Abstract
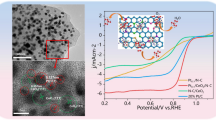
Pt/C catalysts containing four different morphologies of CeO2 as co-catalysts were synthesized in this work, and their electrocatalytic performance for hydrogen evolution reaction (HER) was investigated. As compared with the Pt/C catalyst, these four catalysts containing CeO2 all exhibited improved catalytic activity. Among them, the Pt/C catalyst containing spherical CeO2 with a diameter of 30 ~ 60 nm (Pt/C-CeO2(s2)) possesses the best catalytic activity, displaying an over-potential of 258 mV at 10 mA cm−2 and a Tafel slope of 42 mV dec−1. According to the characterization results of structure, morphology, and elemental valence state, the enhancement of catalytic activity is ascribed to the small particle size and good dispersion degree of Pt, as well as the strong interaction between the exposed (111) crystal plane of small spherical CeO2 and Pt, which leads to a significant increase in metallic Pt content. Moreover, the Pt/C-CeO2(s2) catalyst also demonstrates outstanding long-term stability besides exceptional catalytic activity. The results clearly illustrate that CeO2 with diverse shapes and sizes can remarkably influence the catalytic performance of loaded Pt particles in the HER process.






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Zheng Z, Li N, Wang CQ, Li DY, Zhu YM, Wu G (2012) Ni-CeO2 composite cathode material for hydrogen evolution reaction in alkaline electrolyte. Int J Hydrog Energy 37:13921–13932
Yao N, Meng R, Wu F, Fan ZY, Cheng GZ, Luo W (2020) Oxygen-vacancy-induced CeO2/Co4N heterostructures toward enhanced pH-universal hydrogen evolution reactions. Appl Catal B-Environ 277:8
Song HJ, Sung MC, Yoon H, Ju B, Kim DW (2019) Ultrafine a-phase molybdenum carbide decorated with platinum nanoparticles for efficient hydrogen production in acidic and alkaline media. Adv Sci 6:8
Liu HH, Yan ZH, Chen X, Li JH, Zhang L, Liu FM, Fan GL, Cheng FY (2020) Electrodeposition of Pt-decorated Ni(OH)2/CeO2 hybrid as superior bifunctional electrocatalyst for water splitting. Research 2020:11
Liu JC, Xu GR, Zhen HR, Zhai H, Li CP (2022) Directed self-assembled pathways of 3D rose-shaped PtNi@CeO2 electrocatalyst for enhanced hydrogen evolution reaction. J Alloy Compd 931:8
Meng K, Wen SX, Liu LJ, Jia ZJ, Wang Y, Shao ZG, Qi T (2019) Vertically grown MoS2 nanoplates on VN with an enlarged surface area as an efficient and stable electrocatalyst for HER. ACS Appl Energ Mater 2:2854–2861
Chen JL, Yu TQ, Zhai ZX, Qian GF, Yin SB (2023) Coupling interface engineering with electronic interaction toward high-efficiency H2 evolution in pH-universal electrolytes. J Energy Chem 80:535–541
Ma D, Meng K, Ma J, Jia Z, Wang Y, Liu L, Zhu G, Qi T (2019) The catalytic performance enhancement of Ni2P electrocatalysts for hydrogen evolution reaction by carbon-based substrates. Int J Hydrogen Energy 44:31960–31968
You J, Jia Z, Wang Y, Wang D, Song J, Tian L, Qi T (2022) Carbon dots modified molybdenum disulfide as a high-efficiency hydrogen evolution electrocatalyst. Int J Hydrogen Energy 47:34898–34908
Guo X, Li MG, Qiu LY, Tian FY, He L, Geng S, Liu YQ, Song Y, Yang WW, Yu YS (2023) Engineering electron redistribution of bimetallic phosphates with CeO2 enables high-performance overall water splitting. Chem Eng J 453:9
**a BY, Wu HB, Wang X, Lou XW (2012) One-pot synthesis of cubic PtCu3 nanocages with enhanced electrocatalytic activity for the methanol oxidation reaction. J Am Chem Soc 134:13934–13937
Hua D, Zhang LY, Meng K, Jia ZJ, Wang Y, Qi T (2020) Electrocatalysis of Pd-Er bimetallic catalysts for methanol oxidation in alkaline media. Ionics 26:3459–3464
Zhou W, Jia J, Lu J, Yang L, Hou D, Li G, Chen S (2016) Recent developments of carbon-based electrocatalysts for hydrogen evolution reaction. Nano Energy 28:29–43
Ge R, Huo J, Sun M, Zhu M, Li Y, Chou S, Li W (2021) Surface and interface engineering: molybdenum carbide-based nanomaterials for electrochemical energy conversion. Small 17:e1903380
