distance dispersal. Hence, the threats might be potentially extensively underestimated.
Phenuiviridae: Severe Fever with Thrombocytopenia Syndrome Virus (Dabie bandavirus)
In 2009, researchers have detected a novel virus in Henan, Hubei, Shandong, Anhui, Jiangsu, and Liaoning provinces in China [52]. The virus could infect human beings and cause a febrile disease called severe fever with thrombocytopenia syndrome (SFTS). The clinical manifestations mainly include fever, thrombocytopenia, and leukopenia syndromes. Therefore, the virus was initially named severe fever with thrombocytopenia syndrome virus (SFTSV) and classified into the genus Phlebovirus and family Phenuiviridae. However, it was later renamed by the International Committee on Taxonomy of Viruses (ICTV) in 2019. This novel virus was finally denominated Dabie bandavirus and reclassified into the genus Bandavirus, family Phenuiviridae, and order Bunyavirales.
H. longicornis and I. sinensis have been proven to transovarially and transstadially transmit Dabie bandavirus [93]. While D. silvarum could transmit the virus transovarial rather than transstadial [94]. Additionally, Haemaphysalis flava, R. microplus, Amblyomma testudinarium, D. nuttalli, H. asiaticum kozlovi, H. concinna, I. persulcatus, and Ixodes nipponensis can harbor the virus [95]. Distributed in the Asia Pacific region, H. longicornis is the primary vector for Dabie bandavirus. However, recently, the United States reported the invasion of H. longicornis with increasing numbers infesting sheep [96], in addition to human beings bitten as well [97]. Migratory birds, as the natural hosts of H. longicornis have been suspected to indirectly expand the distribution of H. longicornis by carrying ticks during long-distance migration. There is evidence supporting the relationship between H. longicornis and the flyways of migratory birds [98]. However, according to the ecological niche modeling, H. longicornis have also been predicted to threaten more extensive regions, including Europe, South America, and Africa [1]. However, their vector competence has not been evaluated.
Due to the complexity of eco-social systems and the interconnectedness of pork industries around the world, interrupting the global spread of ASFV is quite difficult. The present strain in China was genotype I ASFV, which shared high similarity with NH/P68 and OURT88/3 [134], human beings, animals, and the environment are like the 3 arms of a triangle, whereby their relationship and interaction should be tackled. This highlighted the synergistic benefit of closer cooperation. Therefore, 3 vaccine frameworks need to be developed. Framework I vaccines are intended for the protection of economically valuable animals. Framework II vaccines are used to protect domestic animals and human beings. Finally, framework III vaccines are applicable for the protection of wild animals. This relies on further basic research of tick-borne pathogens' epitopes and therapeutic antibodies. With ready-made vaccines and abundant basic research, the prevention and control of emerging and re-emerging tick-borne pathogens will be rapid and accurate.
However, relying solely on vaccines is not enough. The comprehensive prevention and control of tick-borne pathogens are also dependent on individual and collective protection, vector control, comprehensive surveillance, accurate diagnosis, and symptomatic treatment. So far, 124 tick species and 103 tick-borne pathogens have been reported in China. A total of 29 tick-borne pathogens were associated with human infections, and the number is still increasing. This further highlighted the importance of assessing the transmission risk of vector ticks. Additionally, according to the assessment of vector ticks and tick-borne pathogens’ distributions [1], the suitable habitats were 14–476% larger than reported areas, indicating that the detection is seriously insufficient. At last, the possible role of migratory birds in China should also be identified to realize the comprehensive prevention and control of ticks and tick-borne pathogens.
Availability of data and material
Not applicable.
References
Zhao GP, Wang YX, Fan ZW, Ji Y, Liu MJ, Zhang WH, Li XL, Zhou SX, Li H, Liang S, Liu W, Yang Y, Fang LQ (2021) Map** ticks and tick-borne pathogens in China. Nat Commun 12:1075. https://doi.org/10.1038/s41467-021-21375-1
Socolovschi C, Mediannikov O, Raoult D, Parola P (2009) The relationship between spotted fever group Rickettsiae and ixodid ticks. Vet Res 40:34. https://doi.org/10.1051/vetres/2009017
Kasaija PD, Estrada-Peña A, Contreras M, Kirunda H, de la Fuente J (2021) Cattle ticks and tick-borne diseases: a review of Uganda’s situation. Ticks Tick Borne Dis 12:101756. https://doi.org/10.1016/j.ttbdis.2021.101756
Li Z, Liu J, Zhao S, Ma Q, Liu A, Li Y, Guan G, Luo J, Yin H (2021) Theileria annulata Subtelomere-Encoded Variable Secreted Protein-TA05575 Binds to Bovine RBMX2. Front Cell Infect Microbiol 11:644983. https://doi.org/10.3389/fcimb.2021.644983
Couzin-Frankel J (2019) Lyme disease research gets a needed boost. Science (New York, N.Y.) 364:221. https://doi.org/10.1126/science.364.6437.221
Mac S, da Silva SR, Sander B (2019) The economic burden of Lyme disease and the cost-effectiveness of Lyme disease interventions: a sco** review. PLoS ONE 14:e0210280. https://doi.org/10.1371/journal.pone.0210280
Hilton E, DeVoti J, Benach JL, Halluska ML, White DJ, Paxton H, Dumler JS (1999) Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am J Med 106:404–409. https://doi.org/10.1016/s0002-9343(99)00046-7
Curcio SR, Tria LP, Gucwa AL (2016) Seroprevalence of Babesia microti in Individuals with Lyme Disease. Vector Borne Zoonotic Dis 16:737–743. https://doi.org/10.1089/vbz.2016.2020
