Abstract
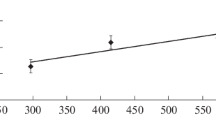
This research presents a study of the thermal synthesis of titanium carbide (TiC) within the Fe-Ti-C system, utilizing a physically grounded semi-empirical mathematical model. The model enables estimation of TiC formation, including nucleation, growth, and the associated time-temperature regime. It incorporates numerical descriptions of thermal, diffusion, and chemical processes, accounting for phase transformations and their dependencies on chemical composition. The study’s key focus is on assessing the impact of system parameters, including chemical composition and furnace temperature, on the kinetics of TiC thermal synthesis. Simulation results demonstrate the profound influence of Fe content on combustion temperature and particle sizes, aligning closely with experimental data. The research not only examines average size values but also delves into carbide particle size distribution. The findings provide valuable insights into optimizing Fe-TiC composites production processes and the developed computer model serves as a useful tool for preliminary investigations, and it complements experimental research, offering a holistic understanding of system behavior and responses to parameter variations.







Similar content being viewed by others
Data Availability
All data that support the findings of this study are included within the article (and any supplementary files).
References
D.J. Miller and J.A. Pask, Liquid-phase sintering of TiC-Ni composites, J. Am. Ceram. Soc., 1983, 66, p 841–846. https://doi.org/10.1111/j.1151-2916.1983.tb10998.x
H. Nakanishi, I. Tanaka, T. Okamoto, Y. Miyamoto, and O. Yamada, Reaction heat sintering using HIP—densification of TiC-Ni composites, J. Jpn. Soc. Powder Powder Metall., 1990, 37, p 105.
M.R. Rahimipour and M. Sobhani, Evaluation of centrifugal casting process parameters for in situ fabricated functionally gradient Fe-TiC composite, Met. Mater. Trans. Mater. Process. Sci., 2013, 44, p 1120–1123. https://doi.org/10.1007/s11663-013-9903-z
I.S. Deschamps, D. dos Santos Avila, E. Vanzuita Piazera, R.R.C. Dudley Cruz, C. Aguilar, and A. NelmoKlein, Design of in situ metal matrix composites produced by powder metallurgy a critical review, Metals, 2022, 12, p 2073. https://doi.org/10.3390/met12122073
M. Jõeleht, The influence of sintering temperature of reactive sintered (Ti, Mo)C-Ni cermets, Mater. Sci., 2015, 21(21), p 435–438. https://doi.org/10.5755/j01.ms.21.3.7179
A.N. Tabachenko, T.A. Panteleeva, and V.I. Itin, Interaction of titanium and carbon burning in the presence of a solution-melt, Combust. Explos. Shock Waves, 1984, 20, p 387–391.
S.D. Dunmead, D.W. Readey, C.E. Selmer, and J.B. Hol, Kinetics of combustion synthesis in the Ti-C and Ti-C-Ni Systems, J. Am. Ceram. Soc., 1989, 72, p 2318–2324. https://doi.org/10.1111/j.1151-2916.1989.tb06083.x
Y. Choi and S.-W. Rhee, Effect of iron and cobalt addition on TiC combustion synthesis, J. Mater. Res., 1993, 8, p 3202–3209. https://doi.org/10.1557/JMR.1993.3202
K. Feng, C. Bai, Y. Yang, W. Wang, and F. Ji, Combustion synthesis of TiC–Fe composites under the action of an electric field, ISIJ Int., 2007, 42, p 648–651. https://doi.org/10.2355/isi**ternational.47.648
S. **, H. Su, and G. Sha, Atom probe tomography analysis of TiCx powders synthesized by SHS in Al/Fe/Cu–Ti–C systems, Materials, 2019, 12, p 1–10. https://doi.org/10.3390/ma12244095
A. Saidi, A. Chrysanthou, and J.V. Wood, Characteristics of the combustion synthesis of TiC and Fe-TiC composites, J. Mater. Sci., 1994, 29, p 4993–4998. https://doi.org/10.1007/BF01151089
J. Wang and S.J. Fu, Fe–Ti–C composite produced by self-propagating high temperature synthesis, Powder Metall., 2008, 51, p 295–297. https://doi.org/10.1179/174329007X204894
H. Zhu, K. Dong, H. Wang, J. Huang, J. Li, and Z. **e, Reaction mechanisms of the TiC/Fe composite fabricated by exothermicp dispersion from Fe–Ti–C element system, Powder Technol., 2013, 246, p 456–461. https://doi.org/10.1016/j.powtec.2013.06.002
A.G. Merzhanov, Fundamentals, achievements, and perspectives for development of solid-flame combustion, Russ. Chem. Bull., 1997, 46, p 1–27.
