Abstract
Different commercial carbonaceous materials, two made of activated carbons and one of multiwalled nanotubes, were used to prepare cathodes for primary aluminum-air cells and compared with the more expensive platinum-dispersed carbon, usually used as cathode for many types of metal-air cells. The aluminum-air cells used in the electrochemical tests were assembled with alkaline gel polymer electrolytes without any separator. Cells with cathodes made of a cheap activated carbon showed better electrochemical performances than those obtained with platinum-based cathodes. Notably, their discharge capacities were improved and the discharge voltages were always higher than 0.2 V. These improved performances were mainly attributed to the better electrocatalytic activity of the activated carbon as it results from polarization measurements, probably due to the presence of defects, as evidenced from Raman spectra. Three-electrode discharge tests were used to measure the electrode potentials and their impact to the overall cell electrochemical performances. During the discharge, in all cases, an increase of the anodic potential towards more positive values was observed, while the cathodic potential remained almost constant. Thus, the final failure of the cells was mainly due to the degradation of the anodic interface. This indicates the possibility to further increase the cell capacity by adopting suitable mitigation strategies of anodic parasitic reaction or different electrolyte design, with the final aim to realize efficient, cheap, and eco-friendly aluminum-air cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many safety and environmental issues, related to the supply of quite rare raw materials and to the production and widespread use of lithium-ion batteries, have forced researchers to find new electrochemical energy conversion devices, possibly based on more abundant and eco-sustainable materials. Among these, metal-air batteries represent a solution with great potential [1]. In these devices, the redox reaction that provides faradaic current occurs between a metal anode and atmospheric oxygen. Specifically, the anode is oxidized in the discharge, releasing electrons to an external circuit, while the oxygen of the air, diffusing through the porous carbon-based cathode, accepts the electrons and combines with metal ions to form discharge products. During the charging, the reactions reverse. Therefore, the air cathode plays a crucial role in metal-air batteries because acts non only as a medium to assure a suitable gas diffusion, but also as a site for accumulating discharge products. In particular, carbon-based air cathodes with a wide hierarchical pore size distribution show improved electrochemical performances [2], due to the more efficient transport of oxygen to the electrolyte interface and also to their electrocatalytic activity for the two foremost reactions occurring in metal-air batteries, namely the oxygen reduction reaction (ORR) and the oxygen evolution reaction (OER) [8]. For what concerns the carbon based materials, their ORR electrocatalytic activity is associated with the presence of defects in the graphitic layers, as edges or point defects caused by vacancy or impurity, that serve as active sites to dissociate O2 into atomic oxygen which reacts with H2O to forms hydroxide ions in the redox reactions [9]. Therefore, the introduction of defects into carbonaceous materials used as cathodes results in more active sites for electrochemical reactions where nucleation of reaction products occurs, possibly resulting also in the decreasing of particle size [10]. As a result, increased discharge capacity and low cycle overpotentials are observed in many metal-air batteries prepared with defective carbon-based cathode [11,12,13,14].
Aluminum is a very interesting metal to be used as anode in metal-air cells, due to its high specific capacity (2980 mAh g−1), its abundance on the Earth’s crust and its recyclability.
Quasi-solid and solid polymer electrolytes represent the materials of choice for the realization of safer aluminum-air batteries [15], as well as for other multivalent metal-air batteries [16]. Recently, with the aim to realize aluminum-air batteries based on environmentally friendly materials [17, 18], we have developed new quasi-solid and solid gel electrolytes based on aqueous solutions and synthetic biodegradable or natural polymers, such as polyvinyl alcohol and xanthan gum [19,20,21,22,23,24]. These gel polymer electrolytes are characterized by good ionic conductivity, almost comparable with those of aqueous solutions [21], showing also interesting self-corrosion inhibition properties of the metal anode [23]. In all our previous studies, mainly focused on new electrolytes, we always used benchmark cathodes, prepared with commercial powders made of platinum dispersed on carbon (Pt/C,10 wt. % Pt, Merck), to allow easier comparison with literature data.