Wass L, Grankvist A, Mattsson M, Gustafsson H, Krogfelt K, Olsen B, Nilsson K, Mårtensson A, Quarsten H, Henningsson AJ, Wennerås C (2018) Serological reactivity to Anaplasma phagocytophilum in neoehrlichiosis patients. Eur J Clin Microbiol Infect Dis 37:1673–1678. https://doi.org/10.1007/s10096-018-3298-3
States SL, Huang CI, Davis S, Tufts DM, Diuk-Wasser MA (2017) Co-feeding transmission facilitates strain coexistence in Borrelia burgdorferi, the Lyme disease agent. Epidemics 19:33–42. https://doi.org/10.1016/j.epidem.2016.12.002
Klitgaard K, Højgaard J, Isbrand A, Madsen JJ, Thorup K, Bødker R (2019) Screening for multiple tick-borne pathogens in Ixodes ricinus ticks from birds in Denmark during spring and autumn migration seasons. Ticks and tick-borne diseases 10:546–552. https://doi.org/10.1016/j.ttbdis.2019.01.007
Gray JS, Estrada-Peña A, Zintl A (2019) Vectors of Babesiosis. Annu Rev Entomol 64:149–165. https://doi.org/10.1146/annurev-ento-011118-111932
Krause PJ (2019) Human babesiosis. Int J Parasitol 49:165–174. https://doi.org/10.1016/j.ijpara.2018.11.007
Theel ES, Pritt BS (2016) Parasites. Microbiol Spectr. https://doi.org/10.1128/microbiolspec.DMIH2-0013-2015
Jia N, Zheng YC, Jiang JF, Jiang RR, Jiang BG, Wei R, Liu HB, Huo QB, Sun Y, Chu YL, Fan H, Chang QC, Yao NN, Zhang WH, Wang H, Guo DH, Fu X, Wang YW, Krause PJ, Song JL, Cao WC (2018) Human babesiosis caused by a Babesia crassa-like pathogen: a case series. Clin Infect Dis 67:1110–1119. https://doi.org/10.1093/cid/ciy212
Madison-Antenucci S, Kramer LD, Gebhardt LL, Kauffman E (2020) Emerging tick-borne diseases. Clin Microbiol Rev 33:e00083-e118. https://doi.org/10.1128/CMR.00083-18
Wang J, Zhang S, Yang J, Liu J, Zhang D, Li Y, Luo J, Guan G, Yin H (2019) Babesia divergens in human in Gansu province, China. Emerg Microbes Infect 8:959–961. https://doi.org/10.1080/22221751.2019.1635431
Oakes VJ, Yabsley MJ, Schwartz D, LeRoith T, Bissett C, Broaddus C, Schlater JL, Todd SM, Boes KM, Brookhart M, Lahmers KK (2019) Theileria orientalis Ikeda genotype in cattle, virginia, USA. Emerg Infect Dis 25:1653–1659. https://doi.org/10.3201/eid2509.190088
Ma Q, Liu J, Li Z, **ang Q, Wang J, Liu A, Li Y, Yin H, Guan G, Luo J (2020) Clinical and pathological studies on cattle experimentally infected with Theileria annulata in China. Pathogens (Basel, Switzerland) 9:727. https://doi.org/10.3390/pathogens9090727
Ben-Harari RR (2019) Tick transmission of toxoplasmosis. Expert Rev Anti Infect Ther 17:911–917. https://doi.org/10.1080/14787210.2019.1682550
Zhou Y, Zhang H, Cao J, Gong H, Zhou J (2016) Epidemiology of toxoplasmosis: role of the tick Haemaphysalis longicornis. Infect Dis Poverty 5:14. https://doi.org/10.1186/s40249-016-0106-0
Milne G, Webster JP, Walker M (2020) Toxoplasma gondii: an underestimated threat? Trends Parasitol 36:959–969. https://doi.org/10.1016/j.pt.2020.08.005
An R, Tang Y, Chen L, Cai H, Lai DH, Liu K, Wan L, Gong L, Yu L, Luo Q, Shen J, Lun ZR, Ayala FJ, Du J (2018) Encephalitis is mediated by ROP18 of Toxoplasma gondii, a severe pathogen in AIDS patients. Proc Natl Acad Sci USA 115:e5344–e5352. https://doi.org/10.1073/pnas.1801118115
Megli CJ, Coyne CB (2022) Infections at the maternal-fetal interface: an overview of pathogenesis and defence. Nat Rev Microbiol 20:67–82. https://doi.org/10.1038/s41579-021-00610-y
**n S, Su R, Jiang N, Zhang L, Yang Y (2020) Low prevalence of antibodies against Toxoplasma gondii in Chinese populations. Front Cell Infect Microbiol 10:302. https://doi.org/10.3389/fcimb.2020.00302
Smith NC, Goulart C, Hayward JA, Kupz A, Miller CM, van Dooren GG (2021) Control of human toxoplasmosis. Int J Parasitol 51:95–121. https://doi.org/10.1016/j.ijpara.2020.11.001
Kurokawa C, Lynn GE, Pedra J, Pal U, Narasimhan S, Fikrig E (2020) Interactions between Borrelia burgdorferi and ticks. Nat Rev Microbiol 18:587–600. https://doi.org/10.1038/s41579-020-0400-5
DeHart TG, Kushelman MR, Hildreth SB, Helm RF, Jutras BL (2021) The unusual cell wall of the Lyme disease spirochaete Borrelia burgdorferi is shaped by a tick sugar. Nat Microbiol 6:1583–1592. https://doi.org/10.1038/s41564-021-01003-w
Eisen L (2020) Vector competence studies with hard ticks and Borrelia burgdorferi sensu lato spirochetes: a review. Ticks Tick Borne Dis 11:101359. https://doi.org/10.1016/j.ttbdis.2019.101359
Kullberg BJ, Vrijmoeth HD, van de Schoor F, Hovius JW (2020) Lyme borreliosis: diagnosis and management. BMJ (Clinical research ed) 369:m1041. https://doi.org/10.1136/bmj.m1041
Sanchez E, Vannier E, Wormser GP, Hu LT (2016) Diagnosis, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA 315:1767–1777. https://doi.org/10.1001/jama.2016.2884
Leimer N, Wu X, Imai Y, Morrissette M, Pitt N, Favre-Godal Q, Iinishi A, Jain S, Caboni M, Leus IV, Bonifay V, Niles S, Bargabos R, Ghiglieri M, Corsetti R, Krumpoch M, Fox G, Son S, Klepacki D, Polikanov YS, Freliech CA, McCarthy JE, Edmondson DG, Norris SJ, D’Onofrio A, Hu LT, Zgurskaya HI, Lewis K (2021) A selective antibiotic for Lyme disease. Cell 184:5405-5418.e16. https://doi.org/10.1016/j.cell.2021.09.011
Gomes-Solecki M, Arnaboldi PM, Backenson PB, Benach JL, Cooper CL, Dattwyler RJ, Diuk-Wasser M, Fikrig E, Hovius JW, Laegreid W, Lundberg U, Marconi RT, Marques AR, Molloy P, Narasimhan S, Pal U, Pedra JHF, Plotkin S, Rock DL, Rosa P, Telford SR, Tsao J, Yang XF, Schutzer SE (2020) Protective immunity and new vaccines for Lyme disease. Clin Infect Dis 70:1768–1773. https://doi.org/10.1093/cid/ciz872
Wadman M (2021) To fight Lyme disease, vaccine targets ticks. Science 374:925. https://doi.org/10.1126/science.acx9658
Zhang YY, Sun YQ, Chen JJ, Teng AY, Wang T, Li H, Hay SI, Fang LQ, Yang Y, Liu W (2022) Map** the global distribution of spotted fever group rickettsiae: a systematic review with modelling analysis. Lancet. https://doi.org/10.1016/S2589-7500(22)00212-6
Epidemiology and statistics (2022) Rocky Mountain spotted fever (RMSF) CDC. https://www.cdc.gov/rmsf/stats/index.html (Accessed June 2022).