A.G. Merzhanov, The theory of stable homogeneous combustion of condensed substances, Combust. Flame, 1969, 13, p 143–156.
O. Verezub, Z. Kálazi, G. Buza, N.V. Verezub, and G. Kaptay, Classification of laser beam induced surface engineering technologies andin situsynthesis of steel matrix surface nanocomposites, Surf. Eng., 2011, 27, p 428–435. https://doi.org/10.1179/174329409X446296
E.O. Mokhnache, G.-S. Wang, L. Geng, K. Balasubramaniam, A. Henniche, and N. Ramdani, In situ (α-Al2O3+ZrB2)/Al composites with network distribution fabricated by reaction hot pressing, Int. J. Miner. Met. Mater., 2016, 22, p 1092–1100.
M. Zhu, Y. Jiang, Y. Sui, and M. Zhou, Study on microstructure and abrasive wear properties of in-situ TiC reinforced high chromium cast iron matrix composite, Mater. Res. Express., 2022 https://doi.org/10.1088/2053-1591/ac5b02
J.B. Holt and Z.A. Munir, Combustion synthesis of titanium carbide: theory and experiment, J. Mater. Sci., 1986, 21, p 251–259.
M.X. Zhang, Q.D. Hu, B. Huang, J.Z. Li, and J.G. Li, Study of formation behavior of TiC in the Fe–Ti–C system during combustion synthesis, Int. J. Refract. Met. Hard Mater., 2011, 29, p 356–360. https://doi.org/10.1016/j.ijrmhm.2011.01.001
V.V. Kaverinsky, G.A. Bagliuk, and Z.P. Sukhenko, Numerical simulation of in situ reaction synthesis of TiC reinforced aluminum matrix composite from elemental Al-Ti-C powders, J. Mater. Eng. Perform., 2023 https://doi.org/10.1007/s11665-023-08650-6
A.A. Vasilyev and P.A. Golikov, Carbon diffusion coefficient in alloyed ferrite, Mater. Phys. Mech., 2019, 39, p 111–119. https://doi.org/10.18720/MPM.3912018_17
J. Agren, A revised expression for the diffusivity of carbon in binary Fe-C austenite, Scr. Metall., 1986, 20, p 1507–1510.
A. Vasilyev, Carbon diffusion coefficient in complexly alloyed austenite, Mater. Sci. Technol., 2018, 2018, p 537–551. https://doi.org/10.13140/2.1.5050.9125
L. Scotti and A. Mottura, Interstitial diffusion of O, N, and C in α-Ti from first-principles: analytical model and kinetic Monte Carlo simulations, J. Chem. Phys., 2016, 144, 084701. https://doi.org/10.1063/1.4942030
F.J.J. Loo and G.F. Bastin, On the diffusion of carbon in titanium carbide, Metall. Trans. A, 1989, 20, p 403–411. https://doi.org/10.1007/bf02653919
A.M. Skrebtsov, A.G. Siaditi, and Y.A. Demchenko, Diffusion of carbon and chromium in steel casting lade during exploitation, Bull. Azov State Tech. Univ., 1999, 8, p 37–40.
