Abstract
The insula, a crucial hub of the human brain network, can be divided into anterior and posterior regions. Previous studies have reported that different insula subregions play various roles in amnestic mild cognitive impairment (aMCI). However, the longitudinal changes in the functional connectivity (FC) of each insula subregion in aMCI patients over time remain unclear. Twenty aMCI patients and 20 healthy controls (HCs) were recruited and underwent resting-state functional magnetic resonance imaging (fMRI) scans and neuropsychological assessments at baseline and at the 15-month follow-up. FMRI data were preprocessed using SPM 12 and the CONN toolbox. Two-way analysis of covariance was used to compare longitudinal changes in the FC of each insula subregion with covariates including sex, age, education, follow-up interval, volume of gray matter, and global correlation (GCOR). Pearson’s correlation was used to evaluate the relationship between insula subregional FC and neuropsychological performance in aMCI patients. In aMCI patients, the right anterior insula exhibited significantly increased FC with the left anterior cingulate cortex, whereas the left posterior insula exhibited decreased FC with the right precuneus compared with HCs. Furthermore, FC between the right anterior insula and left anterior cingulate cortex was significantly correlated with global cognition at follow-up. The current findings revealed different functional alterations in the insula subregions and provided new insights into the neurodegenerative process in aMCI patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
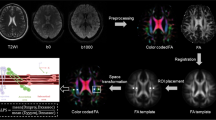
Amnestic mild cognitive impairment (aMCI), characterized by cognitive decline without any notable effect on daily activities, is considered the preclinical stage of Alzheimer’s disease (AD) (Petersen et al., 2018). One key feature of aMCI is abnormalities in neural network connectivity between different brain regions, which increases the risk of progression to AD (Biswal et al., 1995; **, 30(9), 2731–2745. https://doi.org/10.1002/hbm.20705 ." href="/article/10.1007/s11682-024-00899-2#ref-CR28" id="ref-link-section-d9623685e474">2009; ** (SPM) 12 software (https://www.fil.ion.ucl.ac.uk/spm). The first ten images of each fMRI dataset were discarded to remove any fluctuations in the initial MRI signal. Images of each individual subject were corrected for slice timing and realignment. All subjects included in the present study exhibited head motion less than 1.5 mm in any of the x, y, or z directions; less than 1.5° in any angular dimension; and volume-level mean framewise displacement (FD) less than 0.30 (with a mean FD across all subjects of 0.16 ± 0.08) (Power et al., 2012). The resulting images were normalized to the Montreal Neurological Institute (MNI) space, resampled to 2 mm × 2 mm × 2 mm, and smoothed with a 4-mm full width at half maximum (FWHM) isotropic Gaussian kernel. After smoothing, subsequent preprocessing was conducted using the functional connectivity (CONN) toolbox v17c (Whitfield-Gabrieli & Nieto-Castanon, 2012). The images were bandpass filtered to 0.008–0.09 Hz to reduce the influence of noise. Further denoising steps included regression and linear detrending. Possible contamination from white matter and cerebrospinal fluid (CSF) signals (Behzadi et al., 2007) and six motion parameters and their first-order derivatives were regressed out from the whole-brain images.
Definition of insula subregions
Using a data-driven clustering technique, Deen et al. parcellated the human insular lobe into 2, 3, and 4 subregions for each hemisphere, and found that the 2 subregions of insula, anterior and posterior, closely resembled the clustering of DTI-based connectivity patterns (Deen et al., 2011). According to this study, two insular subregions in each hemisphere were defined in the present study. First, the left and right insula were defined anatomically by drawing insular gray matter on the MNI152 standard brain, and whole-brain connectivity maps were computed for each voxel in the insula. After normalization, these resting-state time series were used as regressors in general linear model-based analyses for each subject, and the resulting beta maps were averaged across subjects to obtain the subject-averaged beta map for a given voxel in the insula. Then, these subject-averaged beta maps were treated as vectors, and k-means clustering analysis (k = 2) was applied using squared Euclidean distance as the distance measure, separately for the left and right insula. The k-means algorithm was repeated 100 times, and the solution that minimized within-cluster variance was chosen. Finally, the insula was divided into anterior and posterior subregions (see Fig. 1).
