Abstract
Domestic pigs have become increasingly popular as a model for human diseases such as neurological diseases. Drug discovery platforms have increasingly been used to identify novel compounds that combat neurodegeneration. Currently, bioactive molecules such as melatonin have been demonstrated to offer a neuroprotective effect in several studies. However, a neurodegenerative platform to study novel compounds in a porcine model has not been fully established. In this study, characterized porcine induced neural stem cells (iNSCs) were used for evaluation of the protective effect of melatonin against chemical and pathogenic stimulation. First, the effects of different concentrations of melatonin on the proliferation of porcine iNSCs were studied. Second, porcine iNSCs were treated with the appropriate concentration of melatonin prior to induced degeneration with dimethyl sulfoxide or Zika virus (ZIKV). The results demonstrated that the percentages of Ki67 expression in porcine iNSCs cultured in 0.1, 1, and 10 nM melatonin were not significantly different from that in the control groups. Melatonin at 1 nM protected porcine iNSCs from DMSO-induced degeneration, as confirmed by a dead cell exclusion assay and mitochondrial membrane potential (ΔΨm) analysis. In addition, pretreatment with melatonin reduced the percentage of dead porcine iNSCs after ZIKV infection. Melatonin increased the ΔΨm, resulting in a decrease in cell degeneration. However, pretreatment with melatonin was unable to suppress ZIKV replication in porcine iNSCs. In conclusion, the present study demonstrated the anti-degenerative effect of melatonin against DMSO- and ZIKV-induced degeneration in porcine iNSCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pig is a promising animal model for several human diseases, especially xenotransplantation. The similarities in the anatomy and functions of pig organs with humans make this species a bioreactor for generating biological compounds or tissues for human medicine (Yang et al. 2013; Bourret et al. 2016). With progress in stem cell technology, pig or porcine stem cells can be isolated from different organs and tissues, such as the placenta (Bharti et al. 2016), adipose tissue (Zhang et al. 2016), and embryos (Rasmussen et al. 2011). In general, porcine neural stem cells (NSCs) can be directly isolated from brain tissue (Zheng et al. 2010). However, to be able to obtain brain tissue, the animal has to be euthanized, which does not meet the criteria of the 3Rs rule of animals used for research. Moreover, brain-derived NSCs exhibit limited cell proliferation after prolonged passage (Wright et al. 2006). Therefore, directed reprogramming of somatic cells toward NSCs is an alternative procedure for providing NSCs for basic and preclinical research. Porcine neural stem cells not only are beneficial as human disease models but can also be used for drug discovery and vaccine development in veterinary medicine. Yang and colleagues (2013) transplanted porcine neural progenitors into induced spinal cord injury rats, and the results demonstrated that the cells survived after transplantation and the rats recovered from the injury. In addition to brain tissue, researchers successfully derived porcine NSCs from different sources, including the transdifferentiation of mesenchymal stem cells (MSCs) (Subbarao et al. 2015) and pluripotent stem cells (PSCs) (Kim et al. 2019; Chakritbudsabong et al. 2021a; Setthawong et al. 2021) and the directed reprogramming of somatic cells toward neural stem cells known as induced neural stem cells (iNSCs).
Several exogenous chemical substances, such as pesticides, drugs, or pathogens, might cause oxidative stress–induced degeneration of neural cells, resulting in neuropathological conditions (Niedzielska et al. 2016; Liang et al. 2019). For example, heavy metals such as mercury affect the development of neural tissues (Abbott and Nigussie 2021). Exposure of neural stem cells to mercury leads to increased lipid peroxidase, reactive oxygen species (ROS), and the subsequent degeneration of cells (Abbott and Nigussie 2021). Dimethyl sulfoxide (DMSO), a chemical widely used for cryopreservation of several cell types, including neural stem cells, impairs the oligodendrocyte cell fate of adult neural stem cells (O'Sullivan et al. 2019). Although DMSO is widely used for cryopreservation of several cell types, including stem cells, it has been reported that DMSO has some toxic effect on MSCs (Lee and Park 2017). DMSO was shown to decrease the cell viability and osteogenic differentiation of human gingiva-derived stem cells (Lee and Park 2019). Therefore, DMSO can be used to study the induced degeneration of stem cells. Despite chemical toxicity, some pathogens, especially viruses, harmfully affect the nervous system. For example, human immunodeficiency virus (HIV) is associated with chronic inflammation and the depletion of neural stem cells (Hill et al. 2019), while Zika virus (ZIKV) impairs neural development and severely causes microcephaly in humans (Ferreira and Garcez 2019; Ledur et al. 2020). In addition, pigs have been demonstrated to be a potential reservoir animal for ZIKV. The neonatal pig can be experimentally infected with ZIKV and shows signs of viremia and viruria (Darbellay et al. 2017). Therefore, it is of interest to use ZIKV for the pathogenic induction of porcine iNSC degeneration.
Melatonin, or N-acetyl-5-methoxytryptamine, produced in the pineal gland, shows antioxidant and neuroprotective effects. Melatonin supports the elimination of triglycerides, increases high-density lipoprotein (HDL), and possibly prevents the onset of Alzheimer’s disease (Cardinali et al., 2014). Researchers have discovered that melatonin suppresses free radicals in neural cells through the G protein-coupled melatonin receptors type 1 (MT1) and type 2 (MT2) (Liu et al. 2016a, b). Supplementation with melatonin in induction media increases the neural differentiation of mouse neural stem cells (Shu et al. 2016). Melatonin improves the cell proliferation and neural differentiation of PC12 cells (Liu et al. 2016a, b) and reduces mitochondrial degeneration of murine fibroblasts through AMPK-PPAR gamma-dependent mechanisms (Guven et al. 2016). In pigs, the effect of melatonin has been studied in the reproductive organs. Melatonin reduces free radicals, enhances proliferation and maturation of oocytes and granulosa cells, increases ovarian vascularization, and improves in vitro fertilization rates (Chen et al. 2017; Wang et al. 2017).