Herein, cathodes made of three different commercial carbons were tested in primary aluminum-air cells assembled with Xanthan-based alkaline gel electrolytes, and compared to the Pt/C benchmark cathodes used in the same cells. In particular, we tested two low-cost activated carbons and a commercial multiwalled carbon nanotubes. The aim was to continue further with the study of batteries made of environmentally friendly materials and therefore using, possibly, also noble metal-free cathodes. The study was effected by discharge tests in three-electrode configuration and electrochemical impedance spectroscopy of cells, while scanning electron microscopy/energy dispersive X-ray analysis, laser Raman spectroscopy, adsorption/desorption, and polarization measurements were used for the characterization of materials.
Materials and methods
The xanthan-based electrolyte was prepared by mixing and kneading powders of xanthan gum (powders from Xanthomonas campestris, Merck) in 11 M KOH solution at a ratio of 700 mg mL−1 at room temperature. The resulting gels were yellowish and gummy materials (Fig. 1a), quite resistant to compression (Fig. 1b and c). The fully characterization of this electrolyte is reported in ref. [21, 22].
Air cathodes were prepared using carbon cloth (H2-Planet, 0.35 mm thick, 116 g m−2) as support. Discs of 13 mm diameter were cut from the carbon cloth foils and pasted with different carbon based suspensions. The suspensions were prepared by mixing polyvinylidene fluoride (Merck, powder form) to the carbon powders in a weight ratio of 1:10, and by adding 1 cm3 of solvent (1-methyl-2- pyrrolidinone, Merck) per 220 mg of solid mixture. The carbon-based materials used for the suspensions were platinum-dispersed actived carbon (Pt/C, 10 wt. % Pt, provided by Merck in powder form), multiwalled carbon nanotubes (MWCNTs, from Nanostructured and Amorphous Materials Inc., 20–40 nm diameter, 1–2 µm length, 95% purity, 40–600 m2/g) and two different powdered activated charcoal, one produced from wood (product number 05105, Merck, hereafter named AC1) and the other from generic natural-organic material (product number161551, Merck, hereafter named AC2). After drying at 80 °C for 4 h, the final Pt concentration in the Pt/C cathodes was 2.5% wt of the cathode total weight. The amount of the carbon pasted on each cathode was about 12 mg, while the total weight of the cathodes was about 45 mg. The cathodes prepared with different carbons and the cells assembled with different cathodes were named X-cathodes and X-cells respectively, where X = AC1, MWCNTs, AC2, and Pt/C.
Ultrapure aluminium (Puratronic, 0.5 mm thick, 99.998%, Alfa Aesar) was used as the anode in the form of a 13-mm diameter disc in an aluminum-air cells. The weight of anodes varied between 150 and 170 mg.
The aluminum-air cells were assembled by pressing, in an open support, 2.6 mm thick electrolyte between anode and cathode using 10 mm diameter Teflon spacers (Fig. 1).
The carbonaceous materials were characterized by laser Raman spectroscopy by using the radiation emitted by a He–Ne laser (633 nm) as excitation radiation. The laser radiation was filtered with a band pass filter (Thorlabs-FL632.8–3—Ø1" Laser Line Filter, CWL = 632.8 ± 0.6 nm, FWHM = 3 ± 0.6 nm) and focused on the samples with 150-mm quartz lens. A bandpass filter (Chroma RET270lp, OD ≥ 6 at λ < 269 nm) was used to remove the scattered laser light and to pass the Stokes-shifted Raman signals from 675 to 724 nm (1000–2000 cm−1). The filtered radiation was then collected by a 200-mm-F/4 quartz objective lens and focused on the input slit of the spectrograph (Acton SpectraPro® SP-2300 spectrometer, 1200 grooves/mm, 300 mm, triple grating). The resolution of the acquired spectra was 0.5 nm (10 cm−1). The spectra were acquired by using a 700 nm blazed grating, a back-illuminated CCD (Princeton Instruments, PIXIS 100B) and WinSpec/32© software. The optimized parameters giving the best S/N ratio of the spectra were 20 s CCD exposure time and 10 accumulations. The Raman spectra were fitted using the Origin© software.
