Abstract
The competitive growth mechanisms of ternary eutectic and peri-eutectic transitions of liquid Fe–Ni–Ti alloys were investigated by drop tube technique. As liquid undercooling and cooling rate increased, the microstructure of eutectic Ni40.6Fe36.4Ti23 alloy transformed from the mixture of primary Ni3Ti phase and ternary eutectic into complete ternary eutectic. The predominant growth mode of independently grown Fe2Ti intermetallic phase varied from nonfaceted to faceted growth, while the γ-Fe(Ni) and Ni3Ti phases kept cooperative growth and formed a lamellar structure owing to the coherent interface along \((1 \overline{1}1)_{\gamma }\) and \((0001)_{{{\text{Ni}}_{{\text{3}}} {\text{Ti}}}} \). The peri-eutectic transformation in Fe66.5Ni17.6Ti15.9 alloy was suppressed due to the preferential growth of the primary α′-Fe phase as droplet size decreased. A needle-like Fe2Ti phase precipitated from the supersaturated α′-Fe phase during rapid solidification, and the α-Fe/Fe2Ti interface was characterized as semicoherent. The wear rate and friction coefficient of the ternary eutectic alloy firstly increased and then decreased as controlled by the combined factors of the microstructural transition and grain refinement. For the peri-eutectic alloy, the refinement of microstructure significantly improved the friction performance after rapid solidification. Moreover, the ternary eutectic alloy had a better wear resistance than peri-eutectic alloy, ascribed to the hardening intermetallic phases and the refined microstructure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The process of many commercial materials involves multiphase transition, and their properties are determined by the microstructure formed during the solidification and subsequent processing stages.[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18] One of the essential challenges to materials science is to understand multiphase transition mechanisms and how they can be controlled via deliberate selection of processing parameters. In the case of ternary alloys, the phase transitions involving liquid phase and three solid phases are summarized into three types, i.e., eutectic transition (L → a + b + c), peri-eutectic transition (L + a → b + c), and peritectic transition (L + a + b → c). During the ternary eutectic transition, the three component atoms in the liquid phase in the growth front are redistributed and arranged. The growth mode includes the cooperative growth of the three solid phases (a-b-c) and the pairwise cooperation growth (a-b, b-c, c-a). The exact kind of pattern depends on the volume fractions, the diffusion coupling in the liquid between each of the phases, and the three solid/liquid interfacial energies. Ruggiero and Rutter[19] and Lewis et al.[20] predicted five idealized structures of ternary eutectic as combinations of fibrous and lamellar phases based on geometrical considerations. Many of their predicted patterns were observed in the systems Bi–In–Sn and Ag–Cu–Sn. Indeed, there are more complex structures, such as the “brick-like” structure in Al–Ag–Cu eutectic alloy, in which the two intermetallic phases, Ag2Al and Al2Cu show a close association, often forming aligned ribbons composed of alternating rod-like phases, separated by lamellar regions of a solid-solution aluminum phase.[21] The phases form a three-dimensional interpenetrating Chinese script network structure in the Al2O3–Y3Al5O12–ZrO2 ternary eutectic ceramic.[22]
Peri-eutectic transition, another multiphase transition in ternary alloys, has seldom been considered in the literature due to its complication. Sha et al.[8] reported the metastable peri-eutectic transition, L + Al13Fe4→α-Al + β-AlFeSi, in wrought Al alloys during Bridgman solidification at a growth velocity of lower than 30 mm min−1. The growth by direct reaction of Al13Fe4 with liquid is the main mechanism contributing to β-AlFeSi formation owing to the diffusion in the liquid is much faster than that in solid. Ruan et al.[23] investigated the peri-eutectic transition (L + (Cu)→ (Ag) + Cu3Sb) in Cu46.4Ag38Sb15.6 alloy. They confirmed the solidification product of peri-eutectic transition (L + a → b + c) generates in three manners: the formation of (b + c) eutectic by the reaction between L and a phases, the direct growth of anomalous (a + b + c) structure, or (b + c) eutectic from the melt. However, the detailed mechanism of peri-eutectic transition needs to be further understood, such as the competitive nucleation and growth among three solid phases and the relationship between the formation of primary phase and peri-eutectic transitions.
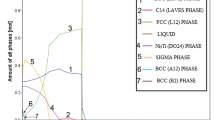
Ternary eutectic (L → Fe2Ti + Ni3Ti + γ-Fe(Ni)) and peri-eutectic (L + α-Fe → Fe2Ti + γ-Fe(Ni)) transitions are the two main phase transition types in ternary Fe–Ni–Ti alloy, as shown in Figure 1(a). Some investigations have reported the phase equilibria among Fe2Ti, Ni3Ti, and γ-Fe(Ni) phases.[24,25,26,27] In our previous work, the ternary eutectic consisted of a fibrous Fe2Ti phase and lamellar γ-Fe(Ni) + Ni3Ti phases was found.[27] However, the alloy undercooling only reaches 141 K in electromagnetic levitation (EML) experiments. The microstructure evolution and mechanical property of the ternary eutectic in higher undercooling are still unknown. Moreover, there are few reports on the solidification mechanism and mechanical properties of peri-eutectic alloy in Fe–Ni–Ti system, especially under rapid solidification conditions.