Pascucci I, Antognini E, Canonico C, Montalbano MG, Necci A, di Donato A, Moriconi M, Morandi B, Morganti G, Crotti S, Gavaudan S (2021) One health approach to rickettsiosis: a five-year study on spotted fever group rickettsiae in ticks collected from humans, animals and environment. Microorganisms 10:35. https://doi.org/10.3390/microorganisms10010035
Zhao S, Yang M, Liu G, Hornok S, Zhao S, Sang C, Tan W, Wang Y (2020) Rickettsiae in the common pipistrelle Pipistrellus pipistrellus (Chiroptera: Vespertilionidae) and the bat soft tick Argas vespertilionis (Ixodida: Argasidae). Parasit Vectors 13:10. https://doi.org/10.1186/s13071-020-3885-x
Li J, Hu W, Wu T, Li HB, Hu W, Sun Y, Chen Z, Shi Y, Zong J, Latif A, Wang L, Yu L, Yu XJ, Liu BY, Liu Y (2018) Japanese spotted fever in eastern China, 2013. Emerg Infect Dis 24:2107–2109. https://doi.org/10.3201/eid2411.170264
Li H, Cui XM, Cui N, Yang ZD, Hu JG, Fan YD, Fan XJ, Zhang L, Zhang PH, Liu W, Cao WC (2016) Human infection with novel spotted fever group Rickettsia genotype, China, 2015. Emerg Infect Dis 22:2153–2156. https://doi.org/10.3201/eid2212.160962
Li H, Li XM, Du J, Zhang XA, Cui N, Yang ZD, Xue XJ, Zhang PH, Cao WC, Liu W (2020) Candidatus Rickettsia xinyangensis as cause of spotted fever group rickettsiosis, **nyang, China, 2015. Emerg Infect Dis 26:985–988. https://doi.org/10.3201/eid2605.170294
Liu H, Li Q, Zhang X, Li Z, Wang Z, Song M, Wei F, Wang S, Liu Q (2016) Characterization of rickettsiae in ticks in northeastern China. Parasit Vectors 9:498. https://doi.org/10.1186/s13071-016-1764-2
Han R, Yang J, Niu Q, Liu Z, Chen Z, Kan W, Hu G, Liu G, Luo J, Yin H (2018) Molecular prevalence of spotted fever group rickettsiae in ticks from Qinghai Province, northwestern China. Infect Genet Evol 57:1–7. https://doi.org/10.1016/j.meegid.2017.10.025
Qin XR, Han HJ, Han FJ, Zhao FM, Zhang ZT, Xue ZF, Ma DQ, Qi R, Zhao M, Wang LJ, Zhao L, Yu H, Liu JW, Yu XJ (2019) Rickettsia japonica and novel Rickettsia species in ticks, China. Emerg Infect Dis 25:992–995. https://doi.org/10.3201/eid2505.171745
Yuan TT, Ma L, Jiang BG, Fu WM, Sun Y, Jia N, Jiang JF (2020) First confirmed infection of candidatus Rickettsia tarasevichiae in rodents collected from northeastern China. Vector Borne Zoonotic Dis (Larchmont, NY) 20:88–92. https://doi.org/10.1089/vbz.2019.2443
Zhuo M, Calev H, Saunders SJ, Li J, Stillman IE, Danziger J (2019) Acute kidney injury associated with human granulocytic anaplasmosis: a case report. Am J Kidney Dis 74:696–699. https://doi.org/10.1053/j.ajkd.2019.03.428
Oliva Chávez AS, Wang X, Marnin L, Archer NK, Hammond HL, Carroll E, Shaw DK, Tully BG, Buskirk AD, Ford SL, Butler LR, Shahi P, Morozova K, Clement CC, Lawres L, Neal A, Mamoun CB, Mason KL, Hobbs BE, Scoles GA, Barry EM, Sonenshine DE, Pal U, Valenzuela JG, Sztein MB, Pasetti MF, Levin ML, Kotsyfakis M, Jay SM, Huntley JF, Miller LS, Santambrogio L, Pedra J (2021) Tick extracellular vesicles enable arthropod feeding and promote distinct outcomes of bacterial infection. Nat Commun 12:3696. https://doi.org/10.1038/s41467-021-23900-8
Cabezas-Cruz A, Espinosa P, Alberdi P, de la Fuente J (2019) Tick-pathogen interactions: the metabolic perspective. Trends Parasitol 35:316–328. https://doi.org/10.1016/j.pt.2019.01.006
Best A, Abu Kwaik Y (2019) Nutrition and bipartite metabolism of intracellular pathogens. Trends Microbiol 27:550–561. https://doi.org/10.1016/j.tim.2018.12.012
Matei IA, Estrada-Peña A, Cutler SJ, Vayssier-Taussat M, Varela-Castro L, Potkonjak A, Zeller H, Mihalca AD (2019) A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasit Vectors 12:599. https://doi.org/10.1186/s13071-019-3852-6
Zhang L, Liu Y, Ni D, Li Q, Yu Y, Yu XJ, Wan K, Li D, Liang G, Jiang X, **g H, Run J, Luan M, Fu X, Zhang J, Yang W, Wang Y, Dumler JS, Feng Z, Ren J, Xu JD (2008) Nosocomial transmission of human granulocytic anaplasmosis in China. JAMA 300:2263–2270. https://doi.org/10.1001/jama.2008.626
Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, **g HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX (2011) Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med 364:1523–1532. https://doi.org/10.1056/NEJMoa1010095
Wormser GP (2016) Accuracy of diagnosis of human granulocytic anaplasmosis in China. Emerg Infect Dis 22:1728–1731. https://doi.org/10.3201/eid2210.160161
Sykes DB, Zhang EW, Karp Leaf RS, Nardi V, Turbett SE (2020) Case 10–2020: an 83-year-old man with pancytopenia and acute renal failure. N Engl J Med 382:1258–1266. https://doi.org/10.1056/NEJMcpc1916250
Clemente TM, Ratnayake R, Samanta D, Augusto L, Beare PA, Heinzen RA, Gilk SD (2022) Coxiella burnetii sterol-modifying protein Stmp1 regulates cholesterol in the intracellular niche. MBio 13:e0307321. https://doi.org/10.1128/mbio.03073-21