N2 adsoprtion/desorption experiments were carried out at − 196 °C with a Quantachrome autosorb-iQ, after degassing for 6 h at 150 °C. Specific surface area of the samples was calculated by the Brunauer–Emmett–Teller (BET) method. Pore size distribution (PSD) of each sample was obtained by quenched solid density functional theory (QSDFT), which provides pore size information over the complete range of micro- and mesopores and accounts for the heterogeneity and surface roughness in carbon materials [25]. The calculation model assumed slit-like pore and were applied on the desorption branch.
A scanning electron microscope (SEM, Phenom ProX) equipped with energy-dispersive X-ray spectrometer (EDX) detector was used for morphological and elementary analysis of cathode and anode surfaces.
The electrochemical performances of aluminum-air cells and cathodes were studied by using an Autolab PGSTAT302N electrochemical workstations. The ORR performances of different cathodes contacted with solid alkaline electrolytes were evaluated by using a three-electrode cell setup where the cathode was the working electrode, 1cm2 platinum plate was the counter electrode and Ag/AgCl (saturated KCl) was the reference electrode. The I-V curves were acquired by starting from OCV and varying the potential towards more negative values at scan rate of 1 mV s−1. The discharge tests were effected in a three electrode cell configurations by using the reference electrode (Ag/AgCl, saturated KCl). The electrochemical impedance spectroscopy (EIS) tests were performed in a two electrode cell configuration by setting the workstations in potentiostatic mode, with the applied frequency ranging from 1 MHz to 50 mHz and a potential amplitude perturbation of 10 mV. The impedance spectra were taken at open-circuit voltage (OCV). The spectra were simulated by using the NOVA software.
Results and discussion
Raman spectroscopy, BET analysis, and surface morphology of cathodes
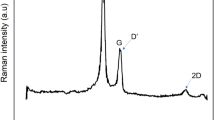
In the range 1000–2000 cm−1 from the laser excitation energy, Raman spectra of carbonaceous materials show two main bands centered at about 1580 and 1350 cm−1 [26,27,28,29]. The band at 1580 cm−1, referred as G band, identify the carbon atom vibrations against each other in ordered layers and is characteristic of graphene and graphitic materials. When disorder due to defects is introduced into the graphitic structures, this band broaden and additional bands arise at about 1200, 1357, and 1620 cm−1. The defects include point defects, due to vacancies or impurities, and boundary or edge. These bands are referred to as D1, D2, and D’ band (D denote disorder-induced). An additional broad band between 1500 and 1550 cm.−1 (hereafter referred to as D3 band) is assigned to amorphous graphitic phase, characteristic of interstitial disorder. It is generally accepted that the area ratio of the D2 and G bands give a good estimation of the order in carbonaceous materials. In particular, the D2 and G bands in Raman spectra are usually used to quantify the crystallite size (La) of 3D graphite through the relation [27]
where La is given in nm, EL is the laser excitation energy, given in eV, the constant 560 is given in units of eV4/nm and ID and IG are the D and G peak areas respectively.
However, it should be considered that the D2 band intensity depends on defect type and density, as these affect the scattering cross-section. In addition, controlled experiments on graphene demonstrate that the D2 position is correlated with specific do** [28]. Therefore, in not controlled experiments and without knowing the preparation procedure of the carbonaceous materials, the evaluation of La from 1) have to be considered qualitative. In Fig. 2 (black lines), the Raman spectra of the sampled carbonaceous powders are reported.
The D2 and G band are clearly observed in all samples. The band at 1550 cm−1, is not observed in MWCNTs spectrum that, instead, shows two not-overlapped bands at 1330 and 1580 cm−1 with a shoulder at 1620 cm−1.