Rapid solidification has become an important approach to achieve potential performances and to develop new metallic materials.[28,29,30,31,32,33,34] During rapid solidification, the nucleation sequence, growth way, structure morphology, and phase selection of the various phases may exhibit new laws that are completely different from those in traditional solidification. Such as grain refinement, segregation reduction, and solution expansion. The optimization of solidification structure can significantly improve the performance of alloy. For example, Oloyede et al.[32] reported the microhardness of hypoeutectic gray cast iron increase with cooling rate and undercooling using drop tube technology. Therefore, the solidification mechanism and applied performances of the alloy involving multiphase transition under rapid solidification are critical scientific issues.
Drop tube technology, an effective method for rapid solidification of alloy melt, realizes the combination of high undercooling and large cooling rate.[33,34] In this work, the ternary eutectic and peri-eutectic transitions in ternary Fe–Ni–Ti alloy were investigated using drop tube technology. The competitive growth mechanism, growth mode transition, and phase interface characteristics in ternary eutectic and peri-eutectic alloys were discussed. Finally, the effect of undercooling and cooling rate on the tribological properties of the two alloys was evaluated by the nanoscratch tests.
Experimental Procedure
The rapid solidification experiments were performed in a 3 m drop tube. Figure 1(a) shows the equilibrium phase diagram of Fe–Ni–Ti, in which the compositions of Ni40.6Fe36.4Ti23 and Fe66.5Ni17.6Ti15.9 alloy were marked. The master alloys were prepared from high-purity Ni (99.999 pct), high-purity Fe (99.999 pct), and high-purity Ti (99.99 pct) by arc melting under an Ar atmosphere, and the mass loss was less than 3‰. During the experiments, the samples were placed in a quartz tube with a 0.3 mm orifice in the base. The quartz tube was then fixed at the top of drop tube. Before the melting process, the drop tube was evacuated to 2 × 10−4 Pa and backfilled with 50 pct helium and 50 pct argon gas to 105 Pa. The sample was melted and superheated to about 200 K above its liquidus temperature by a high-frequency induction supply. After that, the alloy melt was ejected through the orifice by pressurized argon gas. The droplets with the diameter range of 110 to 900 μm were undercooled and rapidly solidified during free fall.
The particles were sectioned, polished, and etched with a solution of 3 mL HF + 8 ml HNO3 + 30 ml H2O for about 6 seconds. The phase transition and phase constitutions of the alloy were determined by a Netzsch 404C DSC and 7000 Shimadzu polycrystalline XRD. The sample microstructures were analyzed by an FEI Verios G4 scanning electron microscope (SEM) which was equipped with an INCA 300 energy dispersive spectrometry (EDS). The nanocrystalline structure was observed using an FEI Talos F200X TEM, and the TEM samples were cut from the droplets by a dual-beam focused ion beam (FIB) in a FIB-SEM Helios G4 CX instrument.
The tribological property of rapidly solidified alloys was analyzed by nanoscratch tests using a Hysitron TI980 nanomechanics test system in constant load mode. A spherical diamond tip with an effective radius of ~1.16 μm was used for scratching. During the experiment, the normal load was ramped up to 3 mN at a rate of 0.6 mN s−1 at the marked location. Then the tip was moved 10 μm on the cross section of the droplets at a rate of 0.67 μm s−1. To obtain the average value and prevent possible overlap of the deformed zone, at least 5 scratches were performed at each sample with a 20 μm separation distance. The surface profiles of the scratches were characterized using an in-situ scanning probe microscopy (SPM). The pile-up heights and residual penetration depth were determined from the topography profiles of the scratches.
Results and Discussion
Phase Constitution and Thermal Analysis
Figure 1(b) illustrates the XRD patterns of master alloys. Ni40.6Fe36.4Ti23 eutectic alloy consisted of Fe2Ti, Ni3Ti, and γ-Fe(Ni) phases while Fe66.5Ni17.6Ti15.9 peri-eutectic alloy consisted of α-Fe, γ-Fe(Ni), and Fe2Ti phases. The DSC curves and microstructural morphologies of arc-melting and DSC samples are shown in Figure 2. The liquidus temperature of Ni40.6Fe36.4Ti23 eutectic alloy was analyzed to be 1418 K. On the DSC curve of Ni40.6Fe36.4Ti23 eutectic alloy, there were two small peaks on the right side of the ternary eutectic transition peaks. These were caused by the melting and solidification of a small amount of primary Ni3Ti phase. Therefore, the composition of the alloy is more likely to be located in the Ni3Ti phase region where is near the ternary eutectic point. Within the ternary Fe2Ti + Ni3Ti + γ-Fe(Ni) eutectic in master alloy shown in Figure 2(b), the Fe2Ti phase grew independently in a fibrous structure while the Ni3Ti and γ-Fe(Ni) phases formed with lamellar morphology. In DSC sample, the three phases grew cooperatively. During cooperative growth, a certain phase leads the nucleation and growth in the melt, and the excess solute discharged from the crystalline phase promotes the nucleation of other phases. Generally, the phases formed by this alternate nucleation growth have a specific orientation relationship. In macroscopic morphology, they have the same growth direction.