A neurotoxicity platform has been developed in human models using human pluripotent stem cell–derived neural stem cells. However, such a platform is still lacking in the pig. Therefore, it is of interest to develop a platform for investigating the protective effect of melatonin on porcine iNSC degeneration after induction with DMSO or Zika virus under in vitro culture conditions.
Materials and methods
Cell line and ethics statement
Porcine iNSC, VSMUi-002E is a cell line that has been derived from the directed reprogramming of skin fibroblasts to neural stem cells (Chakritbudsabong et al. 2021b). The cell line was provided by assistant professor Sasitorn Rungarunlert, Faculty of Veterinary Science, Mahidol University, Thailand. All procedures involving the use of biological materials, including ZIKV, were approved by the Institutional Biosafety Committee of the Faculty of Veterinary Science, Mahidol University, Thailand (IBC/MUVS-B-003/2563).
Preparation of Zika virus
Zika virus strains SV0127/14 and SV0010/15 were kindly provided by the Armed Forces Research Institution of Medical Sciences and Ministry of Public Health, Thailand. Both ZIKV strains were propagated in Vero cells, which were derived from an African green monkey kidney. The cytopathic effect (CPE) was observed on days 2–3 postinfection. The supernatant from the ZIKV cultures was collected and centrifuged to remove cell debris. The titers of ZIKV in the culture supernatants, represented as plaque-forming units (PFU), were evaluated using a plaque assay in a Vero cell monolayer. The ZIKV culture supernatant was aliquoted and stored as the ZIKV stocks at − 80 °C until use. The multiplicity of infection (M.O.I.) was calculated based on the plaque assay results before performing the experiment.
In vitro culture of porcine induced neural stem cells
Frozen porcine iNSCs were thawed and cultured on a Matrigel-coated culture dish. The growth medium consisted of DMEM/F12 (Gibco, Thermo Fisher Scientific, Waltham, MA, Cat. No. 11320033), N2 (Gibco, Thermo Fisher Scientific, Cat. No. 17502048), B27 (Gibco, Thermo Fisher Scientific, Cat. No. 17504044), 20 ng/ml epidermal growth factor (EGF; Gibco, Thermo Fisher Scientific, Cat. No. PHG0311L), 20 ng/ml basic fibroblast growth factor (bFGF; Invitrogen, Carlsbad, CA, Cat. No. RP-8627), and penicillin/streptomycin (Gibco, Thermo Fisher Scientific, Cat. No. 15140122) (Rasmussen et al. 2011). The growth medium was replaced daily. Cells were cultured at 37 °C and 5% CO2 in a humidified atmosphere. Confluent porcine iNSCs were propagated by subculturing with TrypLE Select (Gibco, Thermo Fisher Scientific, Cat. No. 12563011) and replated onto the new culture dish. The porcine iNSC #15 cell line at passages 15–20 was used in the present study.
Differentiation of porcine induced neural stem cells
Porcine iNSCs were allowed to grow in growth medium for 4 d or until they reached 90% confluency. Then, the confluent porcine iNSCs differentiated spontaneously in differentiation medium consisting of DMEM/F12, B27, N2, and penicillin/streptomycin without growth factor supplementation (Rungsiwiwut et al. 2013). The cells were allowed to differentiate for 3 wk before detection of neural differentiation markers.
Gene expression analysis
Total RNA was extracted by using a GeneJet RNA extraction kit (Thermo Fisher Scientific, Cat. No. K0731) according to the manufacturer’s instructions. RNA was reverse transcribed to cDNA by using the SuperScript®III Reverse Transcription system (Thermo Fisher Scientific, Cat. No. 18080093) according to the manufacturer’s instructions. PCR was performed to amplify genes of interest using KOD One™ PCR Master Mix-Blue (Toyobo, Osaka, Japan, Cat. No. KMM-201). The primer sequences used were based on a previous study, which included Pax6, Sox2, and Nestin, with GAPDH used as a housekee** gene (Table 1). The PCR conditions consisted of 30 cycles at a predenaturation temperature of 98 °C for 3 min, a denaturation temperature of 98 °C for 10 s, an annealing temperature for 60 s, and an extension temperature of 68 °C for 18 s. Finally, the PCR products were run on 2% agarose gels and visualized with Quick-Load® Low Molecular Weight DNA Ladder (New England Biolab Inc., England, Cat. No. N0474).
Immunocytochemistry staining
Cells were fixed with 4% paraformaldehyde for 15 min. Fixed cells were permeabilized with 0.1% Triton X-100 in PBS, blocked with 5% fetal bovine serum in PBS for 1 h, and subsequently incubated with primary antibodies. For the detection of neural stem cell and differentiation markers, the primary antibodies used in the present study were antibodies against Pax6, Sox2, Nestin, β-tubulin III, and glial fibrillary acid protein (GFAP). For cell proliferation detection, cell proliferation was assessed by using a mouse anti-Ki67 primary antibody. Cells were incubated overnight at 4 °C and then incubated with compatible fluorochrome-conjugated secondary antibodies. Diamidino-2-phenylindole (DAPI) was used as a counterstain for nuclear detection. The results were visualized by fluorescence microscopy.