For a better identification of band parameters (center, linewidth and area), a fit was performed by considering a combination of Lorentzian-shaped bands for D1, D2, G, and D’ and a Gaussian-shaped band for D3 [26]. The D’ line was considered in the fit only for the MWCNTs spectrum. In the other spectra the insertion of D’ line in the fit caused not-convergence. In Table 1, the fit parameters are reported with the La values as retrieved from 1).
Apart the MWCNTs which show the characteristic nanotube’s Raman spectrum with well separated D2 and G bands, clearly different from other carbonaceous materials, the linewidths of the Raman bands of the other samples, fitted with four D bands and the G band, well correlate with the size of crystallites, namely they increase by decreasing crystallite size. Notably, the AC2 spectrum is very similar to that of the Pt/C, while AC1 spectrum is quite different, with narrower D1 and G band, down-shifted D band and higher ratio of the peak area IG/ID. These spectral features indicate that AC1 exhibits a higher structural order and degree of graphitization than the AC2 and Pt/C samples. Therefore, AC1 powders could be electrically more conductive than the other samples [30]. In addition, the shift of D-band may be correlated with specific defects or do** [31].
Figure 3a displays the adsorption/desorption isotherm of the four carbons. Figure 3b shows the relative PSDs.
All carbonaceous materials, with the exception of MWCNTs, show a type IV isotherm with an H4 hysteresis loop, which is typical of slit-like pores in complex materials containing micro- and mesoporosity, such as hierarchical carbons [32]. Concerning MWCNTs, the isotherm is peculiar of this kind of material and totally different from that of other carbon materials. It is due to a multi-stage adsorption process in different types of pores: inner micropores (< 2 nm), small inner mesopores (3–4 nm) and aggregated pores (20–40 nm). Aggregated pores are those formed by the confined space among the isolate nanotubes in a relatively stable aggregate structure. Aggregated pores normally contribute to more than 78% of the total adsorption amount [33].
From the PSDs of Fig. 3b, it appears that all the carbonaceous materials show a similar micro/mesoporous network, with sharp maxima at 0.43–0.47 and 1.50–1.55 nm. AC2 shows a further mesoporosity in the range 2–5 nm. This kind of hierarchical micro-mesoporous structure can favor ionic diffusion [34]. Concerning MWCNTs, the fitting model for PDS distribution was applied only for slit-like micropore (0.56 nm) and small mesopore (1.6–2.9 nm). The pore volume of both inner micro and mesopores is one order of magnitude smaller. Furthermore, the structure is not hierarchical. The BET surface area of the samples is reported in Table 2.
From Table 2, it results that the surface area of AC2 is higher than the other activated carbons, also in accordance with the higher mesoporosity, while the surface area of AC1 is similar to that of Pt/C samples.
To evaluate the homogeneity of the cathodes prepared with the different carbons, all the samples were visualized under the scanning electron microscope. In Fig. 4, the SEM images of the cathodes prepared with the sampled carbonaceous materials are reported.
The cathode prepared with the Pt/C powders has a microscopic structure consisting of three-dimensional pieces of carbon on which platinum nanoparticles appear well dispersed. The typical dimensions of the pieces of carbon are of the order of tens of microns. Incorporated into the cathode structure, these carbon pieces form very wide channels for air entry.
AC1-cathode has a structure with smaller and more numerous channels and it is characterized by the presence of micrometric splinters of different sizes, typical of the woody origin of these carbons [35].
MWCNTs-cathode has a very closed structure as the nanotubes tend to aggregate when mixed in the solvent used for the preparation of the cathodes.
AC2-cathode has a mixed structure of splinters or flakes and three-dimensional pieces. Overall it appears denser than that prepared with AC1.
The EDX analysis, effected for the search for impurities, showed that only the cathodes prepared with AC2 have impurities, in particular phosphorus and sodium, in atomic concentrations lower than 1%.