For Fe66.5Ni17.6Ti15.9 peri-eutectic alloy, as the alloy melt directly reached the peri-eutectic transition point with the decreased temperature, the melt was in the L, α-Fe, Fe2Ti, and γ-Fe(Ni) four-phase equilibrium region as shown in phase diagram, and all three solid phases had the opportunity to grow first. Obviously, α-Fe phase formed first and then the peri-eutectic transition occurred. Moreover, the first heating cycle showed that there was a solid-state phase transformation at 1056 K, but it did not appear in the second heating cycle. According to the solidified structure after two heating cycles, in equilibrium solidification (DSC sample), the primary α-Fe phase was firstly generated from the melt at 1526 K, corresponding to the first exothermic peak in the DSC curve. Then the peri-eutectic transformation L + α-Fe → Fe2Ti + γ-Fe(Ni) took place at 1461 K. However, the primary α′-Fe phase was supersaturated in non-equilibrium conditions (arc melting). The needle-like Fe2Ti phase precipitated from the α′-Fe phase at 1056 K, as seen in Figure 2(e). According to the EDS analysis results, the Fe, Ni, and Ti contents in the α-Fe phase were 78.52, 13.73, and 7.75 at. pct, and those in γ-Fe(Ni) phase were 58.87, 27.67, and 13.46 at. pct, respectively.
Microstructure Evolution During Rapid Solidification
Cooling rate and undercooling of alloy droplets
Droplet diameter is believed to dominate the microstructural evolution and crystal growth, which corresponds to the coupling effect of high undercooling and large cooling rate. However, the temperature of droplets is difficult to measure in real time due to the high falling speed and small size. Fortunately, theoretical models have been built to calculate the undercooling and cooling rate.[35,36] Using the Newtonian model, the cooling rate of alloy droplets can be estimated by
where ρ is the density of the alloy, Cp the specific heat, D the droplet diameter, ε the surface emissivity, σ the Stefan–Boltzmann constant, T the droplet diameter, T0 the ambient temperature, and h the convective heat transfer coefficient. On the other hand, the undercooling was calculated by a heat transfer model proposed by Lee et al.[35] The parameters used in the calculations are listed in Table I.
The results are shown in Figure 3. For Ni40.6Fe36.4Ti23 alloy, the cooling rate increased from 1.15 × 103 to 4.23 × 104 K s−1 and the undercooling increased from 67 K to 304 K, as the droplet diameter decreased from 834 to 140 μm. The maximum values of cooling rate and undercooling of Fe66.5Ni17.6Ti15.9 alloy were 4.80 × 104 K s−1 and 308 K with a droplet diameter of 112 μm, respectively.
Promotion of ternary eutectic growth
The microstructure evolution of rapidly solidified Ni40.6Fe36.4Ti23 eutectic alloy is illustrated in Figure 4(a). As droplet size decreased, the microstructure transformed from the mixture of primary Ni3Ti phase and ternary eutectic to complete ternary Fe2Ti + Ni3Ti + γ-Fe(Ni) eutectic, as elucidated by the schematic solidification pathways in Figure 5.
During microgravity solidification, when the droplet diameter is larger than 400 μm, the primary Ni3Ti phase increased as the droplet diameter decreased and grew as a block structure. Then the Fe2Ti phase nucleated and grew along the primary Ni3Ti phase serving as the heterogeneous nuclei and ternary eutectic formed between the Ni3Ti phases. Thus, the microstructure was composed of a mixture of primary Ni3Ti dendrites with fragmented Fe2Ti phase and ternary eutectic. The volume fraction of ternary eutectic decreased from 67 to 13 pct as droplet diameter declined to 430 μm, whereas the primary Ni3Ti phase increased by two times, as illustrated in Figure 8(a).