Zhang Y, Fu J, Liu S, Wang L, Qiu J, van Schaik EJ, Samuel JE, Song L, Luo ZQ (2022) Coxiella burnetii inhibits host immunity by a protein phosphatase adapted from glycolysis. Proc Natl Acad Sci USA 119:e2110877119. https://doi.org/10.1073/pnas.2110877119
Jansen W, Cargnel M, Boarbi S, Mertens I, Van Esbroeck M, Fretin D, Mori M (2022) Belgian bulk tank milk surveillance program reveals the impact of a continuous vaccination protocol for small ruminants against Coxiella burnetii. Transbound Emerg Dis 69:e141–e152. https://doi.org/10.1111/tbed.14273
Abraham H, Kamboj AK, Saleh OA (2022) Fevers, acute hepatitis, and hypercoagulability in an immunocompetent host. Gastroenterology 162:54–55. https://doi.org/10.1053/j.gastro.2021.09.058
Koehler LM, Kloppert B, Hamann HP, El-Sayed A, Zschöck M (2019) Comprehensive literature review of the sources of infection and transmission routes of Coxiella burnetii, with particular regard to the criteria of “evidence-based medicine.” Comp Immunol Microbiol Infect Dis 64:67–72. https://doi.org/10.1016/j.cimid.2019.02.004
Buijs SB, Bleeker-Rovers CP, van Roeden SE, Kampschreur LM, Hoepelman A, Wever PC, Oosterheert JJ (2021) Still new chronic Q fever cases diagnosed 8 years after a large Q fever outbreak. Clin Infect Dis 73:1476–1483. https://doi.org/10.1093/cid/ciab476
Eldin C, Mélenotte C, Mediannikov O, Ghigo E, Million M, Edouard S, Mege JL, Maurin M, Raoult D (2017) From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev 30:115–190. https://doi.org/10.1128/CMR.00045-16
Buijs SB, van Roeden SE, van Werkhoven CH, Hoepelman A, Wever PC, Bleeker-Rovers CP, Oosterheert JJ (2021) The prognostic value of serological titres for clinical outcomes during treatment and follow-up of patients with chronic Q fever. Clin Microbiol Infect 27:1273–1278. https://doi.org/10.1016/j.cmi.2021.03.016
Ni J, Lin H, Xu X, Ren Q, Aizezi M, Luo J, Luo Y, Ma Z, Chen Z, Tan Y, Guo J, Liu W, Qu Z, Wu Z, Wang J, Li Y, Guan G, Luo J, Yin H, Liu G (2020) Coxiella burnetii is widespread in ticks (Ixodidae) in the **njiang areas of China. BMC Vet Res 16:317. https://doi.org/10.1186/s12917-020-02538-6
Woldeyohannes SM, Perkins NR, Baker P, Gilks CF, Knibbs LD, Reid SA (2020) Q fever vaccine efficacy and occupational exposure risk in Queensland, Australia: a retrospective cohort study. Vaccine 38:6578–6584. https://doi.org/10.1016/j.vaccine.2020.08.006
Fratzke AP, Jan S, Felgner J, Liang L, Nakajima R, Jasinskas A, Manna S, Nihesh FN, Maiti S, Albin TJ, Esser-Kahn AP, Davies DH, Samuel JE, Felgner PL, Gregory AE (2021) Subunit vaccines using TLR triagonist combination adjuvants provide protection against Coxiella burnetii while minimizing reactogenic responses. Front Immunol 12:653092. https://doi.org/10.3389/fimmu.2021.653092
Kawahara M, Rikihisa Y, Isogai E, Takahashi M, Misumi H, Suto C, Shibata S, Zhang C, Tsuji M (2004) Ultrastructure and phylogenetic analysis of 'Candidatus Neoehrlichia mikurensis’ in the family Anaplasmataceae, isolated from wild rats and found in Ixodes ovatus ticks. Int J Syst Evol Microbiol 54:1837–1843. https://doi.org/10.1099/ijs.0.63260-0
Wennerås C (2015) Infections with the tick-borne bacterium Candidatus Neoehrlichia mikurensis. Clin Microbiol Infect 21:621–630. https://doi.org/10.1016/j.cmi.2015.02.030
Wass L, Grankvist A, Bell-Sakyi L, Bergström M, Ulfhammer E, Lingblom C, Wennerås C (2019) Cultivation of the causative agent of human neoehrlichiosis from clinical isolates identifies vascular endothelium as a target of infection. Emerg Microbes Infect 8:413–425. https://doi.org/10.1080/22221751.2019.1584017
Welinder-Olsson C, Kjellin E, Vaht K, Jacobsson S, Wennerås C (2010) First case of human “Candidatus Neoehrlichia mikurensis” infection in a febrile patient with chronic lymphocytic leukemia. J Clin Microbiol 48:1956–1959. https://doi.org/10.1128/JCM.02423-09
Grankvist A, Andersson PO, Mattsson M, Sender M, Vaht K, Höper L, Sakiniene E, Trysberg E, Stenson M, Fehr J, Pekova S, Bogdan C, Bloemberg G, Wennerås C (2014) Infections with the tick-borne bacterium “Candidatus Neoehrlichia mikurensis” mimic noninfectious conditions in patients with B cell malignancies or autoimmune diseases. Clin Infect Dis 58:1716–1722. https://doi.org/10.1093/cid/ciu189
Li H, Jiang JF, Liu W, Zheng YC, Huo QB, Tang K, Zuo SY, Liu K, Jiang BG, Yang H, Cao WC (2012) Human infection with Candidatus Neoehrlichia mikurensis, China. Emerg Infect Dis 18:1636–1639. https://doi.org/10.3201/eid1810.120594