Electrochemical characterization
The electrochemical equations governing an alkaline aluminum-air cell and the standard electrode potentials are
and the total reaction is:
In addition, a competing reaction occurs on aluminum anode causing self-corrosion
In saturated condition, Al(OH)4− precipitates and forms aluminum hydroxide on electrode surfaces and in the electrolyte. The parasitic reaction 5) determines, in addition to the production of H2, also that of Al(OH)3, as in reaction 4). Namely, both the electrochemical and parasitic reactions contribute to the failure of the cell caused by the oxide/hydroxide coating on anode or by the electrolyte clogging due to formed solid products. The more efficient the electrochemical reaction 4) is, the more reduced the parasitic reaction 5) is, and therefore, it is reduced the production of oxides released by the parasitic reaction, that alter the cell functioning and contribute to the decrease of its capacity. For this reason, it becomes essential to couple efficient air cathodes to aluminum anodes. An air cathode is efficient when it has the right physical structure, to effectively diffuse the air oxygen and to accumulate the reaction products without or at least with little obstruction, an appreciable electrical conductivity and a good catalytic activity.
The catalytic activity may be evaluated through polarization studies effected by linear sweep voltammetry (LSV). The LSV is a polarization technique that gives indications on the activity of a cathode for oxygen reactions as a whole, but it does not decouple the two main processes, namely intrinsic activity and mass transfer capability, that must be equally considered as determining factors affecting the electrocatalytic performance of ORR catalysts [36]. To decouple the processes and obtain kinetic information from LSV curves, rotating disk electrode setup apparatus and kinetic models based on the Koutecky–Levich (K–L) equation should be used [37, 38]. On the other hand, a three-electrode setup of polarization measurements let to compare the ORR performance of different cathodes, contacted with solid electrolytes, in more practical conditions [39]. Anyway, LSV is generally acknowledged as simple and effective method for qualitatively assessing the activity of a cathode for oxygen reactions. The typical LSV curve is S-shaped and different slopes of the “S” show how the reaction is controlled on an electrode by applying a given potential. In the case of the ORR for example, at potential very close to the OCV, the rate of the reaction is quite slow and the current density increases slightly as the potential decreases. When an onset in the potential (Eonset) is reached, the reaction accelerates as the potential decrease at lower values, resulting in a remarkable increase of the current density. The curves show an inflection at certain potential values (half waves potential or E1/2). By further decreasing the voltage, the current tends to plateau values. The two parameters, Eonset and E1/2, are usually used to qualitatively verify the activities of catalysts for ORR. The more positive these potential values are, the more active the material is for ORR. The LSV curves of different cathodes contacted with solid alkaline hydrogel are reported in Fig. 5. All the curves in Fig. 5 clearly show the typical ORR polarization curve trend [ Goel P, Dobhal D, Sharma