Abstract
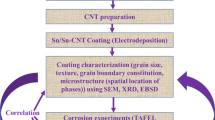
Direct electrodeposition method was used to co-electrodeposit different volume fractions of carbon nanotubes in FeCuCrNiCo high entropy alloy matrix. Phase constitution, morphology, wettability, protective oxide film chemistry, and corrosion behavior of the composite coatings were studied as a function of volume fraction of carbon nanotubes (CNTs). Pristine HEA coating contained mixture of body-centered cubic (bcc) and face-centered cubic (fcc) phases which transformed into nearly single-phase body-centered cubic microstructure with the incorporation of CNTs upto a certain optimum volume fraction. The phase heterogeneity, however, re-appeared for higher CNT additions. The coating morphology showed a transition from one containing mixture of dendritic and granular features to a more compact, smooth, and fine-grained globular matrix with CNT incorporation. A monotonic increase in the water contact angle was also observed with increasing CNT content in the composite coating. Weight loss and potentiodynamic polarization techniques employed for coating corrosion analysis showed that the corrosion behavior of pristine coating was highly sensitive to the amount of reinforced CNTs. Addition of an optimum CNT amount in HEA coating (produced from an electrolyte with 12.5 mg/L of dispersed CNTs) led to a considerable decrease (85.6 pct) in the corrosion rate (compared to the pristine HEA coating). In addition to improved morphology, phase homogenization, and increased contact angle, the reason for this significant improvement was also attributed to the evolution of protective oxides like Cr2O3 and NiO in case of HEA-CNT composite with optimum CNT concentration. For higher CNT additions, a drastic decrease in the protection efficiency was observed because of re-appearance of phase heterogeneity. This promoted galvanic coupling and due to the presence of surface defects in forms of cracks arising due to the presence of agglomerated CNTs in the electrodeposited coatings.




















Similar content being viewed by others
Change history
19 June 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11661-023-07102-z
References
B. Cantor, I.T.H. Chang, P. Knight, and A.J.B. Vincent: Mater. Sci. Eng. A, 2004, vol. 375–377, pp. 213–18
D.B. Miracle and O.N. Senkov: Acta Mater., 2017, vol. 122, pp. 448–511
K.M. Youssef, A.J. Zaddach, C. Niu, D.L. Irvin, and C.C. Koch: Mater. Res. Lett., 2015, vol. 3, pp. 95–99
A.A. Rempel and B.R. Gelchinski: Steel Transl., 2020, vol. 50, pp. 243–47
Y. Shi, B. Yang, and P.K. Liaw: Metals, 2017, vol. 7, pp. 1–8
X.L. Shang, Z.J. Wang, H.E. Feng, J.C. Wang, J.J. Li, and J.K. Yu: Sci. China Technol. Sci, 2018, vol. 61, pp. 189–96
Y.F. Ye, Q. Wang, J. Lu, C.T. Liu, and Y. Yang: Mater. Today Commun., 2016, vol. 19, pp. 349–62
Y.J. Hsu, W.C. Chiang, and J.K. Wu: Mater. Chem. Phys., 2005, vol. 92, pp. 112–17
Y. Garip: Corros. Sci., 2022, vol. 206, p. 110497
X.-W. Qiu and C.-G. Liu: J. Alloys Compd., 2013, vol. 553, pp. 216–20