Negredo A, de la Calle-Prieto F, Palencia-Herrejón E, Mora-Rillo M, Astray-Mochales J, Sánchez-Seco MP, Bermejo Lopez E, Menárguez J, Fernández-Cruz A, Sánchez-Artola B, Keough-Delgado E, Ramírez de Arellano E, Lasala F, Milla J, Fraile JL, Ordobás Gavín M, Martinez de la Gándara A, López Perez L, Diaz-Diaz D, López-García MA, Delgado-Jimenez P, Martín-Quirós A, Trigo E, Figueira JC, Manzanares J, Rodriguez-Baena E, Garcia-Comas L, Rodríguez-Fraga O, García-Arenzana N, Fernández-Díaz MV, Cornejo VM, Emmerich P, Schmidt-Chanasit J, Arribas JR, Crimean Congo Hemorrhagic Fever@Madrid Working Group (2017) Autochthonous Crimean-Congo hemorrhagic fever in Spain. New Engl J Med 377:154–161. https://doi.org/10.1056/NEJMoa1615162
Fillâtre P, Revest M, Tattevin P (2019) Crimean-Congo hemorrhagic fever: an update. Medecine et maladies infectieuses 49:574–585. https://doi.org/10.1016/j.medmal.2019.09.005
Moroso M, Verlhac P, Ferraris O, Rozières A, Carbonnelle C, Mély S, Endtz HP, Peyrefitte CN, Paranhos-Baccalà G, Viret C, Faure M (2020) Crimean-Congo hemorrhagic fever virus replication imposes hyper-lipidation of MAP1LC3 in epithelial cells. Autophagy 16:1858–1870. https://doi.org/10.1080/15548627.2019.1709765
Hawman DW, Haddock E, Meade-White K, Williamson B, Hanley PW, Rosenke K, Komeno T, Furuta Y, Gowen BB, Feldmann H (2018) Favipiravir (T-705) but not ribavirin is effective against two distinct strains of Crimean-Congo hemorrhagic fever virus in mice. Antiviral Res 157:18–26. https://doi.org/10.1016/j.antiviral.2018.06.013
Papa A (2019) Diagnostic approaches for Crimean-Congo hemorrhagic fever virus. Expert Rev Mol Diagn 19:531–536. https://doi.org/10.1080/14737159.2019.1615450
Mishra AK, Hellert J, Freitas N, Guardado-Calvo P, Haouz A, Fels JM, Maurer DP, Abelson DM, Bornholdt ZA, Walker LM, Chandran K, Cosset FL, McLellan JS, Rey FA (2022) Structural basis of synergistic neutralization of Crimean-Congo hemorrhagic fever virus by human antibodies. Science 375:104–109. https://doi.org/10.1126/science.abl6502
Fels JM, Maurer DP, Herbert AS, Wirchnianski AS, Vergnolle O, Cross RW, Abelson DM, Moyer CL, Mishra AK, Aguilan JT, Kuehne AI, Pauli NT, Bakken RR, Nyakatura EK, Hellert J, Quevedo G, Lobel L, Balinandi S, Lutwama JJ, Zeitlin L, Geisbert TW, Rey FA, Sidoli S, McLellan JS, Lai JR, Bornholdt ZA, Dye JM, Walker LM, Chandran K (2021) Protective neutralizing antibodies from human survivors of Crimean-Congo hemorrhagic fever. Cell 184:3486-3501.e21. https://doi.org/10.1016/j.cell.2021.05.001
Moming A, Yue X, Shen S, Chang C, Wang C, Luo T, Zhang Y, Guo R, Hu Z, Zhang Y, Deng F, Sun S (2018) Prevalence and phylogenetic analysis of Crimean-Congo hemorrhagic fever virus in ticks from different ecosystems in **njiang, China. Virologica Sinica 33:67–73. https://doi.org/10.1007/s12250-018-0016-3
Zhang Y, Shen S, Fang Y, Liu J, Su Z, Liang J, Zhang Z, Wu Q, Wang C, Abudurexiti A, Hu Z, Zhang Y, Deng F (2018) Isolation, characterization, and phylogenetic analysis of two new Crimean-Congo hemorrhagic fever virus strains from the northern region of **njiang province, China. Virologica Sinica 33:74–86. https://doi.org/10.1007/s12250-018-0020-7
Spengler JR, Bergeron É, Spiropoulou CF (2019) Crimean-Congo hemorrhagic fever and expansion from endemic regions. Curr Opin Virol 34:70–78. https://doi.org/10.1016/j.coviro.2018.12.002
Mancuso E, Toma L, Polci A, d’Alessio SG, Di Luca M, Orsini M, Di Domenico M, Marcacci M, Mancini G, Spina F, Goffredo M, Monaco F (2019) Crimean-Congo hemorrhagic fever virus genome in tick from migratory bird, Italy. Emerg Infect Dis 25:1418–1420. https://doi.org/10.3201/eid2507.181345
Bondaryuk AN, Andaev EI, Dzhioev YP, Zlobin VI, Tkachev SE, Kozlova IV, Bukin YS (2022) Delimitation of the tick-borne flaviviruses. Resolving the tick-borne encephalitis virus and lou**-Ill virus paraphyletic taxa. Mol Phylogenet Evol 169:107411. https://doi.org/10.1016/j.ympev.2022.107411
Dai X, Shang G, Lu S, Yang J, Xu J (2018) A new subtype of eastern tick-borne encephalitis virus discovered in Qinghai-Tibet Plateau. China Emerg Microbes Infect 7:74. https://doi.org/10.1038/s41426-018-0081-6
Ruzek D, Avšič Županc T, Borde J, Chrdle A, Eyer L, Karganova G, Kholodilov I, Knap N, Kozlovskaya L, Matveev A, Miller AD, Osolodkin DI, Överby AK, Tikunova N, Tkachev S, Zajkowska J (2019) Tick-borne encephalitis in Europe and Russia: review of pathogenesis, clinical features, therapy, and vaccines. Antiviral Res 164:23–51. https://doi.org/10.1016/j.antiviral.2019.01.014
Ličková M, Fumačová Havlíková S, Sláviková M, Slovák M, Drexler JF, Klempa B (2020) Dermacentor reticulatus is a vector of tick-borne encephalitis virus. Ticks Tick Borne Dis 11:101414. https://doi.org/10.1016/j.ttbdis.2020.101414
Holding M, Dowall S, Hewson R (2020) Detection of tick-borne encephalitis virus in the UK. Lancet 395:411. https://doi.org/10.1016/S0140-6736(20)30040-4
Yoshii K (2019) Epidemiology and pathological mechanisms of tick-borne encephalitis. J Vet Med Sci 81:343–347. https://doi.org/10.1292/jvms.18-0373