RC (2020) Aluminum–air batteries: A viability review. J Energy Storage 28:101287. https://doi.org/10.1016/j.est.2020.101287 Zhou W, Zhang H, Nie H, Ma Y, Zhang Y, Zhang H (2015) Hierarchical micron-sized mesoporous/macroporous graphene with well-tuned surface oxygen chemistry for high capacity and cycling stability Li–O2 battery. ACS Appl Mater Interfaces 7(5):3389–3397. https://doi.org/10.1021/am508513m **ao Y, Dai A, Hu C, Lin Y, Connell JW, Dai L (2018) Carbon-Based, Metal-Free Catalysts for Metal–Air Batteries. In: Dai L (ed) Carbon-Based Metal-Free Catalysts: Design and Applications, First Edition. Wiley-VCH Verlag GmbH & Co, KGaA, 2, pp 555–596 Li L, Yu D, Li P, Huang H, **e D, Lin CC, Hu F, Chen HY, Peng S (2021) Interfacial electronic coupling of ultrathin transition-metal hydroxide nanosheets with layered MXenes as a new prototype for platinum-like hydrogen evolution. Energy Environ Sci 14(12):6419–6427. https://doi.org/10.1039/D1EE02538D Deng L, Hu F, Ma M, Huang SC, **ong Y, Chen HY, Li L, Peng S (2021) Electronic modulation caused by interfacial Ni-O-M (M = Ru, Ir, Pd) bonding for accelerating hydrogen evolution kinetics. Angew Chem 133:22450–22456. https://doi.org/10.1002/ange.202110374 Huang H, Yu D, Hu F, Huang SC, Song J, Chen HY, Li LL, Peng S (2022) Clusters Induced Electron Redistribution to Tune Oxygen Reduction Activity of Transition Metal Single-Atom for Metal-Air Batteries. Angew Chem 134(12):e202116068. https://doi.org/10.1002/ange.202116068 Hu F, Yu D, Ye M, Wang H, Hao Y, Wang L, Li L, Han X, Peng S (2022) Lattice-Matching Formed Mesoporous Transition Metal Oxide Heterostructures Advance Water Splitting by Active Fe–O–Cu Bridges. Adv Energy Mater 12(19):2200067. https://doi.org/10.1002/aenm.202200067 Song J, Qiu S, Hu F, Ding Y, Han S, Li L, Chen HY, Han X, Sun C, Peng S (2021) Sub-2 nm thiophosphate nanosheets with heteroatom do** for enhanced oxygen electrocatalysis. Adv Funct Mater 31(19):2100618. https://doi.org/10.1002/adfm.202100618 Fang Z, Li L, Dixon DA, Fushimi RR, Dufek EJ (2021) Nature of Oxygen Adsorption on Defective Carbonaceous Materials. J Phys Chem C 125(37):20686–20696. https://doi.org/10.1021/acs.jpcc.1c06741 Huang S, Fan W, Guo X, Meng F, Liu X (2014) Positive role of surface defects on carbon nanotube cathodes in overpotential and capacity retention of rechargeable lithium–oxygen batteries. ACS Appl Mater Interfaces 6(23):21567–21575. https://doi.org/10.1021/am506564n Gui F, ** Q, **ao D, Xu X, Tan Q, Yang D, Li B, Ming P, Zhang C, Chen Z, Siahrostami S, **ao Q (2022) High-Performance Zinc-Air Batteries Based on Bifunctional Hierarchically Porous Nitrogen-Doped Carbon. Small 18(8):2105928. https://doi.org/10.1002/smll.202105928 Lin H, Liu Z, Mao Y, Liu X, Fang Y, Liu Y, Wang D, **e J (2016) Effect of nitrogen-doped carbon/Ketjenblack composite on the morphology of Li2O2 for high-energy-density Li–air batteries. Carbon 96:965–971. https://doi.org/10.1016/j.carbon.2015.10.057 Xu Y, Zhang Y, Guo Z, Ren J, Wang Y, Peng H (2015) Flexible, stretchable, and rechargeable fiber-shaped zinc–air battery based on cross-stacked carbon nanotube sheets. Angew Chem 127(51):15610–15614. https://doi.org/10.1002/ange.201508848 Wang M, Lai Y, Fang J, Li J, Qin F, Zhang K, Lu H (2015) N-doped porous carbon derived from biomass as an advanced electrocatalyst for aqueous aluminium/air battery. Int J Hydrog Energy 