As the droplet size was between 200 and 400 μm, the primary Ni3Ti phase distributed homogenously and there was almost no eutectic structure and the volume fraction of Ni3Ti phase reached 70.3 pct. Once the droplet diameter was reduced to less than 200 μm, the nucleation and growth competition among the three phases increased, and the nucleation superiority of Ni3Ti phase was lost. Consequently, a ternary Fe2Ti + Ni3Ti + γ-Fe(Ni) eutectic was promoted to form. Unlike the ternary eutectic in the master alloy and big droplets, which was composed of a continuous fibrous Fe2Ti phase grown in nonfaceted way and the lamellar γ-Fe(Ni) + Ni3Ti phases. The ternary eutectic in small droplets consisted of separated Fe2Ti blocks with a predominant growth mode of faceted way, and lamellar γ-Fe(Ni) + Ni3Ti phases. The variations of cooling rate and undercooling with droplet size are believed to dominate the microstructural transition.
Nonfaceted and faceted growth of Fe2Ti phase
The Fe2Ti phase in ternary Fe2Ti + Ni3Ti + γ-Fe(Ni) eutectic grew in the manner of either nonfaceted and faceted way as seen in Figure 6. In the master alloy and droplets with a diameter of greater than 400 μm, the Fe2Ti phase grew into a fibrous structure which exhibited typical nonfaceted characteristics. As the droplet size was less than 200 μm in the drop tube, most Fe2Ti phases grew in faceted way with triangular and quadrilateral shapes, as demonstrated in Figures 4(a3) and 6(b).
According to the solid-liquid interface model proposed by Jackson,[38] the nonfaceted growth is favored when Jackson’s factor α = ΔH/(TLR) is less than 2. If α exceeds 2, faceted growth takes place. Here, ΔH is the fusion heat, R the gas constant, and TL the liquidus temperature. Based on this, α of the Fe2Ti phase was calculated to be 2.13, and this indicated that the Fe2Ti phase would grow in a faceted way. Actually, both nonfaceted and faceted growth modes displayed in the experiment. Nonfaceted growth was predominant in the master alloy and the droplet with D > 400 μm, while at large undercooling and cooling rate, faceted growth was the main growth mode. This confirms that the rapid solidification causes the transition of crystal growth mode. Ternary eutectic growth involves competitive nucleation and cooperative growth of three phases from the melt, which is more complex than single-phase growth. The difference of nucleation rate, volume fraction, growth mode, and growth rate of the three solid phases will affect the distribution and arrangement of the three atoms during solidification. This may inhibit the faceted growth of the Fe2Ti phase. At large undercooling, the Fe2Ti phase grew more independently and then grew into faceted phase as the theory predicted.
Suppression of peri-eutectic transition in small droplets
Figure 4(b) shows the microstructure evolution of Fe66.5Ni17.6Ti15.9 peri-eutectic alloy with droplet size. The alloy droplets with a diameter ranging from 325 to 903 μm were composed of primary α-Fe dendrites with needle-like Fe2Ti precipitate and Fe2Ti + γ-Fe(Ni) pseudobinary eutectic. The volume fraction of pseudobinary eutectic decreased from 50.37 to 8.04 pct as the droplet diameter decreased to 210 μm. However, there was no pseudobinary eutectic in the droplet with a diameter of 112 μm, which implied the suppression of peri-eutectic transition at high undercooling and large cooling rate.
The two solidification pathways are elucidated by the schematic illustration in Figure 7. In the case of the droplets with D > 150 μm, the primary α′-Fe phase first nucleated and grew from the melt as a dendrite structure. As the temperature decreased, Fe2Ti+γ-Fe(Ni) pseudobinary eutectic was formed by the peri-eutectic transition L + α′-Fe → Fe2Ti + γ-Fe(Ni). At the last solidification stage, the needle-like Fe2Ti phase precipitated from the supersaturated α′-Fe phase. For the droplets with D < 150 μm, for example, the droplet with a diameter of 112 μm, the residual melt directly solidified into Fe2Ti phase after the growth of primary equiaxed α′-Fe phase, as the second path in Figure 7. The peri-eutectic transition was completely suppressed in this case. Moreover, the microstructural characteristic of α-Fe phase transformed from coarse dendrites to equiaxed grains owing to the drastic nucleation rate. The average grain size of α-Fe phase was refined from 8.89 to 1.56 μm, and the volume fraction increased to 57.46 pct, as plotted in Figure 8(b). However, there was still Fe2Ti phase but much refined precipitated from the α′-Fe phase.
As is well known that the driving force for rapid solidification will become strong at a large undercooling and cooling rate. Therefore, the occurrence and the suppression of the peri-eutectic transition is determined by competition between the incubation time of the peri-eutectic transition tp and the time essential for completion solidification of α′-Fe phase ta.[39] When D > 150 μm, solidification of α′-Fe phase was longer owing to low undercooling and cooling rate, tp < ta, thus permitting, the peri-eutectic transition, whereas if D < 150 μm, solidification that was subjected to a high cooling rate and undercooling was completed more rapidly, i.e., tp > ta. Consequently, most of the melt has directly solidified into α′-Fe phase before the peri-eutectic transition can occur, which results in the suppression of the peritectic reaction. Thus, the preferential growth of the primary α′-Fe phase suppresses the peri-eutectic transition in small droplets.