Gray matter volume analysis
Structural data processing was performed using SPM12, including normalization to the standard MNI space, segmentation, modulation, and smoothing with an 8-mm FWHM Gaussian kernel. The bilateral hippocampi were defined using the Anatomy Toolbox in SPM12. The bilateral hippocampal volumes and insula subregional volumes were then extracted from the smoothed modulated gray matter maps.
Functional connectivity analysis
Seed-to-voxel correlation analysis was conducted using the CONN toolbox v17c. The mean time series of each insula subregion seed was computed as the reference time course for each individual. Correlation maps were generated by Pearson correlation analysis between each seed time series and those of the other brain voxels. Fisher’s r-to-z transformation was applied for group analysis to improve the normality of the correlation coefficients (Lowe et al., 1998).
Statistical analysis
Demographic data and neuropsychological assessments were analyzed using SPSS 22. Student’s t tests were used to compare continuous variables, including age, years of education, neuropsychological assessment scores, and gray matter volumes. Chi-square tests were used to examine differences in sex distribution between the groups.
The patterns of voxelwise insula subregional FC within each group were determined using one sample t tests with no covariates. The results were thresholded at a cluster-level p < 0.05 and underwent familywise error (FWE) correction. Then, a 2 (time: follow-up versus baseline) × 2 (group: aMCI versus HCs) flexible factorial design was employed, with four conditions, aMCI baseline (aMCI1), aMCI follow-up (aMCI2), HCs baseline (HCs1), and HCs follow-up (HCs2), to detect the main and interaction effects. The covariates used in the between-group analysis included age, sex, educational level, follow-up interval, insula subregion gray matter volume, and global correlation (GCOR) (Saad et al., 2013). GCOR was automatically calculated in the denoising step in CONN. GCOR is the brain-wide average correlation coefficient between every pair of voxels across the entire brain, and it is a conservative approach that can reduce global variations. The results were thresholded at a cluster-level p < 0.05 and FWE corrected. The brain regions were visualized with xjView (http://www.alivelearn.net/xjview).
For each insula subregion, the main effects of “group” were defined as [(HCs1 + HCs2) vs. (aMCI1 + aMCI2)], and the main effects of “time” were defined as [(aMCI1 + HCs1) vs. (aMCI2 + HCs2)]. The “group” × “time” interaction effects were evaluated for increased FC of the insula subregions in aMCI patients relative to HCs by [(aMCI2 - aMCI1) - (HCs2 - HCs1)] and decreased FC of the insula subregions in aMCI patients relative to HCs by [(aMCI1 - aMCI2) - (HCs1 - HCs2)].
Post hoc pairwise comparisons and correlation analyses were conducted using SPSS 22. The regions showing significant “group” × “time” interaction effects were defined as regions of interest (ROIs), and the connectivity strength of each ROI was extracted for comparison analysis and correlation analysis. First, independent sample t tests were used to compute the group differences in FC between HCs and aMCI patients at baseline and follow-up. Then, paired-sample t tests were performed to explore the longitudinal FC changes within each group. Finally, Pearson’s correlations were performed to evaluate (1) the relationship between the FC of each ROI and neuropsychological data in aMCI patients at baseline, (2) the relationship between the FC of each ROI and neuropsychological data in aMCI patients at follow-up, and (3) the relationship between the changes in FC in each ROI and changes in neuropsychological data in aMCI patients over time.
Results
Demographic and neuropsychological tests
The demographic data and neuropsychological scores of all the subjects are shown in Table 1. There were no significant differences in age, sex, educational level, or insula subregional volumes between aMCI patients and HCs, whereas significant volumetric differences in the bilateral hippocampi were found. Compared to HCs, aMCI patients showed significant cognitive decline, as revealed by the MMSE, MoCA, AVLT, and CDR scores at baseline and follow-up (p < 0.001 for all comparisons). As the disease progressed, more severe memory impairment was confirmed by a lower cognitive level at follow-up than at baseline, as assessed by the MMSE (p < 0.017) and MoCA (p < 0.048) scores. Two aMCI subjects converted to mild AD, whereas the other aMCI subjects and all the HCs remained stable (Table 1).