40(46):16230–16237. https://doi.org/10.1016/j.ijhydene.2015.09.054 Gaele MF, Di Palma TM (2022) Polymer Electrolytes for Al-Air Batteries: Current State and Future Perspectives. Energy Fuels 36:12875–12895. https://doi.org/10.1021/acs.energyfuels.2c02453 Lu J, Jaumaux P, Wang T, Wang C, Wang G (2021) Recent Progress in Quasi-Solid and Solid Polymer Electrolytes for Multivalent Metal Ion Batteries. J Mater Chem A 9:24175–24194. https://doi.org/10.1039/D1TA06606D Singh R, Rhee HW (2019) The rise of bio-inspired energy devices. Energy Storage Mater 23:390–408. https://doi.org/10.1016/j.ensm.2019.04.030 Torres FG, De-la-Torre GE, Gonzales KN, Troncoso OP (2020) Bacterial-polymer-based electrolytes: recent progress and applications. ACS Appl Energy Mater 3(12):11500–11515. https://doi.org/10.1021/acsaem.0c02195 Gaele MF, Migliardini F, Di Palma TM (2021) Dual solid electrolytes for aluminium-air batteries based on polyvinyl alcohol acidic membranes and neutral hydrogels. J Solid State Electrochem 25:1207–1216. https://doi.org/10.1007/s10008-021-04900-6 Gaele MF, Di Palma TM (2022) Rechargeable Aluminum-Air Batteries Based on Aqueous Solid-State Electrolytes. Energy Technol 10(4):2101046. https://doi.org/10.1002/ente.202101046 Di Palma TM, Migliardini F, Caputo D, Corbo P (2017) Xanthan and κ-carrageenan based alkaline hydrogels as electrolytes for Al/air batteries. Carbohydr Polym 157:122–127. https://doi.org/10.1016/j.carbpol.2016.09.076 Di Palma TM, Migliardini F, Gaele MF, Corbo P (2019) Physically cross-linked xanthan hydrogels as solid electrolytes for Al/air batteries. Ionics 25(9):4209–4217. https://doi.org/10.1007/s11581-019-02965-y Migliardini F, Di Palma TM, Gaele MF, Corbo P (2018) Solid and acid electrolytes for Al-air batteries based on xanthan-HCl hydrogels. J Solid State Electrochem 22:2901–2916. https://doi.org/10.1007/s10008-018-4003-2 Di Palma TM, Migliardini F, Gaele MF, Corbo P (2021) Aluminum-air Batteries with solid hydrogel electrolytes: Effect of pH upon cell performance. Anal Lett 54(1–2):28–39. https://doi.org/10.1080/00032719.2019.1708923 Neimark AV, Lin Y, Ravikovitch PI, Thommes M (2009) Quenched solid density functional theory and pore size analysis of micro-mesoporous carbons. Carbon 47(7):1617–1628. https://doi.org/10.1016/j.carbon.2009.01.050 Sadezky A, Muckenhuber H, Grothe H, Niessner R, Pöschl U (2005) Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 43(8):1731–1742. https://doi.org/10.1016/j.carbon.2005.02.018 Cançado LG, Takai K, Enoki T, Endo M, Kim YA, Mizusaki H, Jorio A, Coelho LN, Magalhães-Paniago R, Pimenta MA (2006) General equation for the determination of the crystallite size L a of nanographite by Raman spectroscopy. Appl Phys Lett 88(16):163106. https://doi.org/10.1063/1.2196057 Araujo PT, Terrones M, Dresselhaus MS (2012) Defects and impurities in graphene-like materials. Mater Today 15(3):98–109. https://doi.org/10.1016/S1369-7021(12)70045-7 Bokobza L, Bruneel JL, Couzi M (2015) Raman spectra of carbon-based materials (from graphite to carbon black) and of some silicone composites. C 1(1):77–94. https://doi.org/10.3390/c1010077 Kinoshita K (1988) Carbon: electrochemical and physicochemical properties. United States Kwiecinska B, Suarez-Ruiz I, Paluszkiewicz C, Rodriques S (2010) Raman spectroscopy of selected carbonaceous samples. Int J Coal Geol 84(3–4):206–212. https://doi.org/10.1016/j.coal.2010.08.010 