Characteristics of phase interface in eutectic and peri-eutectic alloys
To further investigate the lattice structure and phase interface of rapidly solidified alloys, TEM analysis was carried out. The experimental sample of ternary Ni40.6Fe36.4Ti23 alloy was prepared by FIB from the alloy droplet with a diameter of 140 μm. Figure 9(a) exhibits the HADDF image of ternary Fe2Ti + Ni3Ti + γ-Fe(Ni) eutectic. Obviously, the Fe2Ti phase had smooth boundaries, indicating a faceted growth way. From the HRTEM image and SAED pattern along the \([01{\kern 1pt} {\kern 1pt} \overline{1} {\kern 1pt} {\kern 1pt} 0]\) axis in Figure 9(b), the Fe2Ti phase was hcp structure and the spacing of (0002) and \((\overline{2} 110)\) were 0.386 and 0.240 nm. The lamellar Ni3Ti and γ-Fe(Ni) phases can be also observed in Figure 9(a). According to the HRTEM image and SAED pattern at the γ-Fe(Ni)/Ni3Ti phase interface, the orientations of γ-Fe(Ni) and Ni3Ti phases satisfied \((1\overline{1} 1)_{\gamma }\)//\( (0001)_{{{\text{Ni}}_{{\text{3}}} {\text{Ti}}}} \) and \([011]_{\gamma }\)//\( [2\bar{1}\bar{1}0]_{{{\text{Ni}}_{{\text{3}}} {\text{Ti}}}} \). The spacing of \((1{\kern 1pt} {\kern 1pt} \overline{1} {\kern 1pt} {\kern 1pt} 1)\) and \((1{\kern 1pt} {\kern 1pt} 1{\kern 1pt} {\kern 1pt} \overline{1} )\) in the γ-Fe(Ni) phase were 0.206 and 0.218 nm, that of (0001) and \((0{\kern 1pt} \overline{1} {\kern 1pt} {\kern 1pt} 1{\kern 1pt} 0)\) in the Ni3Ti phase were 0.847 and 0.436 nm. Atomic arrangement marked by yellow and blue spheres reveals that there was no absence of atoms at the γ-Fe(Ni)/Ni3Ti phase interface. The schematic diagram of the interface is displayed in Figure 9(d). The lattice misfit δ can be estimated by Reference 40:
where ds and dc are the plane spacing in γ-Fe(Ni) and Ni3Ti phases along the \((1\overline{1} 1)_{\gamma }\), which were 0.206 and 0.212 nm. The lattice misfit was calculated to be 2.87 pct, indicating it was a coherent interface with low interface energy. This well explains why the γ-Fe(Ni) and Ni3Ti kept cooperative growth and form a lamellar structure.
Figure 10 illustrates the microstructures of Fe66.5Ni17.6Ti15.9 peri-eutectic alloys processed by arc melting and the solidified droplet with a diameter of 112 μm. The Fe2Ti precipitate was significantly refined at a high undercooling and large cooling rate. The HRTEM image and SAED pattern at the α-Fe/Fe2Ti phase interface are shown in Figures 10(c) and (d). The spacing of \((\overline{1} 2\overline{1} )\) and (110) in the α-Fe phase were 0.116 and 0.207 nm, and that of (0002) and \((\overline{2} 110)\) in the Fe2Ti phase were 0.376 and 0.235 nm. The inverse FFT of \((11{\kern 1pt} {\kern 1pt} 0)_{\alpha }\), \((\overline{1} \overline{1} {\kern 1pt} {\kern 1pt} 0{\kern 1pt} )_{\alpha }\), \((2\overline{1} {\kern 1pt} {\kern 1pt} \overline{1} {\kern 1pt} 0)_{{{\text{Ni}}_{{3}} {\text{Ti}}}}\), and \((\overline{2} 110)_{{{\text{Ni}}_{{3}} {\text{Ti}}}}\) demonstrates that the interface contains edge dislocations. The spacing of misfit dislocation is given by
where b is the Burgers vector generated on the α-Fe side and is equal to d(110)α = 0.207 nm. With a dislocation misfit of 12.67 pct, the spacing of misfit dislocation was found to be 1.63 nm. The distance between the crystal planes of the two phases was quite different, so the atoms cannot correspond to each other, such as the γ-Fe(Ni) and Ni3Ti phases. Therefore, the α-Fe/Fe2Ti phase interface was characterized as a semicoherent interface.