Detection of porcine induced neural stem cell degeneration
Detection of degenerative porcine iNSCs was carried out by propidium iodide (PI)/Hoechst counter-staining and the trypan blue exclusion method. Cells were counted to calculate the degenerated proportion.
Treatment of porcine induced neural stem cells with melatonin
Confluent porcine iNSCs were allowed to grow in growth medium supplemented with 0.1, 1, or 10 nM melatonin (Sigma-Aldrich, Cat. No. M5250). Cells were cultured for 24 h and later subjected to subsequent experiments. For detection of proliferation, cells were fixed and immunostained for Ki67. To study the protective effect of melatonin in DMSO- (PanReac AppliChem, Darmstadt, Germany, Cat. No. A3672,0100) or ZIKV-induced degeneration, the growth medium was removed, and the culture was washed with PBS, followed by replacement with growth media containing DMSO or ZIKV as described below.
Induction of degeneration of porcine induced neural stem cells with dimethyl sulfoxide
Porcine iNSCs were subjected to degeneration induced by DMSO. DMSO concentrations of 1, 5, and 10% (v/v) were added to the culture medium. Cells were cultured with DMSO for 30 min before determination of degeneration. The experimental protocol is shown in Fig. 3A.
Induction of degeneration of porcine induced neural stem cells with Zika virus
Porcine iNSCs were allowed to grow until reaching confluency before supplementation with melatonin in their growth medium. The concentrations of melatonin used in the present study were 0.1, 1, and 1 nM. Cells were cultured in melatonin-containing culture medium for 24 h before ZIKV infection at M.O.I. = 0.1 for 2 h. After incubation for 72 h, the live/dead cells were evaluated using the trypan blue exclusion method. The experimental protocol is shown in Fig. 4A.
Mitochondrial membrane potential assay
Porcine iNSCs were evaluated for mitochondrial membrane potential (ΔΨm) using the TMRE-Mitochondrial Membrane Potential Assay Kit (Cat. No. ab113852, Abcam, UK) according to the manufacturer’s recommendation. Fluorescence intensity was evaluated by using a microplate reader (Synergy HT, BIO-TEK, Winooski, VT) with Ex/Em = 549/575 nm.
Plaque assay
To determine the titer of the virus, the plaque assay was adapted from a previous report (Kato et al. 2017). In brief, Vero cells (9.5 × 105 cells/well) were plated in 12-well cell culture plates and inoculated with serial dilutions of viruses. After 4 d of inoculation, the cells were fixed with 4% formaldehyde in PBS and then stained with a 1% crystal violet solution. The titer was determined using the number of plaques and the viral dilution and then reported in PFU/ml.
Statistical analysis
The data are shown as the mean ± S.D. The differences among the groups were analyzed by one-way ANOVA. A p value ≤ 0.05 was considered a significant difference.
Results
Characterization of the porcine induced neural stem cells
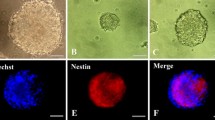
Prior to performing the experiments, the porcine iNSCs were characterized by morphological appearance, expression of genes and proteins that represent neural stem cells and differentiation ability. The results demonstrated that porcine iNSCs exhibited the characteristics of typical neural stem cells, including a radial shape with two or more processes, as shown in Fig. 1A. Under standard culture conditions, the porcine iNSCs expressed Pax6, Sox2, and Nestin, as confirmed by gene expression analysis (Fig. 1B). The immunocytochemistry staining results demonstrated that porcine iNSCs expressed Nestin, Pax6, and Sox2 (Fig. 1C). In addition, after spontaneous differentiation, the porcine iNSCs differentiated into neurons and supporting cells, as confirmed by β-tubulin III and glial fibrillary acid protein (GFAP) immunostaining (Fig. 1D).
Characterization of porcine induced neural stem cells. Phase contrast imaging demonstrated the morphology of porcine induced neural stem cells. Cells grew as a cluster, and cell processes were observed (A). RT–PCR results demonstrated the mRNA expression of Pax6, Sox2, and Nestin. GAPDH was used as the housekee** gene (B). Immunocytochemistry staining results confirmed the expression of Nestin, Pax6, and Sox2 (C). After spontaneous differentiation, the expression of the neuronal marker β-tubulin III and the astrocyte marker GFAP in porcine iNSCs was detected by immunocytochemistry staining (D). Scale bar in A and C = 100 µm, D = 200 µm. RT–PCR, reverse transcription–polymerase chain reaction; mRNA, messenger ribonucleic acid; GFAP, glial fibrillary acidic protein; DAPI, 4′,6-diamidino-2-phenylindole.
Effect of melatonin on the proliferation of porcine induced neural stem cells
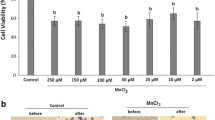
Porcine iNSCs were cultured in culture medium supplemented with different concentrations of melatonin for 24 h prior to detection of cell proliferation. The results revealed that the expression of Ki67, a proliferation marker, was detected in all groups (Fig. 2A). The percentage of Ki67 expression in porcine iNSCs cultured at 0.1, 1, and 10 nM melatonin was not significantly different from that in the control groups (35 ± 3.4, 39 ± 2.7, 41 ± 5.2, and 32 ± 6.0%, respectively, p > 0.05).