Cychosz KA, Guillet-Nicolas R, García-Martínez J, Thommes M (2017) Recent advances in the textural characterization of hierarchically structured nanoporous materials. Chem Soc Rev 46(2):389–414. https://doi.org/10.1039/C6CS00391E Yang QH, Hou PX, Bai S, Wang MZ, Cheng HM (2001) Adsorption and capillarity of nitrogen in aggregated multi-walled carbon nanotubes. Chem Phys Lett 345(1–2):18–24. https://doi.org/10.1016/S0009-2614(01)00848-X Zhai Y, Dou Y, Zhao D, Fulvio PF, Mayes RT, Dai S (2011) Carbon materials for chemical capacitive energy storage. Adv Mater 23(42):4828–4850. https://doi.org/10.1002/adma.201100984 Peng X, Zhang L, Chen Z, Zhong L, Zhao D, Chi X, Zhao X, Li L, Lu X, Leng K, Liu C, Liu W, Tang W, Loh KP (2019) Hierarchically porous carbon plates derived from wood as bifunctional ORR/OER electrodes. Adv Mater 31(16):1900341. https://doi.org/10.1002/adma.201900341 Wang HF, Tang C, Zhang Q (2018) A review of precious-metal-free bifunctional oxygen electrocatalysts: rational design and applications in Zn− air batteries. Adv Funct Mater 28(46):1803329. https://doi.org/10.1002/adfm.201803329 Wang J, Zhao CX, Liu JN, Ren D, Li BQ, Huang JQ, Zhang Q (2021) Quantitative kinetic analysis on oxygen reduction reaction: A perspective. Nano Mater Sci 3(3):313–318. https://doi.org/10.1016/j.nanoms.2021.03.006 Daems N, Breugelmans T, Vankelecom IF, Pescarmona PP (2018) Influence of the composition and preparation of the rotating disk electrode on the performance of mesoporous electrocatalysts in the alkaline oxygen reduction reaction. ChemElectroChem 5(1):119–128. https://doi.org/10.1002/celc.201700907 Sun H, Li Q, Lian Y, Zhang C, Qi P, Mu Q, ** H, Zhang B, Chen M, Deng Z, Peng Y (2020) Highly efficient water splitting driven by zinc-air batteries with a single catalyst incorporating rich active species. Appl Catal B Environ 263:118139. https://doi.org/10.1016/j.apcatb.2019.118139 **a W, Mahmood A, Liang Z, Zou R, Guo S (2016) Earth-abundant nanomaterials for oxygen reduction. Angew Chem Int Ed 55(8):2650–2676. https://doi.org/10.1002/anie.201504830 Trasatti S (2009) ELECTROCHEMICAL THEORY | Electrokinetics. In: J Garche (ed) Encyclopedia of Electrochemical Power Sources, Elsevier, pp 23–31. https://doi.org/10.1016/B978-044452745-5.00021-6 Paul S (2016) Materials and electrochemistry: present and future battery. J Electrochem Sci Technol 7(2):115–131. https://doi.org/10.5229/JECST.2016.7.2.115 Stuve EM (2014) Overpotentials in Electrochemical Cells. In: Kreysa G, Ota Ki, Savinell, RF (eds) Encyclopedia of Applied Electrochemistry, Springer, New York, pp 1445–1453. https://doi.org/10.1007/978-1-4419-6996-5_330 Wang Q, Miao H, Xue Y, Sun S, Li S, Liu Z (2017) Performances of an Al–0.15 Bi–0.15 Pb–0.035 Ga alloy as an anode for Al–air batteries in neutral and alkaline electrolytes. Rsc Adv 7(42):25838–25847. https://doi.org/10.1039/C7RA02918G Barsoukov E, Macdonald JR (2005) Impedance spectroscopy: theory, experiment, and applications, 2nd edn. Wiley, HobokenReferences
Funding
Open access funding provided by Consiglio Nazionale Delle Ricerche (CNR) within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gaele, M.F., Califano, V. & Di Palma, T.M. Efficient cathodes for quasi-solid-state aluminum-air batteries. Ionics 29, 1447–1458 (2023). https://doi.org/10.1007/s11581-023-04896-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-04896-1