Zhou G, Shan Y, Wang L, Hu Y, Guo J, Hu F, Shen J, Gu Y, Cui J, Liu L, Wu X (2019) Photoinduced semiconductor-metal transition in ultrathin troilite FeS nanosheets to trigger efficient hydrogen evolution. Nat Commun 10:399
Yang M, Zhu YR, Lin ZY, Yan XT, Dong B, Zhou YN, Li QZ, Zhou YL, Nan J, Chai YM (2020) Modulation engineering of in situ cathodic activation of FePx based on W-incorporation for the hydrogen evolution reaction. Nanoscale 12:12364–12373
**g Y, Liu H, Yan R, Chen J, Dai H, Liu C, Zhang X-D (2019) Mesoporous CoP nanowire arrays for hydrogen evolution. ACS Appl Nano Mater 2:5922–5930
Zhang J, Liu Y, Sun C, **. ACS Energy Lett 3:779–786
Jian J, Yuan L, Qi H, Sun X, Zhang L, Li H, Yuan H, Feng S (2018) Sn-Ni3S2 ultrathin nanosheets as efficient bifunctional water-splitting catalysts with a large current density and low overpotential. ACS Appl Mater Interfaces 10:40568–40576
Sun J, Lu J, Huang C, Wu Q, **a L, Xu Q, Yao W (2021) Modification of Ni3N with a cobalt-doped carbon shell for high-performance hydrogen evolution in alkaline media. ACS Sustain Chem Eng 9:1994–2002
Rehman AU, Hossain SS, Rahman SU, Ahmed S, Hossain MM (2015) Influence of CeO2 on Pt–Pd/CeO2–OMC catalysts for formic acid oxidation. Electrocatalysis 6:348–356
Liu XW, Zhou KB, Wang L, Wang BY, Li YD (2009) Oxygen vacancy clusters promoting reducibility and activity of ceria nanorods. J Am Chem Soc 131:3140–3141
Resasco J, DeRita L, Dai S, Chada JP, Xu MJ, Yan XX, Finzel J, Hanukovich S, Hoffman AS, Graham GW, Bare SR, Pan XQ, Christopher P (2020) Uniformity is key in defining structure–function relationships for atomically dispersed metal catalysts: the case of Pt/CeO2. J Am Chem Soc 142:169–184
Pastor-Perez L, Ramos-Fernandez EV, Sepulveda-Escribano A (2019) Effect of the CeO2 synthesis method on the behaviour of Pt/CeO2 catalysis for the water-gas shift reaction. Int J Hydrog Energy 44:21837–21846
Ren ZB, Peng F, Li JW, Liang X, Chen BH (2017) Morphology-dependent properties of Cu/CeO2 catalysts for the water-gas shift reaction. Catalysts 7:12
Abdelouahab-Reddam Z, El Mail R, Coloma F, Sepulveda-Escribano A (2015) Effect of the metal precursor on the properties of Pt/CeO2/C catalysts for the total oxidation of ethanol. Catal Today 249:109–116
Montini T, Melchionna M, Monai M, Fornasiero P (2016) Fundamentals and catalytic applications of CeO2-based materials. Chem Rev 116:5987–6041
Wang Y, Wang SY, Wang X (2009) CeO2 promoted electro-oxidation of formic acid on Pd/C nano-electrocatalysts. Electrochem Solid-State Lett 12(5):B73–B76
Chen X, Liao W, Zhong M, Chen J, Yan S, Li W, Wang C, Chen W, Lu X (2022) Rational design of robust iridium-ceria oxide-carbon nanofibers to boost oxygen evolution reaction in both alkaline and acidic media. Nano Res 16:7724–7732
Swathi S, Yuvakkumar R, Senthil Kumar P, Ravi G, Thambidurai M, Dang C, Velauthapillai D (2022) Gadolinium doped CeO2 for efficient oxygen and hydrogen evolution reaction. Fuel 310:122319
Zheng Z, Li N, Wang CQ, Li DY, Zhu YM, Wu G (2012) Ni-CeO2 composite cathode material for hydrogen evolution reaction in alkaline electrolyte. Int J Hydrogen Energy 37:13921–13932
Gao YX, Wang WD, Chang SJ, Huang WX (2013) Morphology effect of CeO2 support in the preparation, metal-support interaction, and catalytic performance of Pt/CeO2 catalysts. ChemCatChem 5:3610–3620
Zhang DF, Zhang CS, Chen YM, Wang QF, Bian LY, Miao J (2014) Support shape effect on the catalytic performance of Pt/CeO2 nanostructures for methanol electrooxidation. Electrochim Acta 139:42–47
Vayssilov GN, Lykhach Y, Migani A, Staudt T, Petrova GP, Tsud N, Skala T, Bruix A, Illas F, Prince KC, Matolin V, Neyman KM, Libuda J (2011) Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles. Nat Mater 10:310–315
Wang Q, Jia W, Liu B, Zhao W, Li C, Zhang J, Xu G (2012) Controllable synthesis of nearly monodisperse spherical aggregates of CeO2 nanocrystals and their catalytic activity for HCHO oxidation. Chem Asian J 7:2258–2267