TEM analysis for a rapidly solidified Fe66.5Ni17.6Ti15.9 peri-eutectic alloy droplet with 112 μm diameter: (a) HAADF image of master alloy, (b) HAADF image of a droplet with a diameter of 112 μm, (c, d) HRTEM image, and SAED pattern of the α-Fe/Fe2Ti interface, the inset is the inverse FFT of \((110)_{\alpha }\), \((\overline{1} \overline{1} 0)_{\alpha }\), \((2{\kern 1pt} \overline{{1{\kern 1pt} }} {\kern 1pt} \overline{1} {\kern 1pt} {\kern 1pt} 0)_{{{\text{Ni}}_{{3}} {\text{Ti}}}}\), and \((\overline{2} 110)_{{{\text{Ni}}_{{3}} {\text{Ti}}}}\)
Tribological Properties of Rapidly Solidified Alloys
Friction coefficient and wear rate vs droplet size
Influenced by the two factors of undercooling and cooling rate, the microstructure of the alloy droplets changed, resulting in the variation of friction performances of the droplets with different sizes. The cross-section profile of the scratches is plotted in Figure 11(a). It is evident that the scratch groove in the Ni40.6Fe36.4Ti23 alloy droplet with a diameter of 548 μm is shallower than that in the Fe66.5Ni17.6Ti15.9 alloy droplet with a diameter of 633 μm, and the pile-up on the Fe66.5Ni17.6Ti15.9 alloy is more significant. These indicate that the Ni40.6Fe36.4Ti23 alloy has a better wear resistance owing to the more refined microstructure, and there are a lot of intermetallic compounds of Ni3Ti and Fe2Ti in the eutectic alloy, while the peri-eutectic alloy is mainly composed of α-Fe solid-solution phase. The average pile-up height hp and residual depth hr are shown in Figure 11(b). As the droplet diameter decreased, the pile-up height of Fe66.5Ni17.6Ti15.9 alloy decreased from 44.33 to 22.14 nm, and the residual depth decreased from 34.60 to 28.11 nm. However, for the Ni40.6Fe36.4Ti23 alloy, the pile-up height and residual depth firstly increased and then decreased.
Micromechanical properties of rapidly solidified alloy droplets: (a) scratch profiles, the insets are the typical AFM images, (b) pile-up height hp and residual depth hr vs droplet size D, (c, d) friction coefficient f along scratch direction x, (e) average friction coefficient fm vs D. (f) wear rate W vs D
The friction coefficient against droplet diameter for Ni40.6Fe36.4Ti23 and Fe66.5Ni17.6Ti15.9 alloys are shown in Figures 11(c) through (e). The average friction coefficient of Ni40.6Fe36.4Ti23 alloy first increased and then decreased. This is ascribed to that the eutectic structure has a higher resistance owing to the more homogeneous microstructure and refined grains. The tribological property then increased because the structure in smaller droplets was further refined, as illustrated in Figure 4(a3). For Fe66.5Ni17.6Ti15.9 peri-eutectic alloy, the fluctuation of friction coefficient lessened as droplet size decreased, revealing a more homogeneous and refined microstructure. The average friction coefficient decreased from 0.18 to 0.13 with reduced droplet diameter. This confirms that the refinement of microstructure significantly improves the friction performance of the alloy. Rapid solidification results in a high concentration of defects, such as vacancies, dislocations, and bond deformations, at the grain boundaries, which hinders dislocation motion and plastic deformation. Moreover, the semicoherent precipitated Fe2Ti phase in the α-Fe matrix also increases the wear resistance. The droplets with smaller friction coefficient are anticipated to yield higher wear resistance for a potential tribological application.
The wear resistance can be quantitatively evaluated by the wear rate, defined as the volume removed per unit scratch distance, which is written as follows[41]:
where V is the wear volume, and x is the scratch distance. Hence, the wear rate can be directly measured from the cross-sectional profiles of scratches, and the results are summarized in Figure 11(f). As droplet diameter was reduced, the wear rate of Ni40.6Fe36.4Ti23 alloy first increased and then decreased, and that of Fe66.5Ni17.6Ti15.9 alloy linearly decreased to 6.65 × 103 nm2. The smaller alloy droplets had a better wear resistance because of the refinement strengthening during rapid solidification. Generally, the material with larger grains is mainly intergranular fractures during plastic deformation with more wear. When the grains become smaller, the transgranular fracture will dominate the deformation mode, and the wear decreases.
Effect of yield strength and young’s modulus on scratch profile
To further reveal the friction mechanism of different alloys, the static analysis was carried out using finite element (FEM) analysis in ABAQUS. Such numerical investigations can be established to study the influence of the yield strength and Young’s modulus on scratch behavior. The mechanical parameters used in the simulation were obtained by quasi-static compression experiments. The Yield strength σs of Ni40.6Fe36.4Ti23 and Fe66.5Ni17.6Ti15.9 alloys were 1532 and 1124 MPa. The Elastic modulus E of the two alloys were 23 and 14 GPa. In order to ensure the analysis under the premise of the consistency of the related conditions, the stress distribution of the two alloys was statistically calculated when the indenter was loaded with a normal force of 3 mN and then produced a lateral displacement of 10 μm.