Within-group FC of the insula subregions
The within-group FC maps of the insula subregions in HCs and aMCI patients exhibited similar patterns at baseline and follow-up (Supplemental Figures S1-S4). Significant connectivity was identified between the left AIC and the bilateral ACC, paracingulate gyrus, putamen, frontal orbital cortex, inferior frontal gyrus, and temporal pole. The right AIC was positively associated with the bilateral ACC, central opercular cortex, frontal orbital cortex, and right junction region of the temporal, frontal, and parietal lobes. The bilateral PIC exhibited significant connectivity with the bilateral ACC; central opercular cortex; precentral, postcentral, and supramarginal gyri; parietal operculum cortex; and junction region of the temporal, frontal and parietal lobes.
Between-group differences in longitudinal insula subregional FC changes
No significant differences were found in the main effect of “group” or the main effect of “time”.
The differences in the longitudinal changes in insula subregional FC between aMCI patients and HCs were explored in the “group” × “time” interaction effect. Compared with HCs, aMCI patients showed significantly increased FC between the right AIC and left ACC, as well as significantly decreased FC between the left PIC and right precuneus (Table 2; Fig. 2).
Longitudinal alterations in insula subregional FC in aMCI patients and HCs. (a) Increased FC between the right AIC and left ACC in aMCI patients compared to HCs at follow-up vs. baseline (interaction effect). (b) Decreased FC between the left PIC and right precuneus in aMCI patients compared to HCs at follow-up vs. baseline (interaction effect). FC, functional connectivity; ACC, anterior cingulate cortex; AIC, anterior insula cortex; PIC, posterior insula cortex
Post hoc pairwise comparisons for FC between the right AIC and the left ACC showed that (1) at baseline, aMCI patients exhibited significantly decreased FC compared with HCs; (2) at follow-up, no significant group differences in FC were found between aMCI patients and HCs; (3) compared with baseline, aMCI patients demonstrated increased FC at follow-up; and (4) compared with baseline, HCs demonstrated decreased FC at follow-up.
Post hoc pairwise comparisons for FC between the left PIC and the right precuneus showed that (1) at baseline, aMCI patients exhibited significantly increased FC compared with HCs; (2) at follow-up, aMCI patients exhibited significantly decreased FC compared with HCs; (3) compared with baseline, aMCI patients demonstrated decreased FC at follow-up; and (4) compared with baseline, HCs demonstrated increased FC at follow-up.
Relationship between insula subregional FC and cognitive performance
In aMCI patients, Pearson’s correlations were performed between the FC values of the two abovementioned ROIs and the MMSE, MoCA, AVLT-immediate recall, AVLT-delayed recall, and AVLT-recognition scores. These correlation analyses were applied for baseline data, follow-up data, and longitudinal changes. Therefore, statistical significance was set at p < 0.05 and Bonferroni corrected for multiple comparisons (p < 0.0016, 30 multiple comparisons). In the aMCI group, the FC between the right AIC and left ACC was significantly correlated with MMSE score (r = 0.721 p < 0.001) at follow-up, suggesting that stronger FC may contribute to the maintenance of cognitive ability (Fig. 3).
Discussion
The current study demonstrated distinct longitudinal changes in insula subregional FC in aMCI patients by analyzing longitudinal FC data acquired from aMCI patients and HCs. There were two primary findings in aMCI patients compared to HCs over time: the FC between the right AIC and left ACC increased, whereas the FC between the left PIC and right precuneus decreased. In addition, at follow-up, the FC between the right AIC and left ACC was positively associated with global cognition in aMCI patients, as measured by the MMSE score.