Effect of different concentrations of melatonin on the proliferation of porcine induced neural stem cells. Confluent porcine iNSCs were cultured with different concentrations of melatonin for 24 h before detection of the Ki67-positive cells. The expression of Ki67 (green), a proliferation marker, was detected in all groups of porcine iNSCs (A). The percentage of Ki67 expression in porcine iNSCs cultured at 0.1, 1, and 10 nM was not significantly different from that in the control groups (35 ± 3.4, 39 ± 2.7, 41 ± 5.2, and 32 ± 6.0%, respectively, p > 0.05). Dimethyl sulfoxide–induced degeneration of porcine iNSCs in a concentration-dependent manner. Notable morphological changes, including shrunken processes of the cells and detachment from the surface of the culture dish, were observed in porcine iNSCs cultured in 5 and 10% (v/v) DMSO (B). Porcine iNSCs cultured in 5% DMSO were shrunken, and the cells remained attached to the culture dish (B). The results of the propidium iodide and Hoechst 33,342 staining demonstrated that the highest number of degenerated cells was found in 10% (v/v) DMSO, while 1% DMSO showed no notable difference in degenerated cells in comparison with the control group (C). Scale bar = 100 µm. DAPI, 4′,6-diamidino-2-phenylindole; PI, propidium iodide.
Effect of dimethyl sulfoxide on the degeneration of porcine induced neural stem cells
The degeneration of porcine iNSCs was induced by supplementation with DMSO in the growth medium for 30 min. Morphological changes were observed in the porcine iNSCs cultured in 1, 5, and 10% (v/v) DMSO. DMSO at a concentration of 10% (v/v) caused notable differences in the cell morphology, including shrunken processes of the cells and detachment from the surface of the culture dish (Fig. 2B). Although the processes of the porcine iNSCs cultured in 5% DMSO had shrunk, the cells remained attached to the culture dish (Fig. 2B). The results of the propidium iodide and Hoechst 33,342 staining demonstrated that the highest number of degenerated cells was found in the 10% (v/v) DMSO treatment, while 1% DMSO showed no notable difference in degenerated cells in comparison with the control group (Fig. 2C). Therefore, 5% DMSO was selected for subsequent experiments.
Protective effect of melatonin against dimethyl sulfoxide–induced cell degeneration
Porcine iNSCs were cultured in growth medium supplemented with melatonin for 24 h and subsequently treated with 5% DMSO for 30 min. After incubation with DMSO, the results showed that dead and floating cells could be observed in all groups (Fig. 3B). The percentage of live cells in the 1 nM (70.65 ± 3.86%) and 10 nM melatonin (74.81 ± 2.64%) pretreated group was significantly higher compared to that in the non-treated group (55.90 ± 8.48%), p < 0.05, respectively (Fig. 3C).
Protective effect of melatonin on dimethyl sulfoxide–induced degeneration. Porcine induced neural stem cells (iNSCs) were induced to degenerate by treatment with 5% (v/v) dimethyl sulfoxide for 30 min. The degenerated cells were detected by trypan blue exclusion assay. The experimental protocol is shown in A. After incubation with DMSO, the results showed that dead and floating cells could be observed in all groups (B).The percentage of live cells in 5% DMSO-treated group (55.90 ± 8.48%) was significantly lower than that of the control group (72.85 ± 3.36%), p < 0.01. The percentages of live cells in 1 nM (70.65 ± 3.86%) and 10 nM melatonin-treated group (74.81 ± 2.64%) were significantly higher compared to those in 5% DMSO-treated group, p < 0.05 (C). The mitochondrial membrane potentials (ΔΨm) of DMSO-treated cells (8310.50 ± 136.47 a.u.) were significantly lower than that of the control group (10,095.50 ± 82.73 a.u.) (D). Pretreatment of porcine iNSCs with 1 nM of melatonin (8986.00 ± 65.05 a.u.) significantly increased the ΔΨm in comparison with that in the no melatonin pretreatment, 5% DMSO-treated group (8310.50 ± 136.47 a.u.) (D). Scale bar = 100 µm. MMP, mitochondrial membrane potential. *p < 0.05; **p < 0.01.
Protective effect of melatonin against Zika virus–induced cell degeneration
In our preliminary experiment, we found that the porcine iNSCs survived ZIKV infection at an M.O.I. of 0.1 (data not shown). Therefore, we selected an M.O.I. of 0.1 for studying the protective effect of melatonin against ZIKV-induced cell degeneration. Porcine iNSCs were pretreated with 0.1, 1, and 10 nM melatonin for 24 h prior to infection with ZIKV for 2 h. After infection with ZIKV for 72 h, the results showed that dead and floating cells could be observed in all groups (Fig. 4B). The percentage of live cells in all melatonin-treated groups was not significantly different from that in the control group but was significantly higher than that in the ZIKV group (42.80 ± 7.52%), particularly in 1 nM (79.30 ± 3.90%) and 10 nM melatonin-treated cells (69.50 ± 4.03%) (p < 0.05), as shown in Fig. 4C.