V.P. Shapovalov and A.N. Kurasov, Diffusion of titanium in iron, Met. Sci. Heat Treat., 1975, 17, p 803–805. https://doi.org/10.1007/bf00703075
P. Klugkist and C. Herzig, Tracer diffusion of titanium in α-iron, Phys. Stat. Sol. A, 1995, 148, p 413–421. https://doi.org/10.1002/pssa.2211480209
E. A. Krivonosova. Modeling of kinetics of modifying phases formation during welding and high concentration energy processing. Bulletin of Tul.S.U. Technical sciences. 6 (2015). 71–83.
L. Scotti and A. Mottura, Diffusion anisotropy of poor metal solute atoms in hcp-Ti, J. Chem. Phys., 2015, 142, p 204–308. https://doi.org/10.1063/1.4921780
W. Gierlotka, G. Lothongkum, B. Lohwongwatana, and C. Puncreoburt, Atomic mobility in titanium grade 5 (Ti6Al4V), J. Min. Metal. B., 2019, 55, p 65–77. https://doi.org/10.2298/JMMB180620030G
L. Scotti and A. Mottura, First-principles investigation of solute diffusion mechanisms in alpha-Ti. In Proceedings of the 13th World Conference on Titanium (2016), pp. 1901–1906. https://doi.org/10.1002/9781119296126.ch318
C. Yu, P. Cao, and M.I. Jones, Microstructural evolution during pressureless sintering of blended elemental Ti-Al-V-Fe titanium alloys from fine hydrogenated-dehydrogenated titanium powder, Metals, 2017, 7, p 285–304. https://doi.org/10.3390/met7080285
S.L. Makurov, Study of iron based melts viscosity by crucible torsional vibrations, Bull. Azov State Tech. Univ., 1997, 2, p 40–42.
S. Li, Y. Chen, J. Kang, Y. Huang, J.A. Gianetto, and L. Yin, Interfacial microstructures and mechanical properties of dissimilar titanium alloy and steel friction stir butt-welds, J. Manuf. Process., 2019, 40, p 160–168. https://doi.org/10.1016/j.jmapro.2019.03.015
C. Yu and P. Cao, Microstructural evolution during pressureless sintering of blended elemental Ti-Al-V-Fe titanium alloys from fine hydrogenated-dehydrogenated titanium powder, Metals, 2017, 7, p 285. https://doi.org/10.3390/met7080285
H. Nakajima, S. Ohshida, K. Nonaka, Y. Yoshida, and F.E. Fujita, Diffusion of iron in β Ti-Fe alloys, Scr. Mater., 1996, 34, p 949–953. https://doi.org/10.1016/1359-6462(95)00617-6
H.W. Mead and C.E. Birchenall, Self-diffusion of iron in Austenite, JOM, 1956, 8, p 1336–1339. https://doi.org/10.1007/bf03377878
F. Buffington, K. Hirano, and M. Cohen, Self diffusion in iron, Acta Metall., 1961, 9, p 434–439. https://doi.org/10.1016/0001-6160(61)90137-7
A. Meyer, L. Hennig, F. Kargl, and T. Unruh, Iron self diffusion in liquid pure iron and iron-carbon alloys, J. Phys. Condens. Matter, 2019, 31, p 395–401. https://doi.org/10.1088/1361-648x/ab2855
H.S. Zurob, C.R. Hutchison, Y. Brechet, and G. Purdy, Modelling recrystallisation of microalloyed austenite: effect of coupling recovery, presipitation and recrystallization, Acta Mater., 2002, 50, p 3075–3092. https://doi.org/10.1016/S1359-6454(02)00097-6
T. Nawaya, W. Back, and A. von Hehl. Tensile properties of α-titanium alloys at elevated temperatures. In MATEC Web of Conferences 321 “The 14th World Conference on Titanium”. (2020). https://doi.org/10.1051/matecconf/202032104016
H.S. Zurob, C.R. Hutchison, Y. Brechet, and G. Purdy, A model for the competition of precipitation and recrystallization in deformed austenite, Acta Mater., 2001, 49, p 4183–4190. https://doi.org/10.1016/S1359-6454(01)00315-9