A higher level of cognition is attributed to coalition and interaction of different neural networks rather than isolated networks (**, 33(6), 1352–1363. https://doi.org/10.1002/hbm.21291 ." href="/article/10.1007/s11682-024-00899-2#ref-CR37" id="ref-link-section-d9623685e1616">2012b). The insula plays a key role in the interaction of sophisticated neural networks. As the disease progresses, aMCI patients may experience different outcomes. Longitudinal studies are more meaningful than cross-sectional studies for investigating the functional neural basis of cognitive deterioration in aMCI patients. The present study revealed that the longitudinal trajectories of insula subregional FC differed between aMCI patients and HCs during follow-up.
Previous studies have described the AIC and ACC as the two most important anchor points of the SN (Menon, 2011). The AIC is responsible for switching between cognitive resources (Touroutoglou et al., 2012), and the ACC is known to be a signal area needed for enhanced cognitive control (Egner, 2009). Unlike disrupted DMN connectivity (Beason-Held, 2011), previous studies reported that SN connectivity was enhanced in patients with AD (Chand et al., 2017b; Zhou et al., 2010). In the present study, the FC between the right AIC and left ACC significantly increased over time in aMCI patients compared to HCs. Furthermore, the FC values were positively associated with the MMSE scores at follow-up. This increased FC between the AIC and ACC might be a compensatory neural mechanism for disease progression and could play a critical role in partially maintaining cognitive function in aMCI patients.
We also found that the FC between the left PIC and right precuneus decreased longitudinally. The precuneus is widely recognized as an essential part of the DMN, which is involved in several cognitive processes (Patel et al., 2015; Zhang & Li, 2012). Furthermore, the precuneus is one of the earliest regions where β-amyloid (Aβ) accumulates (Wolk & Klunk, 2009). Reduced FC of the precuneus has been reported in aMCI patients in previous studies (Binnewijzend et al., 2012; Lee et al., 2016; Qi et al., 2010). Significantly reduced FC between the left PIC and bilateral precuneus was reported in patients with isolated rapid eye movement sleep behavior disorder and was associated with MoCA scores (Byun et al., 2021). The results of the present study showing reduced FC between the left PIC and the right precuneus are consistent with those of previous studies, and this reduced FC may be an underlying mechanism of progressive cognitive decline in aMCI patients.
There were several limitations in this study. First, the sample size was relatively small, and studies with larger sample sizes are needed to replicate the current findings. Second, the mean follow-up interval in aMCI patients and HCs was group-matched, but there were intersubject variations. Although this factor was included as a nuisance covariate in our data analyses, we cannot entirely rule out the influence of the follow-up interval on the current results. Third, without positron emission tomography (PET) examination and CSF analysis, the criteria used in the present study indicated an intermediate likelihood that MCI was due to AD. The A (β-amyloid) – T (tau) – N (neurodegeneration) system should be referred to in future studies (Albert et al., 2011). Finally, resting-state fMRI can be influenced by pulse and breathing. This physiological information should be recorded during scanning and included in data preprocessing to reduce the influence of these variations in future studies.
Conclusions
The current study revealed different patterns of insula subregional FC changes in aMCI patients over time. These results provide new insights into the neurodegenerative process and may facilitate future research on neural markers of cognitive decline in aMCI patients.
Data availability
The datasets analyzed in the current study are not publicly available because the project is still in progress. However, the datasets are available from the corresponding author upon reasonable request.
References
Albert, M. S., Dekosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., Gamst, A., Holtzman, D. M., Jagust, W. J., Petersen, R. C., Snyder, P. J., Carrillo, M. C., Thies, B., & Phelps, C. H. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement, 7(3), 270–279. https://doi.org/10.1016/j.jalz.2011.03.008.
Beason-Held, L. L. (2011). Dementia and the default mode. Current Alzheimer Research, 8(4), 361–365. https://doi.org/10.2174/156720511795745294.
Behzadi, Y., Restom, K., Liau, J., & Liu, T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 37(1), 90–101. https://doi.org/10.1016/j.neuroimage.2007.04.042.