Zhao HY, Wang Y, Tang Q, Wang L, Zhang H, Quan C, Qi T (2014) Pt catalyst supported on titanium suboxide for formic acid electrooxidation reaction. Int J Hydrogen Energy 39:9621–9627
Meher SK, Rao GR (2012) Polymer-assisted hydrothermal synthesis of highly reducible shuttle-shaped CeO2: microstructural effect on promoting Pt/C for methanol electrooxidation. ACS Catal 2:2795–2809
Justin P, Charan PHK, Rao GR (2010) High performance Pt–Nb2O5C electrocatalysts for methanol electrooxidation in acidic media. Appl Catal B-Environ 100:510–515
Hsu NY, Chien CC, Jeng KT (2008) Characterization and enhancement of carbon nanotube-supported PtRu electrocatalyst for direct methanol fuel cell applications. Appl Catal B-Environ 84:196–203
Justin P, Rao GR (2011) Methanol oxidation on MoO3 promoted Pt/C electrocatalyst. Int J Hydrog Energy 36:5875–5884
Mao MY, Ly HQ, Li YZ, Yang Y, Zeng M, Li N, Zhao XJ (2016) Metal support interaction in Pt nanoparticles partially confined in the mesopores of microsized mesoporous CeO2 for highly efficient purification of volatile organic compounds. ACS Catal 6:418–427
Scibioh MA, Kim SK, Cho EA, Lim TH, Hong SA, Ha HY (2008) Pt–CeO2/C anode catalyst for direct methanol fuel cells. Appl Catal B-Environ 84:773–782
Habibi B, Delnavaz N (2015) Pt–CeO2/ reduced graphene oxide nanocomposite for the electrooxidation of formic acid and formaldehyde. RSC Adv 5:73639–73650
Bruix A, Migani A, Vayssilov GN, Neyman KM, Libuda J, Illas F (2011) Effects of deposited Pt particles on the reducibility of CeO2(111). Phys Chem Chem Phys 13:11384–11392
Zeng M, Li Y (2015) Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction. J Mater Chem A 3:14942–14962
Wang H, Maiyalagan T, Wang X (2012) Review on recent progress in nitrogen-doped graphene: synthesis, characterization, and its potential applications. ACS Catal 2:781–794
Cheng X, Li YH, Zheng LR, Yan Y, Zhang YF, Chen G, Sun SR, Zhang JJ (2017) Highly active, stable oxidized platinum clusters as electrocatalysts for the hydrogen evolution reaction. Energy Environ Sci 10:2450–2458
Akbayrak M, Onal AM (2020) Binder-free iridium based electrocatalysts: facile preparation, high activity and outstanding stability for hydrogen evolution reaction in acidic medium. J Colloid Interface Sci 580:11–20
DemirArabaci E, Önal AM, Özkar S (2020) Ceria supported nickel (0) nanoparticles: a highly active and low cost electrocatalyst for hydrogen evolution reaction. J Electrochem Soc 167:106513
Rajalakshmi R, Viswanathan C, Ponpandian N (2021) Sm3+ rare-earth do** in non-noble metal oxide-WO3 grown on carbon cloth fibre as a bifunctional electrocatalyst for high-performance water electrolysis. Sustain Energy Fuels 5:5851
Majhi KC, Yadav M (2022) Neodymium oxide doped neodymium phosphate as efficient electrocatalyst towards hydrogen evolution reaction in acidic medium. J Environ Chem Eng 10:107416
Zhang ZW, Dai YF, He ZH, Wang ZZ, Ji HJ, Lu LH, Wu Y, Bu XH (2022) Ni-MOF with dual organic ligands derived Pt-Ni alloy catalyst for highly efficient hydrogen evolution reaction. Chem Lett 51:716–719
Funding
The authors are grateful for the financial support from the National Key Research and Development Program of China (2020YFC1909001) and the cooperation project between universities in Chongqing and institutes affiliated to the Chinese Academy of Sciences (Grant No. HZ2021013).
Ethics declarations
Ethical approval
Participants in the study agreed to participate and publish without involving potential conflicts of interest and animal testing.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qi, P., You, J., Wang, Y. et al. Pt/C catalysts containing CeO2 with different morphologies for the hydrogen evolution reaction. Ionics 29, 5329–5337 (2023). https://doi.org/10.1007/s11581-023-05214-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05214-5