D.V. Bavbande, R. Mishra, and J.M. Juneja, Studies on the kinetics of synthesis of TiC by calciothermic reduction of TiO2 in presence of carbon, J. Therm. Anal. Calorim., 2004, 78, p 775–780. https://doi.org/10.1155/2015/469121
A.I. Gusev, Phase equilibria, phases and compounds in the Ti–C system, Russ. Chem. Rev., 2002, 71, p 507–532. https://doi.org/10.1070/RC2002v071n06ABEH000721
W. Gąsior and A. Dębski, Enthalpy of formation of intermetallic phases from Fe-Ni-Ti system. Comparative studies, Arch. Metall. Mater., 2012, 4, p 1095–1104. https://doi.org/10.2478/v10172-012-0122-4
B. Dutta, E.J. Palmiere, and C.M. Sellars, Modelling the kinetics of strain induced precipitation in Nb microalloyed steels, Acta Mater., 2001, 49(49), p 785–794.
M. Perez, N. Dumont, and D. Acevedo-Reyes, Implementation of classical nucleation and growth theories for precipitation, Acta Mater., 2008, 56, p 2119–2132. https://doi.org/10.1016/j.actamat.2007.12.050
S. F. Sokolov. Research and modeling of the evolution of microstructure and resistance to deformation of steels during hot forming: dissertation. Ph.D. tech. sciences: 05.16.05, 05.16.01. St. Petersburg (2013).
K. Xu, B.G. Thomas, and R. Malley, Equilibrium model of precipitation in micro-alloyed steels, Metall. Mater. Trans. A, 2011, 42A, p 524–539.
M. Verdier, Y. Brechet, and P. Guyot, Recovery of AlMg alloys: flow stress and strain-hardening properties, Acta Mater., 1999, 47, p 127–134.
Acknowledgments
The presented study was performed within the departmental project 1030 III-5-21 “Scientific and technological principles of synthesis and consolidation of high-wear composites based on alloys of aluminium and titanium reinforced with high-modulus compounds” of the National Academy of Science of Ukraine.
Author information
Authors and Affiliations
Contributions
V.V. Kaverinsky helped in investigation, formal analysis, writing–original draft, visualization, software, methodology. G.A. Bagliuk was involved in conceptualization, resources, validation, writing–review & editing, supervision, project administration. Z.P. Sukhenko contributed to validation, resources, data curation, writing–review & editing, visualization.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
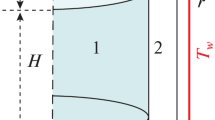
Appendix 1. Heat Transfer Calculation Data and Equations
Α coefficient dictated by the Nusselt number (Nu):
where λ is thermal conductivity coefficient of the environment, W/m·K; l is a characteristic size, m.
The empirical equations used for the Nusselt number estimation:
For the flat horizontal surfaces:
For the cylindrical side surfaces
where PrT-env is Prandtl number of the environment at the common environment temperature; PrT-env is Prandtl number of the environment at the heat transfer surface temperature.
Values of the empirical parameters in Eq 12 and 13 depending on the Pr × Gr product are given in Table
2.
The Grashof number:
where g is gravitation acceleration, m/s2; l is a characteristic size, m; β is the coefficient of volume expansion of the environment, K-1; ΔT is the difference between the object surface and the environment temperatures, K; υ is the kinematic viscosity of the environment, m2/s.
The effective approximate value of volume expansion coefficient β:
where T1 and T2 are, correspondently, the bulk and near the surface (≈ the sample surface temperature) temperatures of the environment.