Binnewijzend, M. A., Schoonheim, M. M., Sanz-Arigita, E., Wink, A. M., van der Flier, W. M., Tolboom, N., Adriaanse, S. M., Damoiseaux, J. S., Scheltens, P., van Berckel, B. N., & Barkhof, F. (2012). Resting-state fMRI changes in Alzheimer’s disease and mild cognitive impairment. Neurobiology of Aging, 33(9), 2018–2028. https://doi.org/10.1016/j.neurobiolaging.2011.07.003.
Biswal, B., Yetkin, F. Z., Haughton, V. M., & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541. https://doi.org/10.1002/mrm.1910340409.
Byun, J. I., Cha, K. S., Kim, M., Lee, W. J., Lee, H. S., Sunwoo, J. S., Shin, J. W., Kim, T. J., Moon, J., Lee, S. T., Jung, K. H., Chu, K., Kim, M. H., Kim, H. J., Shin, W. C., Lee, S. K., & Jung, K. Y. (2021). Altered insular functional connectivity in isolated REM sleep behavior disorder: A data-driven functional MRI study. Sleep Medicine, 79, 88–93. https://doi.org/10.1016/j.sleep.2020.12.038.
Chand, G. B., & Dhamala, M. (2017b). Interactions between the anterior cingulate-insula network and the fronto-parietal network during perceptual decision. Neuroimage, 152, 381–389. https://doi.org/10.1016/j.neuroimage.2017.03.014.
Chand, G. B., Wu, J., Hajjar, I., & Qiu, D. (2017a). Interactions of the Salience Network and its subsystems with the default-Mode and the Central-Executive networks in normal aging and mild cognitive impairment. Brain Connectivity, 7(7), 401–412. https://doi.org/10.1089/brain.2017.0509.
Daselaar, S. M., Prince, S. E., & Cabeza, R. (2004). When less means more: Deactivations during encoding that predict subsequent memory. Neuroimage, 23(3), 921–927. https://doi.org/10.1016/j.neuroimage.2004.07.031.
Deen, B., Pitskel, N. B., & Pelphrey, K. A. (2011). Three systems of insular functional connectivity identified with cluster analysis. Cerebral Cortex, 21(7), 1498–1506. https://doi.org/10.1093/cercor/bhq186.
Egner, T. (2009). Prefrontal cortex and cognitive control: Motivating functional hierarchies. Nature Neuroscience, 12(7), 821–822. https://doi.org/10.1038/nn0709-821.
Kim, H. J., Lee, J. H., Cheong, E. N., Chung, S. E., Jo, S., Shim, W. H., & Hong, Y. J. (2020). Elucidating the risk factors for progression from amyloid-negative amnestic mild cognitive impairment to Dementia. Current Alzheimer Research, 17(10), 893–903. https://doi.org/10.2174/1567205017666201130094259.
Lee, E. S., Yoo, K., Lee, Y. B., Chung, J., Lim, J. E., Yoon, B., & Jeong, Y. (2016). Default Mode Network Functional Connectivity in Early and late mild cognitive impairment: Results from the Alzheimer’s Disease Neuroimaging Initiative. Alzheimer Disease and Associated Disorders, 30(4), 289–296. https://doi.org/10.1097/WAD.0000000000000143.
Liu, Z., Wei, W., Bai, L., Dai, R., You, Y., Chen, S., & Tian, J. (2014). Exploring the patterns of acupuncture on mild cognitive impairment patients using regional homogeneity. PLoS One, 9(6), e99335. https://doi.org/10.1371/journal.pone.0099335.
Liu, X., Chen, X., Zheng, W., **a, M., Han, Y., Song, H., Li, K., He, Y., & Wang, Z. (2018). Altered functional connectivity of insular subregions in Alzheimer’s Disease. Frontiers in Aging Neuroscience, 10, 107. https://doi.org/10.3389/fnagi.2018.00107.
Lowe, M. J., Mock, B. J., & Sorenson, J. A. (1998). Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage, 7(2), 119–132. https://doi.org/10.1006/nimg.1997.0315.
Menon, V. (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506. https://doi.org/10.1016/j.tics.2011.08.003.