Protective effect of melatonin on Zika virus–induced degeneration. Porcine iNSCs were pretreated with 0.1, 1, and 10 nM melatonin for 24 h before infection with ZIKV. The experimental protocol is shown in A. After infection with ZIKV for 72 h, dead and floating cells were observed in all groups (B). The percentages of live cells in the melatonin-treated groups, 0.1 nM (63.10 ± 9.01%), 1 nM (79.30 ± 3.90%), and 10 nM (69.50 ± 4.03%), were significantly higher than those in the ZIKV group (42.80 ± 7.52%) (C). Mitochondrial membrane potentials (ΔΨm) of 1 (9600.00 ± 229.14 a.u.) and 10 nM (10,230.70 ± 179.51 a.u.) melatonin-pretreated groups were significantly higher than that of the ZIKV-treated group (8774.33 ± 98.90 a.u.) (D). The results of the plaque assay demonstrated that pretreatment of porcine iNSCs was unable to reduce ZIKV replication. Pretreatment with 1 nM melatonin resulted in a significantly higher ZIKV titer than that in the nonmelatonin treatment group (202 ± 31.1 PFU/ml vs. 74 ± 31.1 PFU/ml, p < 0.05, respectively) (E). Melatonin concentrations of 0.1 nM (112 ± 56.3 PFU/ml) and 10 nM (136 ± 71.7 PFU/ml) did not result in significantly higher titers than that in the nonmelatonin treatment group (E). Scale bar = 100 µm. MMP, mitochondrial membrane potential. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
The protective effect of melatonin on mitochondrial membrane potential after DMSO- and ZIKV-induced cell degeneration
The results demonstrated that after DMSO-induced cell degeneration, the ΔΨm of porcine iNSCs pretreated with different concentrations of melatonin were slightly higher than those without melatonin pretreatment (Fig. 3D). In addition, porcine iNSCs pretreated with 1 or 10 nM melatonin prior to infection with ZIKV showed a significantly higher (p < 0.05) ΔΨm than that of the ZIKV group (Fig. 4D).
Melatonin prevents ZIKV-induced cell death
The plaque assay results are shown in Fig. 4E. Pretreatment of the porcine iNSCs with melatonin before ZIKV infection showed no significant difference in virus titer in comparison with the untreated group (p > 0.05). However, cells pretreated with melatonin showed higher viral titers than ZIKV-infected cells, especially 1 nM melatonin, which exhibited the highest viral titer (Fig. 4E).
Discussion
To date, neural stem cells have been intensively used for studying the pathophysiology of neural diseases, drug discovery and cellular therapy (Liu et al. 2012). The pig is considered a suitable animal model for studying some human neural diseases because the anatomy and function of particular organs are similar to those of humans (Lind et al. 2007). Recently, researchers were able to isolate and culture stem cells from different sources, including tissue-specific stem cells, induced pluripotent stem cells, and induced neural stem cells (iNSCs) (Vierbuchen et al. 2010, Their et al. 2012). In this study, before performing the experiments, the porcine iNSCs were subjected to characterization. The results demonstrated that the cells exhibited a bipolar cell morphology with 1 or 2 prominent nuclei. The porcine iNSCs expressed Pax6, Sox2, and Nestin, which are neural stem cell markers, and β-tubulin III and GFAP, cellular markers of differentiation. Furthermore, the proliferation ability of the porcine iNSCs used in the present study was confirmed by positive immunostaining for the nuclear protein Ki67, which is related to cellular proliferation and used as a cell proliferation marker (Schlüter et al. 1993). Although we did not include the characteristics of NSCs derived from porcine brain tissue as a control in the present study, the stemness of porcine iNSCs in the present study was similar to that of porcine neural progenitor cells derived from epiblasts (Rasmussen et al. 2011) or embryonic germ cells (Choi et al. 2020). The characterization results of the porcine iNSCs make these cell types good candidates for the replacement of brain tissue–derived neural cells, as brain tissue–derived neural cells may lose their proliferation and stem cell characteristics after prolonged in vitro culture (Liu et al. 2012).
In the present study, the protective effect of melatonin on porcine iNSCs was evaluated by culturing the cells in growth medium supplemented with different concentrations of melatonin. Our results demonstrated that the maximum concentration of melatonin at 100 nM was not toxic to porcine iNSCs, which was in agreement with a previous study (Serrano et al. 2019). It has been stated elsewhere that melatonin plays an important role in neural stem cell proliferation through melatonin receptor (MT) 1 and extracellular signal-regulated kinase (ERK) 1/2 phosphorylation (Yu et al. 2017). Although there were no significant differences in the cell proliferation rate among the different concentrations of melatonin in the present study, 1 nM melatonin tended to enhance porcine iNSC proliferation. Therefore, 1 nM melatonin was selected for the following reasons. First, the nanomolar concentration range of melatonin used in the present study is considered the physiological concentration, and it can elevate the mRNA expression of neural stem cell markers, including Pax6, Sox2, and Nestin, similar to a previous report (Sharma et al. 2008). Second, an antiproliferative effect might occur, as a higher concentration of melatonin (1 mM) inhibits proliferation and increases the apoptosis of canine mammary gland carcinoma cells (Serrano et al. 2019).