Figures 12(a) and (b) show the von Mises stress distributions of Ni40.6Fe36.4Ti23 and Fe66.5Ni17.6Ti15.9 alloys. A larger stress concentration zone can be seen on the surface of Fe66.5Ni17.6Ti15.9 alloy, and the pile-up was observed. This is due to the lower yield strength of the alloy, which produced greater plastic deformation when scratched. The maximum von Mises stress of Ni40.6Fe36.4Ti23 and Fe66.5Ni17.6Ti15.9 alloys were 1532 and 1124 MPa, respectively. From the shear-stress distribution on the alloy surface in Figures 12(c) and (d), the absolute value of the shear stress on the alloy surface at the front of the indenter was higher than that behind the indenter, owing to the tangential friction of the indenter. For Fe66.5Ni17.6Ti15.9 alloy, the plasticity of the material was better and the friction during scratch was greater; hence, there was more shear-stress concentration under the indenter.
Conclusions
In summary, the rapid solidification mechanisms of eutectic and peri-eutectic transitions in ternary Fe–Ni–Ti alloy have been investigated by drop tube technique. Their tribological properties were analyzed by nanoscratch tests and the following conclusions were drawn:
-
(1)
As liquid undercooling and cooling rate increased, the microstructure of Ni40.6Fe36.4Ti23 eutectic alloy transformed from the mixture of primary Ni3Ti phase and ternary eutectic to complete ternary eutectic. In ternary eutectic, the Fe2Ti intermetallic phase grew independently in two modes, at the droplets with D > 400 μm, nonfaceted growth was predominant, while at smaller droplets, faceted growth took the dominant place. The γ-Fe(Ni) and Ni3Ti phases kept cooperative growth and formed a lamellar structure owing to the coherent phase interface along \((1{\kern 1pt} {\kern 1pt} \overline{1} {\kern 1pt} {\kern 1pt} 1)_{\gamma }\) and \((0001)_{{\text{Ni}}_{{\text{3}}} {\text{Ti}}} \).
-
(2)
The peri-eutectic transition was suppressed under rapid solidification owing to the preferential growth of the primary α′-Fe phase, which varied from coarse dendrites to equiaxed grains owing to the drastic nucleation rate. A needle-like Fe2Ti phase precipitated from the primary supersaturated α′-Fe phase during rapid solidification, and the α-Fe/Fe2Ti phase interface was characterized as a semicoherent interface.
-
(3)
The wear rate and friction coefficient of the ternary eutectic alloy firstly increased due to the preferential growth of the coarse Ni3Ti phase, and then decreased caused by the microstructure refinement. For the peri-eutectic alloy, the grain refinement significantly improved the tribological property after containerless solidification. However, the ternary eutectic alloy had a better wear resistance because of the more refined structure, and a lot of hardening Fe2Ti and Ni3Ti intermetallic phases.
References
M. Rappaz and C.A. Gandin: Acta Metall. Mater., 1993, vol. 41, pp. 345–60.
M.C. Flemings: Metall. Trans. A., 1991, vol. 22, pp. 957–81.
W. Kurz, M. Rappaz, and R. Trivedi: Int. Mater. Rev., 2020, vol. 66, pp. 30–76.
T.K. Akopyan, N.A. Belov, E.A. Naumova, and N.V. Letyagin: Mater. Lett., 2019, vol. 245, pp. 110–13.
A. Choudhury, Y.C. Yabansu, S.R. Kalidindi, and A. Dennstedt: Acta Mater., 2016, vol. 110, pp. 131–41.
G. Zeng, M.D. Callaghan, S.D. McDonald, H. Yasuda, and K. Nogita: J. Alloys Compd., 2019, vol. 797, pp. 804–10.
J. De Wilde, E. Nagels, F. Lemoisson, and L. Froyen: Mater. Sci. Eng. A., 2005, vol. 413–14, pp. 514–20.
G. Sha, K.A.Q. O’Reilly, B. Cantor, J.M. Titchmarsh, and R.G. Hamerton: Acta Mater., 2003, vol. 51, pp. 1883–97.
P. Steinmetz, S. Gadkari, and A. Genau: J. Cryst. Growth., 2019, vol. 507, pp. 425–36.
G. Huber, Y. Brechet, and T. Pardoen: Acta Mater., 2005, vol. 53, pp. 2739–49.
M.C. Flemings: J. Cryst. Growth., 2020, vol. 530, p. 125246.
E. Çadırlı, H. Kaya, D. Räbiger, S. Eckert, and M. Gündüz: J. Alloys Compd., 2015, vol. 647, pp. 471–80.
A. Dennstedt, L. Helfen, P. Steinmetz, B. Nestler, and L. Ratke: Metall. Mater. Trans. A., 2015, vol. 47A, pp. 981–84.
I. Baker, M. Wu, and Z. Wang: Mater. Charact., 2019, vol. 147, pp. 545–57.
G.A. Song, J.S. Lee, J.S. Park, N.S. Lee, W.H. Lee, and K.B. Kim: J. Alloys Compd., 2009, vol. 481, pp. 135–39.