Nellessen, N., Rottschy, C., Eickhoff, S. B., Ketteler, S. T., Kuhn, H., Shah, N. J., Schulz, J. B., Reske, M., & Reetz, K. (2015). Specific and disease stage-dependent episodic memory-related brain activation patterns in Alzheimer’s disease: A coordinate-based meta-analysis. Brain Struct Funct, 220(3), 1555–1571. https://doi.org/10.1007/s00429-014-0744-6.
Patel, G. H., Yang, D., Jamerson, E. C., Snyder, L. H., Corbetta, M., & Ferrera, V. P. (2015). Functional evolution of new and expanded attention networks in humans. Proc Natl Acad Sci U S A, 112(30), 9454–9459. https://doi.org/10.1073/pnas.1420395112.
Petersen, R. C., Stevens, J. C., Ganguli, M., Tangalos, E. G., Cummings, J. L., & Dekosky, S. T. (2001). Practice parameter: Early detection of dementia: Mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology Neurology, 56(9), 1133–1142. https://doi.org/10.1212/wnl.56.9.1133.
Petersen, R. C., Lopez, O., Armstrong, M. J., Getchius, T., Ganguli, M., Gloss, D., Gronseth, G. S., Marson, D., Pringsheim, T., Day, G. S., Sager, M., Stevens, J., & Rae-Grant, A. (2018). Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and implementation Subcommittee of the American. Academy of Neurology Neurology, 90(3), 126–135. https://doi.org/10.1212/WNL.0000000000004826.
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 59(3), 2142–2154. https://doi.org/10.1016/j.neuroimage.2011.10.018.
Qi, Z., Wu, X., Wang, Z., Zhang, N., Dong, H., Yao, L., & Li, K. (2010). Impairment and compensation coexist in amnestic MCI default mode network. Neuroimage, 50(1), 48–55. https://doi.org/10.1016/j.neuroimage.2009.12.025.
Saad, Z. S., Reynolds, R. C., Jo, H. J., Gotts, S. J., Chen, G., Martin, A., & Cox, R. W. (2013). Correcting brain-wide correlation differences in resting-state FMRI. Brain Connectivity, 3(4), 339–352. https://doi.org/10.1089/brain.2013.0156.
Scheltens, P., Leys, D., Barkhof, F., Huglo, D., Weinstein, H. C., Vermersch, P., Kuiper, M., Steinling, M., Wolters, E. C., & Valk, J. (1992). Atrophy of medial temporal lobes on MRI in probable Alzheimer’s disease and normal ageing: Diagnostic value and neuropsychological correlates. Journal of Neurology, Neurosurgery and Psychiatry, 55(10), 967–972. https://doi.org/10.1136/jnnp.55.10.967.
Seo, E. H., & Choo, I. L. (2016). Amyloid-independent functional neural correlates of episodic memory in amnestic mild cognitive impairment. European Journal of Nuclear Medicine and Molecular Imaging, 43(6), 1088–1095. https://doi.org/10.1007/s00259-015-3261-9.
Taylor, K. S., Seminowicz, D. A., & Davis, K. D. (2009). Two systems of resting state connectivity between the insula and cingulate cortex. Human Brain Map**, 30(9), 2731–2745. https://doi.org/10.1002/hbm.20705.
Touroutoglou, A., Hollenbeck, M., Dickerson, B. C., & Feldman, B. L. (2012). Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage, 60(4), 1947–1958. https://doi.org/10.1016/j.neuroimage.2012.02.012.
Wang, L., Zang, Y., He, Y., Liang, M., Zhang, X., Tian, L., Wu, T., Jiang, T., & Li, K. (2006). Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: Evidence from resting state fMRI. Neuroimage, 31(2), 496–504. https://doi.org/10.1016/j.neuroimage.2005.12.033.
Wang, Z., **a, M., Dai, Z., Liang, X., Song, H., He, Y., & Li, K. (2015). Differentially disrupted functional connectivity of the subregions of the inferior parietal lobule in Alzheimer’s disease. Brain Struct Funct, 220(2), 745–762. https://doi.org/10.1007/s00429-013-0681-9.