Pugh SJ, Moïsi JC, Kundi M, Santonja I, Erber W, Angulo FJ, Jodar L (2022) Effectiveness of two doses of tick-borne encephalitis (TBE) vaccine. J Travel Med 29:taab193. https://doi.org/10.1093/jtm/taab193
Rampa JE, Askling HH, Lang P, Zens KD, Gültekin N, Stanga Z, Schlagenhauf P (2020) Immunogenicity and safety of the tick-borne encephalitis vaccination (2009–2019): a systematic review. Travel Med Infect Dis 37:101876. https://doi.org/10.1016/j.tmaid.2020.101876
Chen X, Li F, Yin Q, Liu W, Fu S, He Y, Lei W, Xu S, Liang G, Wang S, Yang G, Qi X, Wang H (2019) Epidemiology of tick-borne encephalitis in China, 2007–2018. PLoS ONE 14:e0226712. https://doi.org/10.1371/journal.pone.0226712
Holding M, Dowall SD, Medlock JM, Carter DP, Pullan ST, Lewis J, Vipond R, Rocchi MS, Baylis M, Hewson R (2020) Tick-borne encephalitis virus, United Kingdom. Emerg Infect Dis 26:90–96. https://doi.org/10.3201/eid2601.191085
Zhuang L, Sun Y, Cui XM, Tang F, Hu JG, Wang LY, Cui N, Yang ZD, Huang DD, Zhang XA, Liu W, Cao WC (2018) Transmission of severe fever with thrombocytopenia syndrome virus by Haemaphysalis longicornis ticks, China. Emerg Infect Dis 24:868–871. https://doi.org/10.3201/eid2405.151435
Hu YY, Zhuang L, Liu K, Sun Y, Dai K, Zhang XA, Zhang PH, Feng ZC, Li H, Liu W (2020) Role of three tick species in the maintenance and transmission of severe fever with thrombocytopenia syndrome virus. PLoS Negl Trop Dis 14:e0008368. https://doi.org/10.1371/journal.pntd.0008368
Casel MA, Park SJ, Choi YK (2021) Severe fever with thrombocytopenia syndrome virus: emerging novel phlebovirus and their control strategy. Exp Mol Med 53:713–722. https://doi.org/10.1038/s12276-021-00610-1
Rainey T, Occi JL, Robbins RG, Egizi A (2018) Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J Med Entomol 55:757–759. https://doi.org/10.1093/jme/tjy006
Wormser GP, McKenna D, Piedmonte N, Vinci V, Egizi AM, Backenson B, Falco RC (2020) First recognized human bite in the United States by the Asian longhorned tick, Haemaphysalis Longicornis. Clin Infect Dis 70:314–316. https://doi.org/10.1093/cid/ciz449
Zhang YZ, Xu J (2016) The emergence and cross species transmission of newly discovered tick-borne Bunyavirus in China. Curr Opin Virol 16:126–131. https://doi.org/10.1016/j.coviro.2016.02.006
Zhao L, Li J, Cui X, Jia N, Wei J, **a L, Wang H, Zhou Y, Wang Q, Liu X, Yin C, Pan Y, Wen H, Wang Q, Xue F, Sun Y, Jiang J, Li S, Cao W (2020) Distribution of Haemaphysalis longicornis and associated pathogens: analysis of pooled data from a China field survey and global published data. Lancet Planetary Health 4:e320–e329. https://doi.org/10.1016/S2542-5196(20)30145-5
Yun SM, Park SJ, Kim YI, Park SW, Yu MA, Kwon HI, Kim EH, Yu KM, Jeong HW, Ryou J, Lee WJ, Jee Y, Lee JY, Choi YK (2020) Genetic and pathogenic diversity of severe fever with thrombocytopenia syndrome virus (SFTSV) in South Korea. JCI Insight 5:e129531. https://doi.org/10.1172/jci.insight.129531
Kobayashi Y, Kato H, Yamagishi T, Shimada T, Matsui T, Yoshikawa T, Kurosu T, Shimojima M, Morikawa S, Hasegawa H, Saijo M, Oishi K, SFTS Epidemiological Research Group Japan (2020) Severe fever with thrombocytopenia syndrome, Japan, 2013–2017. Emerg Infect Dis 26:692–699. https://doi.org/10.3201/eid2604.191011
Tran XC, Yun Y, Van An L, Kim SH, Thao N, Man P, Yoo JR, Heo ST, Cho NH, Lee KH (2019) Endemic severe fever with thrombocytopenia syndrome, Vietnam. Emerg Infect Dis 25:1029–1031. https://doi.org/10.3201/eid2505.181463
Park A, Park SJ, Jung KL, Kim SM, Kim EH, Kim YI, Foo SS, Kim S, Kim SG, Yu KM, Choi Y, Kim JY, Baek YH, Song MS, Kim SR, Kim SY, Jeong HW, Kim SH, Jung JU, Choi YK (2021) Molecular signatures of inflammatory profile and B-cell function in patients with severe fever with thrombocytopenia syndrome. MBio 12:e02583-e2620. https://doi.org/10.1128/mBio.02583-20
Suzuki T, Sato Y, Sano K, Arashiro T, Katano H, Nakajima N, Shimojima M, Kataoka M, Takahashi K, Wada Y, Morikawa S, Fukushi S, Yoshikawa T, Saijo M, Hasegawa H (2020) Severe fever with thrombocytopenia syndrome virus targets B cells in lethal human infections. J Clin Investig 130:799–812. https://doi.org/10.1172/JCI129171
Li H, Lu QB, **ng B, Zhang SF, Liu K, Du J, Li XK, Cui N, Yang ZD, Wang LY, Hu JG, Cao WC, Liu W (2018) Epidemiological and clinical features of laboratory-diagnosed severe fever with thrombocytopenia syndrome in China, 2011–17: a prospective observational study. Lancet Infect Dis 18:1127–1137. https://doi.org/10.1016/S1473-3099(18)30293-7
Takayama-Ito M, Saijo M (2020) Antiviral drugs against severe fever with thrombocytopenia syndrome virus infection. Front Microbiol 11:150. https://doi.org/10.3389/fmicb.2020.00150