Y. Guo, X. Shang, and Q. Liu: Surf. Coat. Technol., 2018, vol. 344, pp. 353–58
X. Li, Y. Feng, B. Liu, D. Yi, X. Yang, W. Zhang, G. Chen, Y. Liu, and P. Bai: J. Alloys Compd., 2019, vol. 788, pp. 485–94
J. Cheng, D. Liu, X. Liang, and Y. Chen: Surf. Coat. Technol., 2015, vol. 281, pp. 109–16
J.-W. Yeh: Eur J Control, 2006, vol. 31, pp. 633–48
A. Aliyu, M.Y. Rekha, and C. Srivastava: Philos. Mag. 2018, vol. 99, pp. 1–18
S. Singh, S.M. Shaikh, M.K.P. Kumar, B.S. Murty, and C. Srivastava: Materialia, 2020, vol. 14, p. 100917
M.J. Popescu, F. Branzoi, I. Constantin, M. Anastasescu, M. Burada, D. Mitric, I. Anasiei, M.-T. Olaru, and V. Constantin: Coatings, 2021, vol. 11, p. 1367
S. Praveen, B.S. Murty, and R.S. Kottada: Mater. Sci. Eng. A, 2012, vol. 534, pp. 83–89
S. Ozturk, F. Alptekin, S. Onal, S.E. Sunbul, O. Sahin, and K. Icin: J. Alloys Compd., 2022, vol. 903, p. 163867
A. Singh, T. Ram Prabhu, A.R. Sanjay, and V. Koti: Mater. Today: Proc., 2017, vol. 4, pp. 3872–881
R.I. Rubel, M.H. Ali, M.A. Jafor, and M.M. Alam: AIMS Mater. Sci., 2019, vol. 6, pp. 756–80
D. Wang, Y. Xu, D. **ao, Q. Qiao, P. Yin, Z. Yang, J. Li, W. Winchester, Z. Wang, and T. Hayat: J. Hazard. Mater., 2019, vol. 371, pp. 83–93
R. Ramachandran and M. Nosonovsky: Phys. Chem, 2015, vol. 17, pp. 24988–4997
T. Zheng, Y. Hu, Y. Zhang, and F. Pan: J. Colloid Interface Sci., 2017, vol. 505, pp. 87–95
K. Baratpour: ASTM, 2004, vol. G31–72, pp. 1–8
R. Mishra and R. Balasubramaniam: Corros. Sci., 2004, vol. 46, pp. 3019–029
S. Wang, J. Zhang, O. Gharbi, V. Vivier, M. Gao, and M.E. Orazem: Nat. Rev. Methods Prim., 2021, vol. 41, pp. 1–21
C. Ji, A. Ma, and J. Jiang: J. Alloys Compd., 2022, vol. 900, p. 163508
M. Liu, X. Cheng, X. Li, Y. Pan, and J. Li: Appl. Surf. Sci., 2016, vol. 389, pp. 1182–191
S. He and D. Jiang: Int. J. Electrochem. Sci., 2018, vol. 13, pp. 5822–849
W.A. Badawy, F.M. Al-Kharafi, and J.R. Al-Ajmi: J. Appl. Electrochem., 2000, vol. 30, pp. 693–704
J.-Y. Jiang, D. Wang, H.-Y. Chu, H. Ma, Y. Liu, Y. Gao, J. Shi, and W. Sun: Materials (Basel), 2017, vol. 10, pp. 1–2
H. Luo, Z. Li, A.M. Mingers, and D. Raabe: Corros. Sci., 2018, vol. 134, pp. 131–39
M. Steimecke, G. Seiffarth, C. Schneemann, F. Oehler, S. Förster, and M. Bron: ACS Catal., 2020, vol. 10, pp. 3595–603
T. **, M.B. Shahzad, D. Xu, Z. Sun, J. Zhao, C. Yang, M. Qi, and K. Yang: Mater. Sci. Eng. C, 2017, vol. 17, pp. 1079–085
M. Seo, G. Hultquist, C. Leygraf, and N. Sato: Corros. Sci., 1986, vol. 26, pp. 949–60
L. Huang, X. Wang, X. Zhao, C.Z. Wang, and Y. Yang: Mater. Chem. Phys., 2021, vol. 259, p. 124007
Acknowledgments
The authors acknowledge the research funding received from the SERB Government of India.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, S., Srivastava, C. Engineering the Protective Oxide Chemistry for Enhanced Corrosion Protection Performance of FeCuCrNiCo-CNT Composite Coatings in 3.5 M NaCl Solution Corrosive Media. Metall Mater Trans A 54, 1398–1413 (2023). https://doi.org/10.1007/s11661-023-06994-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-023-06994-1