Wang, P., Li, R., Yu, J., Huang, Z., Yan, Z., Zhao, K., & Li, J. (2017). Altered distant synchronization of background network in mild cognitive impairment during an executive function Task. Frontiers in Behavioral Neuroscience, 11, 174. https://doi.org/10.3389/fnbeh.2017.00174.
Wang, S., Sun, H., Hu, G., Xue, C., Qi, W., Rao, J., Zhang, F., Zhang, X., & Chen, J. (2021). Altered Insular Subregional Connectivity Associated with cognitions for distinguishing the spectrum of pre-clinical Alzheimer’s Disease. Frontiers in Aging Neuroscience, 13, 597455. https://doi.org/10.3389/fnagi.2021.597455.
Whitfield-Gabrieli, S., & Nieto-Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. https://doi.org/10.1089/brain.2012.0073.
Wolk, D. A., & Klunk, W. (2009). Update on amyloid imaging: From healthy aging to Alzheimer’s disease. Current Neurology and Neuroscience Reports, 9(5), 345–352. https://doi.org/10.1007/s11910-009-0051-4.
**, 33(6), 1352–1363. https://doi.org/10.1002/hbm.21291.
**e, C., Bai, F., Yu, H., Shi, Y., Yuan, Y., Chen, G., Li, W., Chen, G., Zhang, Z., & Li, S. J. (2012a). Abnormal insula functional network is associated with episodic memory decline in amnestic mild cognitive impairment. Neuroimage, 63(1), 320–327. https://doi.org/10.1016/j.neuroimage.2012.06.062.
Zanchi, D., Montandon, M. L., Sinanaj, I., Rodriguez, C., Depoorter, A., Herrmann, F. R., Borgwardt, S., Giannakopoulos, P., & Haller, S. (2017). Decreased Fronto-Parietal and increased default Mode Network activation is Associated with subtle cognitive deficits in Elderly Controls. Neurosignals, 25(1), 127–138. https://doi.org/10.1159/000486152.
Zhang, S., & Li, C. S. (2012). Functional connectivity map** of the human precuneus by resting state fMRI. Neuroimage, 59(4), 3548–3562. https://doi.org/10.1016/j.neuroimage.2011.11.023.
Zhang, J., Wang, J., Xu, X., You, Z., Huang, Q., Huang, Y., Guo, Q., Guan, Y., Zhao, J., Liu, J., Xu, W., Deng, Y., **e, F., & Li, B. (2023). In vivo synaptic density loss correlates with impaired functional and related structural connectivity in Alzheimer’s disease. Journal of Cerebral Blood Flow and Metabolism, 43(6), 977–988. https://doi.org/10.1177/0271678X231153730.
Zhou, J., Greicius, M. D., Gennatas, E. D., Growdon, M. E., Jang, J. Y., Rabinovici, G. D., Kramer, J. H., Weiner, M., Miller, B. L., & Seeley, W. W. (2010). Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain, 133(Pt 5), 1352–1367. https://doi.org/10.1093/brain/awq075.
Funding
This work was supported by grants from the R&D Program of the Bei**g Municipal Education Commission (KM202310025013), the National Natural Science Foundation of China (62076169), the Bei**g Chao-Yang Hospital Multidisciplinary Team Program (CYDXK202201, CYDXK202207), and the Bei**g Hospitals Authority’s Ascent Plan (DFL20220303).
Author information
Authors and Affiliations
Contributions
HL conducted the data collection and analysis and wrote the manuscript. XF conducted the data collection. KL contributed to the conceptualization of the study and revision of the manuscript. CZ contributed to the revision of the manuscript. XJ contributed to the conceptualization and design of the study and revised the manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures in the studies involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, H., Fan, X., Li, K. et al. Increased anterior insula connectivity associated with cognitive maintenance in amnestic mild cognitive impairment: a longitudinal study. Brain Imaging and Behavior (2024). https://doi.org/10.1007/s11682-024-00899-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s11682-024-00899-2