Li H, Zhang LK, Li SF, Zhang SF, Wan WW, Zhang YL, **n QL, Dai K, Hu YY, Wang ZB, Zhu XT, Fang YJ, Cui N, Zhang PH, Yuan C, Lu QB, Bai JY, Deng F, **ao GF, Liu W, Peng K (2019) Calcium channel blockers reduce severe fever with thrombocytopenia syndrome virus (SFTSV) related fatality. Cell Res 29:739–753. https://doi.org/10.1038/s41422-019-0214-z
Dong F, Li D, Wen D, Li S, Zhao C, Qi Y, Jangra RK, Wu C, **a D, Zhang X, Deng F, Chandran K, Zou Z, Yuan F, Zheng A (2019) Single dose of a rVSV-based vaccine elicits complete protection against severe fever with thrombocytopenia syndrome virus. NPJ Vaccines 4:5. https://doi.org/10.1038/s41541-018-0096-y
Kwak JE, Kim YI, Park SJ, Yu MA, Kwon HI, Eo S, Kim TS, Seok J, Choi WS, Jeong JH, Lee H, Cho Y, Kwon JA, Jeong M, Maslow JN, Kim YE, Jeon H, Kim KK, Shin EC, Song MS, Jung UJ, Choi YK, Park SH (2019) Development of a SFTSV DNA vaccine that confers complete protection against lethal infection in ferrets. Nat Commun 10:3836. https://doi.org/10.1038/s41467-019-11815-4
Yoshikawa T, Taniguchi S, Kato H, Iwata-Yoshikawa N, Tani H, Kurosu T, Fujii H, Omura N, Shibamura M, Watanabe S, Egawa K, Inagaki T, Sugimoto S, Phanthanawiboon S, Harada S, Yamada S, Fukushi S, Morikawa S, Nagata N, Shimojima M, Saijo M (2021) A highly attenuated vaccinia virus strain LC16m8-based vaccine for severe fever with thrombocytopenia syndrome. PLoS Pathog 17:e1008859. https://doi.org/10.1371/journal.ppat.1008859
Qin XC, Shi M, Tian JH, Lin XD, Gao DY, He JR, Wang JB, Li CX, Kang YJ, Yu B, Zhou DJ, Xu J, Plyusnin A, Holmes EC, Zhang YZ (2014) A tick-borne segmented RNA virus contains genome segments derived from unsegmented viral ancestors. Proc Natl Acad Sci USA 111:6744–6749. https://doi.org/10.1073/pnas.1324194111
Ladner JT, Wiley MR, Beitzel B, Auguste AJ, Dupuis AP, Lindquist ME, Sibley SD, Kota KP, Fetterer D, Eastwood G, Kimmel D, Prieto K, Guzman H, Aliota MT, Reyes D, Brueggemann EE, St John L, Hyeroba D, Lauck M, Friedrich TC, O’Connor DH, Gestole MC, Cazares LH, Popov VL, Castro-Llanos F, Kochel TJ, Kenny T, White B, Ward MD, Loaiza JR, Goldberg TL, Weaver SC, Kramer LD, Tesh RB, Palacios G (2016) A multicomponent animal virus isolated from mosquitoes. Cell Host Microbe 20:357–367. https://doi.org/10.1016/j.chom.2016.07.011
Souza WM, Fumagalli MJ, Torres Carrasco AO, Romeiro MF, Modha S, Seki MC, Gheller JM, Daffre S, Nunes M, Murcia PR, Acrani GO, Figueiredo L (2018) Viral diversity of Rhipicephalus microplus parasitizing cattle in southern Brazil. Sci Rep 8:16315. https://doi.org/10.1038/s41598-018-34630-1
Kuivanen S, Levanov L, Kareinen L, Sironen T, Jääskeläinen AJ, Plyusnin I, Zakham F, Emmerich P, Schmidt-Chanasit J, Hepojoki J, Smura T, Vapalahti O (2019) Detection of novel tick-borne pathogen, Alongshan virus, in Ixodes ricinus ticks, south-eastern Finland, 2019. Eurosurveillance 24:1900394. https://doi.org/10.2807/1560-7917.ES.2019.24.27.1900394
Kobayashi D, Kuwata R, Kimura T, Shimoda H, Fujita R, Faizah AN, Kai I, Matsumura R, Kuroda Y, Watanabe S, Kuniyoshi S, Yamauchi T, Watanabe M, Higa Y, Hayashi T, Shinomiya H, Maeda K, Kasai S, Sawabe K, Isawa H (2021) Detection of **gmenviruses in Japan with evidence of vertical transmission in ticks. Viruses 13:2547. https://doi.org/10.3390/v13122547
Yu ZM, Chen JT, Qin J, Guo JJ, Li K, Xu QY, Wang W, Lu M, Qin XC, Zhang YZ (2020) Identification and characterization of **gmen tick virus in rodents from **njiang. China. Infect Genet Evol 84:104411. https://doi.org/10.1016/j.meegid.2020.104411
Jia N, Liu HB, Ni XB, Bell-Sakyi L, Zheng YC, Song JL, Li J, Jiang BG, Wang Q, Sun Y, Wei R, Yuan TT, **a LY, Chu YL, Wei W, Li LF, Ye JL, Lv QY, Cui XM, Guan Y, Tong YG, Jiang JF, Lam TTY, Cao WC (2019) Emergence of human infection with **gmen tick virus in China: a retrospective study. EBioMedicine 43:317–324. https://doi.org/10.1016/j.ebiom.2019.04.004
Emmerich P, Jakupi X, von Possel R, Berisha L, Halili B, Günther S, Cadar D, Ahmeti S, Schmidt-Chanasit J (2018) Viral metagenomics, genetic and evolutionary characteristics of Crimean-Congo hemorrhagic fever orthonairovirus in humans, Kosovo. Infect Genet Evol 65:6–11. https://doi.org/10.1016/j.meegid.2018.07.010
Wang ZD, Wang B, Wei F, Han SZ, Zhang L, Yang ZT, Yan Y, Lv XL, Li L, Wang SC, Song MX, Zhang HJ, Huang SJ, Chen J, Huang FQ, Li S, Liu HH, Hong J, ** YL, Wang W, Zhou JY, Liu Q (2019) A new segmented virus associated with human febrile illness in China. N Engl J Med 380:2116–2125. https://doi.org/10.1056/NEJMoa1805068
Wang ZD, Wang W, Wang NN, Qiu K, Zhang X, Tana G, Liu Q, Zhu XQ (2019) Prevalence of the emerging novel Alongshan virus infection in sheep and cattle in Inner Mongolia, northeastern China. Parasit Vectors 12:450. https://doi.org/10.1186/s13071-019-3707-1