Dimethyl sulfoxide (DMSO) is generally known as an effective cryoprotectant and is widely used for cryopreservation of many cell types, including neural stem cells (Milosevic et al. 2005), at a concentration of 10% (v/v). In addition to using DMSO as a cryoprotectant, it has been reported that DMSO enhances the differentiation of human-induced pluripotent stem cells toward hepatocytes, pancreatic progenitor cells, insulin-secreting β-cells, and cardiomyocytes (Li et al. 2018). The results of the present study confirm that 1% v/v DMSO did not stimulate the degeneration of porcine iNSCs. DMSO dramatically increased the degeneration of porcine iNSCs in a concentration-dependent manner. We selected 5% v/v of DMSO for the subsequent experiments. The degenerative process of porcine iNSCs begins with morphological changes, including the shortening of cell processes, formation of a round shape, and detachment from the surface of the culture dish. DMSO might alter the extracellular membrane of the cells, the cell’s membrane structure, resulting in cell adaptation to the surface with passive and active adhesion. Cells can enter the degenerative phase if DMSO is not washed out or the cell membrane structure is not secured (Okano et al. 1995). Under the conditions of the present study, the results demonstrated that melatonin protects against the DMSO-induced degeneration of porcine iNSCs. Moreover, the porcine iNSCs that survived after DMSO-induced degeneration could further spontaneously differentiate into neurons, as confirmed by expression of β-tubulin III and GFAP, an astrocyte marker (Machado et al. 2020). It has been demonstrated that melatonin increases neural stem cell differentiation via MT receptor-mediated activation of the phosphoinositide 3-kinase/Akt signaling pathway, resulting in upregulation of Nestin and MAP2, which are neural differentiation markers (Shu et al. 2016). From the results of the present study, it is implied that melatonin not only promotes survival but also maintains the differentiation ability of porcine iNSCs. Mitochondrial membrane potential (ΔΨm) plays an important role in the maintenance of cell viability and homeostasis (Zorova et al. 2018). A decrease in ΔΨm causes mitochondrial dysfunction and results in the degeneration of cells. The results of the present study demonstrated that 1 nM of melatonin showed a protective effect against DMSO-induced degeneration by increasing the ΔΨm. Thus, pretreatment of porcine iNSCs with 1 nM of melatonin can be beneficial for other degenerative induction in vitro studies.
The pandemic of ZIKV infection has received the attention of scientists because it impairs fetal neural development, resulting in microcephaly (Han et al. 2021). A study in human iPSC-derived neural stem cells demonstrated a high rate of neural cell death (Tang et al. 2016). There is evidence that pigs can be infected by the Zika virus and might be the reservoir of the disease (Nunez-Avellaneda et al. 2021). Therefore, the pig can be a model for the development of new therapeutics and an understanding of the pathogenesis of ZIKV. In the second experiment of our study, we examined whether melatonin can rescue ZIKV-induced degeneration of porcine iNSCs. We found that at 72 h postinfection, melatonin protected against ZIKV-induced degeneration. Thereafter, the cells tended to degenerate and detach from the culture dish. This in vitro phenomenon was also observed in another study (Yang et al. 2020). ZIKV infection induces neural cell apoptosis by activating the protein that modulates Bax and triggers programmed cell death (Han et al. 2021). ZIKV infection induces neural cell apoptosis by activating the protein that modulates Bax and triggers programmed cell death (Han et al. 2021). In the present study, although melatonin was unable to suppress ZIKV replication, as demonstrated by the plaque assay results, melatonin at concentrations of 1 and 10 nM showed a protective effect against ZIKV-induced degeneration by increasing the ΔΨm. Therefore, our in vitro model of pretreatment of porcine iNSCs with melatonin might be beneficial for exploring the molecular mechanism of the replication of ZIKV and how the virus uses neural stem cells as the cell reservoir.
In conclusion, our study demonstrated that melatonin is a potential anti-degeneration supplement for porcine iNSCs. Melatonin protects porcine iNSCs against DMSO- and ZIKV-induced degeneration.
References
Abbott LC, Nigussie F (2021) Mercury toxicity and neurogenesis in the mammalian brain. Int J Mol Sci 22:7520
Bharti D, Shivakumar SB, Subbarao RB, Rho GJ (2016) Research advancements on porcine derived mesenchymal stem cells. Curr Stem Cell Res Ther 11:78–93
Bourret R, Martinez E, Vialla F, Giquel C, Thonnat-Marin A, De Vos J (2016) Human-animal chimeras: ethical issues about farming chimeric animals bearing human organs. Stem Cell Res Ther 7:87
Cardinali DP, Vigo DE, Olivar N, Vidal MF, Brusco LI (2014) Melatonin therapy in patients with Alzheimer’s Disease. Antioxidants (Basel) 3(2):245–277
Chakritbudsabong W, Chaiwattanarungruengpaisan S, Sariya L, Pamonsupornvichit S, Ferreira JN, Sukho P, Gronsang D, Tharasanit T, Dinnyes A, Rungarunlert S (2021a) Exogenous LIN28 is required for the maintenance of self-renewal and pluripotency in presumptive porcine-induced pluripotent stem cells. Front Cell Dev Biol. 9:709286