The temperature dependences of the kinematic viscosity υ and thermal conductivity coefficient of the environment λ were given through a second-degree polynomial with empirical coefficients (16):
The following values of the factors a0, a1 and a2 were used:
For kinematic viscosity: a0 = 1.31 × 10−5, a1 = 9.97 × 10−8, a2 = 6.49 × 10−11
For thermal conductivity: a0 = 0.0246, a1 = 7.57 × 10−5, a2 = − 2.02 × 10−8
For the radiation heat transfer the heat flux from/to the surface:
where εnp is emissivity factor; C0 is black body emissivity, W/m2·K4 C0 = 5.67 × 10−8; Tout is the temperature at the muffle wall surface (≈ inner environment temperature), K; Tsurf is the temperature of the sample surface, K.
The reduced emissivity factor εnp depends of the ones of the sample and the muffle wall:
where ε1 and ε2 are correspondently the emissivity factors of the sample and the muffle wall.
The true values of the emissivity factors are not precisely known and could somewhat differ in real systems. For the modeling purpose they assumed to be dependent on temperature according to the following proposed empirical formulas for their estimation:
So the resulting emissivity factor εnp varied from ~ 0.05 to ~ 0.6 in the considered temperature range.
Appendix 2. Thermophysical Properties Used in the Simulation
Reactions heat effects estimation equations (grounded on the data from Ref 46, 47):
where xC and xFe are, correspondently, atomic fractions of C and Fe in the formed compound assuming that TiC and TiFe2 have wide homogeneity regions, and the value of enthalpy depends on the exact composition.
The temperature dependences of the specific heat (Cp) and density (ρ) values were approximated by second degree polynomial equations like 23:
The values of the empirical factors a0, a1 and a2 are presented in Table
3. The presented values are the result of our approximations made based on data collected from different resources such as internet searches, paper reference books, software databases, etc.
Appendix 3. Equations Used for Particles Nucleation Description
The excess phases’ particles nucleation is described by the equation from Ref 48:
where Nn is a volumetric density of the potential nucleation centers, m-−3; FZ is the Zeldovich factor [49]; β is a factor corresponding to the diffusive accession of the atoms to the nuclei of critical size; ΔGC is a thermodynamic barrier of nucleation, J/mol.
The Zeldovich factor FZ allows to conceder the possibility of the fluctuation dissolution of the nuclei with a size more than the critical one. It can be estimated as follows:
where nc is the number of the effective elemental volumes of the compound in the nuclei.
If to assume a spherical shape of the nuclei nc could be calculated by the following formula 26:
where RC is the critical radius of the nuclei, m; Va—the conventional effective elemental volume of the compound.
The conditional elementary volume Va is calculated as the ratio of the molar volume of a given compound to the Avogadro’s number.
The critical radius RC is calculated according to Ref 50 by Eq 27:
where γp/γ is the specific surface energy on the border particle/matrix, J/m2; ΔGp is the volumetric Gibbs energy change, J/m3.
The value of ΔGp was estimated in the way like in [51]:
where Vm is the molar volume of the compound, m3/mol; XMe and XC* are molar parts of the components (if it is a carbide XC* is the part of carbon); XMeeq and XC*eq are the equilibrium molar parts of the components in the solid solution.
It was assumed that the nuclei appear at the dislocations grid modes. So the potential nucleation sites density was estimated according to Ref 48 by formula 29:
where ρd is dislocation density, m−1.
According to the considerations from Ref 50 and 52 the dislocation density was estimated as follows (30):
where σY is the yield strength of the phase, Pa; μ is the shear modulus, Pa; b is the Burgers vector modulus value, m; α is an empirical factor.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaverinsky, V.V., Bagliuk, G.A. & Sukhenko, Z.P. Numerical Simulation of In Situ Reaction Synthesis of TiC-Reinforced Steels from Elemental Fe-Ti-C Powders. J. of Materi Eng and Perform (2024). https://doi.org/10.1007/s11665-024-09574-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11665-024-09574-5