Zhang X, Wang N, Wang Z, Liu Q (2020) The discovery of segmented flaviviruses: implications for viral emergence. Curr Opin Virol 40:11–18. https://doi.org/10.1016/j.coviro.2020.02.001
Temmam S, Bigot T, Chrétien D, Gondard M, Pérot P, Pommelet V, Dufour E, Petres S, Devillers E, Hoem T, Pinarello V, Hul V, Vongphayloth K, Hertz JC, Loiseau I, Dumarest M, Duong V, Vayssier-Taussat M, Grandadam M, Albina E, Dussart P, Moutailler S, Cappelle J, Brey PT, Eloit M (2019) Insights into the host range, genetic diversity, and geographical distribution of **gmenviruses. mSphere 4:e00645-e719. https://doi.org/10.1128/mSphere.00645-19
Kholodilov IS, Litov AG, Klimentov AS, Belova OA, Polienko AE, Nikitin NA, Shchetinin AM, Ivannikova AY, Bell-Sakyi L, Yakovlev AS, Bugmyrin SV, Bespyatova LA, Gmyl LV, Luchinina SV, Gmyl AP, Gushchin VA, Karganova GG (2020) Isolation and characterisation of alongshan virus in Russia. Viruses 12:362. https://doi.org/10.3390/v12040362
Dixon LK, Stahl K, Jori F, Vial L, Pfeiffer DU (2020) African swine fever epidemiology and control. Ann Rev Animal Biosci 8:221–246. https://doi.org/10.1146/annurev-animal-021419-083741
Zhao D, Liu R, Zhang X, Li F, Wang J, Zhang J, Liu X, Wang L, Zhang J, Wu X, Guan Y, Chen W, Wang X, He X, Bu Z (2019) Replication and virulence in pigs of the first African swine fever virus isolated in China. Emerg Microbes Infect 8:438–447. https://doi.org/10.1080/22221751.2019.1590128
Wang N, Zhao D, Wang J, Zhang Y, Wang M, Gao Y, Li F, Wang J, Bu Z, Rao Z, Wang X (2019) Architecture of African swine fever virus and implications for viral assembly. Science 366:640–644. https://doi.org/10.1126/science.aaz1439
Wang T, Wang L, Han Y, Pan L, Yang J, Sun M, Zhou P, Sun Y, Bi Y, Qiu HJ (2021) Adaptation of African swine fever virus to HEK293T cells. Transbound Emerg Dis 68:2853–2866. https://doi.org/10.1111/tbed.14242
Dixon LK, Sun H, Roberts H (2019) African swine fever. Antiviral Res 165:34–41. https://doi.org/10.1016/j.antiviral.2019.02.018
Olesen AS, Belsham GJ, Bruun Rasmussen T, Lohse L, Bødker R, Halasa T, Boklund A, Bøtner A (2020) Potential routes for indirect transmission of African swine fever virus into domestic pig herds. Transbound Emerg Dis 67:1472–1484. https://doi.org/10.1111/tbed.13538
Pereira de Oliveira R, Hutet E, Paboeuf F, Duhayon M, Boinas F, Perez de Leon A, Filatov S, Vial L, Le Potier MF (2019) Comparative vector competence of the afrotropical soft tick Ornithodoros moubata and palearctic species, O. erraticus and O. verrucosus, for African swine fever virus strains circulating in Eurasia. PLoS ONE 14:e0225657. https://doi.org/10.1371/journal.pone.0225657
Golnar AJ, Martin E, Wormington JD, Kading RC, Teel PD, Hamer SA, Hamer GL (2019) Reviewing the potential vectors and hosts of african swine fever virus transmission in the United States. Vector Borne Zoonotic Dis (Larchmont, NY) 19:512–524. https://doi.org/10.1089/vbz.2018.2387
Sun E, Huang L, Zhang X, Zhang J, Shen D, Zhang Z, Wang Z, Huo H, Wang W, Huangfu H, Wang W, Li F, Liu R, Sun J, Tian Z, **a W, Guan Y, He X, Zhu Y, Zhao D, Bu Z (2021) Genotype I African swine fever viruses emerged in domestic pigs in China and caused chronic infection. Emerg Microbes Infect 10:2183–2193. https://doi.org/10.1080/22221751.2021.1999779
Sanchez-Vicente S, Tagliafierro T, Coleman JL, Benach JL, Tokarz R (2019) Polymicrobial nature of tick-borne diseases. MBio 10:e02055-e2119. https://doi.org/10.1128/mBio.02055-19
McEwen SA, Collignon PJ (2018) Antimicrobial resistance: a one health perspective. Microbiol Spectr. https://doi.org/10.1128/microbiolspec.ARBA-0009-2017
Funding
This work was supported by the Key Research and Development Project of Shaanxi Province (2020NY-017), the Animal Husbandry Special fund of Department of Agriculture and Rural Affairs of Shaanxi Province (XN14) and the State Key Laboratory of Veterinary Etiological Biology, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences (SKLVEB2019KFKT007).
Author information
Authors and Affiliations
Contributions
YL: Conceptualization, Investigation, Visualization, Writing (original draft). JG: Data curation, Investigation, Resources. DZ: Writing (original draft). LM: Investigation. CY: Resources. MS: Writing (review & editing). GL: Writing (review & editing), Project administration, Funding acquisition. QL: Conceptualization, Resources, Writing (review & editing), Supervision, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Ethical Approval
Not applicable.
Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luan, Y., Gou, J., Zhong, D. et al. The Tick-Borne Pathogens: An Overview of China’s Situation. Acta Parasit. 68, 1–20 (2023). https://doi.org/10.1007/s11686-023-00658-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-023-00658-1