J. Hötzer, P. Steinmetz, A. Dennstedt, A. Genau, M. Kellner, I. Sargin, and B. Nestler: Acta Mater., 2017, vol. 136, pp. 335–46.
M. Rappaz, P. Jarry, G. Kurtuldu, and J. Zollinger: Metall. Mater. Trans. A., 2020, vol. 51A, pp. 2651–64.
J.W. Lum, D.M. Matson, and M.C. Flemings: Metall. Mater. Trans. B., 1996, vol. 27B, pp. 865–70.
M.A. Ruggiero and J.W. Rutter: Mater. Sci. Technol., 1997, vol. 13, pp. 5–11.
D. Lewis, S. Allen, M. Notis, and A. Scotch: J. Electron. Mater., 2002, vol. 31, pp. 161–67.
J. Hötzer, M. Jainta, P. Steinmetz, B. Nestler, A. Dennstedt, A. Genau, M. Bauer, H. Köstler, and U. Rüde: Acta Mater., 2015, vol. 93, pp. 194–204.
O. Benamara, M. Cherif, T. Duffar, and K. Lebbou: J. Cryst. Growth., 2015, vol. 429, pp. 27–34.
Y. Ruan and W.J. **e: Intermetallics., 2012, vol. 31, pp. 232–41.
L.I. Duarte, U.E. Klotz, C. Leinenbach, M. Palm, F. Stein, and J.F. Löffler: Intermetallics., 2010, vol. 18, pp. 374–84.
G. Cacciamani, J. De Keyzer, R. Ferro, U.E. Klotz, J. Lacaze, and P. Wollants: Intermetallics., 2006, vol. 14, pp. 1312–25.
Y.U. Heo, M. Takeguchi, K. Furuya, and H.C. Lee: Acta Mater., 2009, vol. 57, pp. 1176–87.
X.W. Li, B.W. Wu, Y. Ruan, and B. Wei: J. Mater. Sci., 2021, vol. 56, pp. 15407–22.
J. Kundin, L. Mushongera, and H. Emmerich: Acta Mater., 2015, vol. 95, pp. 343–56.
Y. Tian, R. Gauvin, and M. Brochu: Metall. Mater. Trans. A., 2016, vol. 47A, pp. 3771–80.
N.T. Brown, E. Martinez, and J. Qu: Acta Mater., 2017, vol. 129, pp. 83–90.
X.X. Wei, X. Lin, W. Xu, Q.S. Huang, M. Ferry, J.F. Li, and Y.H. Zhou: Acta Mater., 2015, vol. 95, pp. 44–56.
O. Oloyede, R.F. Cochrane, and A.M. Mullis: J. Alloys Compd., 2017, vol. 707, pp. 347–50.
A.M. Mullis, O.E. Jegede, T.D. Bigg, and R.F. Cochrane: Acta Mater., 2020, vol. 188, pp. 591–98.
A. Ilbagi, H. Henein, and A.B. Phillion: J. Mater. Sci., 2010, vol. 46, pp. 6235–42.
E.S. Lee and S. Ahn: Acta Metall. Mater., 1994, vol. 42, pp. 3231–43.
P.S. Grant, B. Cantor, and L. Katgerman: Acta Metall. Mater., 1993, vol. 41, pp. 3097–3108.
W.F. Gale and T.C. Totemeir: Smithells Metals Reference Book, 8th ed. Elsevier, Burlington, 2004.
K.A. Jackson: J. Cryst. Growth., 2004, vol. 264, pp. 519–29.
Y.Z. Chen, F. Liu, G.C. Yang, N. Liu, C.L. Yang, and Y.H. Zhou: Scripta Mater., 2007, vol. 57, pp. 779–82.
L. Hu, G. Zhang, W. Hu, G. Gottstein, S. Bogner, and A. Bührig-Polaczek: Acta Mater., 2013, vol. 61, pp. 7155–65.
Y.X. Ye, C.Z. Liu, H. Wang, and T.G. Nieh: Acta Mater., 2018, vol. 147, pp. 78–89.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. U1806219, 52073232 and 52088101), the Science Fund for Distinguished Young Scholars of Shaanxi Province (Grant No. 2020JC-11) and the Science Fund for Scientific and Technological Innovation Team of Shaanxi Province (Grant No. 2021TD-14). The authors are very grateful to Mr. B.W. Wu for his help with the experiments. They also thank Dr. D.L. Geng for his supports and valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, X.W., Ruan, Y. & Wei, B. Promotion or Suppression of Eutectic and Peri-Eutectic Growth in Containerlessly Processed Fe–Ni–Ti Alloys. Metall Mater Trans B 53, 1351–1363 (2022). https://doi.org/10.1007/s11663-022-02498-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02498-2