Chakritbudsabong W, Sariya L, Juntahirun P, Chaisilp N, Chaiwattanarungruengpaisan S, Rungsiwiwut R, Ferreira JN, Rungarunlert S (2021b) Generation of porcine induced neural stem cell using the Sendai virus. Front Vet Sci 8:806785
Chen Z, Zuo X, Li H, Hong R, Ding B, Liu C, Gao D, Shang H, Cao Z, Huang W, Zhang X, Zhang Y (2017) Effect of melatonin on maturation, histone acetylation, autophagy of porcine oocytes and subsequent embryonic development. Anim Sci J 88:1298–1310
Choi K-H, Lee D-K, Oh J-N, Kim S-H, Lee M, Jeong J, Choe GC, Lee C-K (2020) The generation of neural progenitor cells from pig embryonic germ cells. J Anim Reprod Biotechnol 35:42–49
Darbellay J, Lai K, Babiuk S, Berhane Y, Ambagala A, Wheler C, Wilson D, Walker S, Potter A, Gilmour M, Safronetz D, Gerdts V, Karniychuk U (2017) Neonatal pigs are susceptible to experimental Zika virus infection. Emerg Microbes Infect. 6(2):e6
Ferreira RO, Garcez PP (2019) Dissecting the toxic effects of Zika virus proteins on neural progenitor cells. Neuron 101:989–991
Guven C, Taskin E, Akcakaya H (2016) Melatonin prevents mitochondrial damage induced by doxorubicin in mouse fibroblasts through AmpK-Ppar Gamma-dependent mechanisms. Med SciMonit 22:438–446
Han X, Wang J, Yang Y, Qu S, Wan F, Zhang Z, Wang R, Li G, Cong H (2021) Zika virus infection induced apoptosis by modulating the recruitment and activation of pro-apoptotic protein Bax. J Virol 95:e01445-e1520
Hill JD, Zuluaga-Ramirez V, Gajghate S, Winfield M, Persidsky Y (2019) Chronic intrahippocampal infusion of HIV-1 neurotoxic proteins: a novel mouse model of HIV-1 associated inflammation and neural stem cell dysfunction. J Neuroimmune Pharmacol 14:375–382
Kato F, Tajima S, Nakayama E, Kawai Y, Taniguchi S, Shibasaki K, Taira M, Maeki T, Lim CK, Takasaki T, Saijo M (2017) Characterization of large and small-plaque variants in the Zika virus clinical isolate ZIKV/Hu/S36/Chiba/2016. Sci Rep 7:16160
Kim E, Hwang SU, Yoon JD, Kim H, Lee G, Hyun SH (2018) Isolation and characterization of GFAP-positive porcine neural stem/progenitor cells derived from a GFAP-CreERT2 transgenic piglet. BMC Vet Res 14:331
Kim E, Kim M, Hwang SU, Kim J, Lee G, Park YS, Hyun SH (2019) Neural induction of porcine-induced pluripotent stem cells and further differentiation using glioblastoma-cultured medium. J Cell Mol Med 23:2052–2063
Ledur PF, Karmirian K, Pedrosa CDSG, Souza LRQ, Assis-de-Lemos G, Martins TM, Ferreira JCCG, de Azevedo Reis GF, Silva ES, Silva D, Salerno JA, Ornelas IM, Devalle S, Madeiro da Costa RF, Goto-Silva L, Higa LM, Melo A, Tanuri A, Chimelli L, Murata MM, Garcez PP, Filippi-Chiela EC, Galina A, Borges HL, Rehen SK (2020) Zika virus infection leads to mitochondrial failure, oxidative stress and DNA damage in human iPSC-derived astrocytes. Sci Rep 10:1218
Lee H, Park JB (2017) Evaluation of the effects of dimethylsulphoxide on morphology, cellular viability, mRNA, and protein expression of stem cells culture in growth media. Biomed Rep 7(4):291–296
Lee H, Park JB (2019) Dimethyl sulfoxide leads to decreased osteogenic differentiation of stem cells derived from gingiva via Runx2 and Collagen I expression. Eur J Dent 13(2):131–136
Li J, Narayanan C, Bian J, Sambo D, Brickler T, Zhang W, Chetty S (2018) A transient DMSO treatment increases the differentiation potential of human pluripotent stem cells through the Rb Family. PLoS One. 13(12):e0208110
Liang S, Yin N, Faiola F (2019) Human pluripotent stem cells as tools for predicting developmental neural toxicity of chemicals: strategies, applications, and challenges. Stem Cells Dev 28:755–768
Lind NM, Moustgaard A, Jelsing J, Vajta G, Cumming P, Hansen AK (2007) The use of pigs in neuroscience: modeling brain disorders. Neurosci Biobehav Rev 31:728–751
Liu GH, Yi F, Suzuki K, Qu J, Izpisua Belmonte JC (2012) Induced neural stem cells: a new tool for studying neural development and neurological disorders. Cell Res 22:1087–1091
Liu J, Clough SJ, Hutchinson AJ, Adamah-Biassi EB, Popovska-Gorevski M, Dubocovich ML (2016a) MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu Rev Pharmacol Toxicol 56:361–383
Liu Y, Zhang Z, Lv Q, Chen X, Deng W, Shi K, Pan L (2016b) Effects and mechanism of melatonin on the proliferation and neural differentiation of PC12 cells. Biochem Biophys Res Commun 478:540–545
Machado LS, Pieri NCG, Botigelli RC, de Castro RVG, de Souza AF, Bridi A, Lima MA, Fantinato Neto P, Pessôa LVF, Martins SMMK, De Andrade AFC, Freude KK, Bressan FF (2020) Generation of neural progenitor cells from porcine-induced pluripotent stem cells. J Tissue Eng Regen Med 14:1880–1891
Milosevic J, Storch A, Schwarz J (2005) Cryopreservation does not affect proliferation and multipotency of murine neural precursor cells. Stem Cells 23:681–688
Niedzielska E, Smaga I, Gawlik M, Moniczewski A, Stankowicz P, Pera J, Filip M (2016) Oxidative stress in neurodegenerative diseases. Mol Neurobiol 53:4094–4125
Nunez-Avellaneda D, Cetina-Trejo RC, Zamudio-Moreno E, Baak-Baak C, Cigarroa-Toledo N, Reyes-Solis G, Ortega-Pacheco A, Suzán G, Tandugu C, García-Rejón JE, Blitvich BJ, Machain-Williams C (2021) Evidence of Zika virus infection in pigs and mosquitoes. Mexico Emerg Infect Dis 27:574–577
Okano T, Yamada N, Okuhara M, Sakai H, Sakurai Y (1995) Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials 16(4):297–303
O’Sullivan A, Lange S, Rotheneichner P, Bieler L, Aigner L, Rivera FJ, Couillard-Despres S (2019) Dimethylsulfoxide inhibits oligodendrocyte fate choice of adult neural stem and progenitor cells. Front Neurosci 13:1242
Rasmussen MA, Hall VJ, Carter TF, Hyttel P (2011) Directed differentiation of porcine epiblast-derived neural progenitor cells into neurons and glia. Stem Cell Res 7:124–136
Rungsiwiwut R, Manolertthewan C, Numchaisrika AV, Virutamasen P, Techakumphu M, Pruksananonda K (2013) The ROCK inhibitor Y-26732 enhances the survival and proliferation of human embryonic stem cell-derived neural progenitor cells upon dissociation. Cells Tissues Organs 198:127–138
Schlüter C, Duchrow M, Wohlenberg C, Becker MH, Key G, Flad HD, Gerdes J (1993) The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J Cell Biol 123:513–522
Serrano C, Guzmán S, Arias JI, Torres CG (2019) Melatonin decreases in vitro viability and migration of spheres derived from CF41.Mg canine mammary carcinoma cells. BMC Vet Res. 15:390
Setthawong P, Phakdeedindan P, Techakumphu M, Tharasanit T (2021) Molecular signature and colony morphology affect in vitro pluripotency of porcine induced pluripotent stem cells. Reprod Domest Anim 56:1104–1116
Setthawong P, Tharasanit T, Techakumphu M (2019) Effects of activin A on the pluripotency of induced pluripotent stem cells derived from porcine Sertoli cells. Thai J Vet Med 49:183–191
Sharma R, Ottenhof T, Rzeczkowska PA, Niles LP (2008) Epigenetic targets for melatonin: induction of histone H3 hyperacetylation and gene expression in C17.2 neural stem cells. J Pineal Res 45:277–284
Shu T, Wu T, Pang M, Liu C, Wang X, Wang J, Liu B, Rong L (2016) Effects and mechanisms of melatonin on neural differentiation of induced pluripotent stem cells. Biochem Biophys Res Commun 474:566–571
Subbarao RB, Ullah I, Kim EJ, Jang SJ, Lee WJ, Jeon RH, Kang D, Lee SL, Park BW, Rho GJ (2015) Characterization and evaluation of neuronal trans-differentiation with electrophysiological properties of mesenchymal stem cells isolated from porcine endometrium. Int J Mol Sci 16:10934–10951
Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee EM, Christian KM, Didier RA, ** P, Song H, Ming GL (2016) Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 18:587–590
Their M, Wörsdörfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nöthen MM, Brüstle O, Edenhofer F (2012) Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell 10:473–479
Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M (2010) Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463:1035–1041
Wang T, Gao YY, Chen L, Nie ZW, Cheng W, Liu X, Schatten H, Zhang X, Miao YL (2017) Melatonin prevents postovulation oocyte aging and promotes subsequent embryonic development in the pig. Aging (albany NY) 9:1552–1564
Wright LS, Prowse KR, Wallace K, Linskens MH, Svendsen CN (2006) Human progenitor cells isolated from the develo** cortex undergo decreased neurogenesis and eventual senescence following expansion in vitro. Exp Cell Res 312(11):2107–2120
Yang JR, Liao CH, Pang CY, Huang LL, Chen YL, Shiue YL, Chen LR (2013) Transplantation of porcine embryonic stem cells and their derived neuronal progenitors in a spinal injury rat model. Cytotherapy 15:201–208
Yang S, Gorshkov K, Lee EM, Xu M, Cheng YS, Sun N, Soheilian F, de Val N, Ming G, Song H, Tang H, Zheng W (2020) Zika virus-induced neuronal apoptosis via increased mitochondrial fragmentation. Front Microbiol. 11:598203
Yu X, Li Z, Zheng H, Ho J, Chan MT, Wu WK (2017) Protective roles of melatonin in central nervous system diseases by regulation of neural stem cells. Cell Prolif. 50:e12323
Zhang S, Bai C, Zheng D, Gao Y, Fan Y, Li L, Guan W, Ma Y (2016) Identification and characterization of pig adipose-derived progenitor cells. Can J Vet Res 80:309–317
Zheng YM, An ZX, Zhao XE (2010) Osteogenic and neurogenic differentiation of EGFP gene transfected neural stem cells derived from the brain of porcine fetuses at intermediate and late gestational age. Cell Biol Int 34:809–814
Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, Jankauskas SS, Babenko VA, Zorov SD, Balakireva AV, Juhaszova M, Sollott SJ, Zorov DB (2018) Mitochondrial membrane potential. Anal Biochem 552:50–59
Acknowledgements
The authors would like to express our gratitude to the Faculty of Veterinary Science, Mahidol University, for providing the cell line and facility to support our study.
Funding
The present study was financially supported by funding support from the National Science, Research and Innovation Fund (NSRF) via the Program Management Unit for Human Resources & Institutional Development, Research and Innovation [grant no.: B05F630046], Mahidol University [Basic Research Fund: fiscal year 2021], and Srinakharinwirot University [grant no.: 002/2562].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horcharoensuk, P., Yang-en, S., Chakritbudsabong, W. et al. Melatonin attenuates dimethyl sulfoxide– and Zika virus–induced degeneration of porcine induced neural stem cells. In Vitro Cell.Dev.Biol.-Animal 58, 232–242 (2022). https://doi.org/10.1007/s11626-022-00648-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-022-00648-z