Abstract
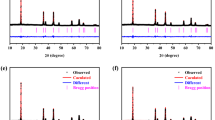
It is exactly the high potential and high energy density that makes the LiNi0.5Mn1.5O4 (LNMO) an attractive material for lithium-ion battery cathode. However, the poor cycle performance of LNMO caused by John-Teller effect during the Li+ ion insertion/desertion process has been a hindrance for its practical application. Herein, the influence of M-doped (M= Cr and Co) on structure, morphology, and electrochemical performances of LiMyNi0.5-yNi1.5O4 spinel materials are deeply investigated to improve the structural stability and cycling ability. The results reveal that the cell volume and of LiNi0.5Mn1.5O4 are decreased with Cr3+ and Co2+ do**; the stability of structure and electrical conductivity is correspondingly improved. The state density diagrams demonstrate that Cr3+ and Co2+ cationic do** have clearly enhanced the interaction between oxygen and transition metals (Ni and Mn) and improved the transition capacity of Li+. The initial discharge specific capacities of LiCo0.12Ni0.38Mn1.5O4 and LiCr0.12Ni0.38Mn1.5O4 samples are 113.3 mAh·g−1 and 107.7 mAh·g−1 respectively at a high rate of 0.5 C, which are higher than that of pure LiNi0.5Mn1.5O4. Additionally, the capacity retention rates of 87.2% and 63.97% come through respectively after 50 cycles while only 59.8% for pure LNMO. The rate of LiCo0.12Ni0.38Mn1.5O4 exhibits a better stability than LiCr0.12Ni0.38Mn1.5O4.






Similar content being viewed by others
References
Wang Y, Pu YJ, Ma ZS, Pan Y, Sun CQ (2016) Interfacial adhesion energy of lithium-ion battery electrodes. Extreme Mech Lett 9:226–236. https://doi.org/10.1016/j.eml.2016.08.002
Meng FB, Guo HJ, Wang ZX, Wang JX, Yan GC, Wu XW, Li XH, Zhou LJ (2019) The influences of SO42- from electrolytic manganese dioxide precursor on the electrochemical properties of Li-rich Mn-based material for Li-ion batteries. IONICS 25:2585–2594. https://doi.org/10.1007/s11581-018-2796-8
Jarvis KA, Deng ZQ, Allard LF, Manthiram A, Ferreira PJ (2011) Atomic structure of a lithium-rich layered oxide material for lithium-ion batteries: evidence of a solid solution Chem Mater 23:3614–3621. https://doi.org/10.1021/cm200831c
Wang CP, Ma ZS, Wang Y, Lu CS (2016) Failure prediction of high-capacity electrode materials in lithium-ion batteries. J Electrochem Soc 163:A1157–A1163. https://doi.org/10.1016/j.electacta.2016.07.039
Wei AJ, Li W, Chang Q, Bai X, He R, Zhang LH, Liu ZF, Wang YJ (2019) Effect of Mg2+/F- co-do** on electrochemical performance of LiNi0.5Mn1.5O4 for 5 V lithium-ion batteries. Electrochim Acta 323:134692. https://doi.org/10.1016/j.electacta.2019.134692
Chang LG, Cao SY, Luo SH, Zhang FS, Li K (2021) Study on synthesis of spinel LiNi0.5Mn1.5O4 cathode material and its electrochemical properties by two-stage roasting. Int J Energy Res 45:8932–8941. https://doi.org/10.1002/er.6426
Uzun D (2015) Boron-doped Li1.2Mn0.6Ni0.2O2 as a cathode active material for lithium-ion battery. Solid State Ionics 281:73–81. https://doi.org/10.1016/j.ssi.2015.09.008
Gao C, Liu HP, Bi SF, Li HL, Ma CS (2020) Investigation the improvement of high voltage spinel LiNi0.5Mn1.5O4 cathode material by anneal process for lithium-ion batteries. Green Energy Environ 6:114–123. https://doi.org/10.1016/j.gee.2020.03.001
Chen YY, Sun Y, Huang XJ (2016) Origin of the Ni/Mn ordering in high-voltage spinel LiNi0.5Mn1.5O4: the role of oxygen vacancies and cation do**. Comp Mater Sci 115:109–116. https://doi.org/10.1016/j.commatsci.2016.01.005
Rana J, Glatthaar S, Gesswein H, Sharma N, Binder JR, Chernikov R, Schumacher G, Banhart J (2014) Local structural changes in LiMn1.5Ni0.5O4 spinel cathode material for lithium-ion batteries. J Power Sources 255:439–449. https://doi.org/10.1016/j.jpowsour.2014.01.037
Kunduraci M, Al-Sharab JF, Amatucci GG (2007) High-power nanostructured LiMn2-xNixO4 high-voltage lithium-ion battery electrode materials: electrochemical impact of electronic conductivity and morphology. Amatucc Chem Mater 18:3585–3592. https://doi.org/10.1021/cm060729s
Wu W, Qin X, Guo JL, Wang JF, Yang HY, Wang L (2017) Influence of cerium do** on structure and electrochemical properties of LiNi(0.5)Mn(1.5)O4 cathode materials. Rare Earths 35:887–895. https://doi.org/10.1016/S1002-0721(17)60991-8
Sanad MMS, Abdellatif HA, Elnaggar EM, El‑Kady GM, Rashad MM (2019) Understanding structural, optical, magnetic and electrical performances of Fe- or Co-substituted spinel LiMn1.5Ni0.5O4 cathode materials. Appl Phys.A: Mater Sci Process 125:139. https://doi.org/10.1007/s00339-019-2445-8
Kiziltas-Yavuz N, Bhaskar A, Dixon D, Yavuz M, Nikolowski K, Lu L, Eichel R, Ehrenberg H (2014) Improving the rate capability of high voltage lithium-ion battery cathode material LiNi0.5Mn1.5O4 by ruthenium do**. J Power Sources 267:533–541. https://doi.org/10.1016/j.jpowsour.2014.05.110
Liu WJ, Shi Q, Qu QT, Gao T, Zhu GB, Shao J, Zheng HH (2017) Improved Li-ion diffusion and stability of LiNi0.5Mn1.5O4 cathode through in-situ co-do** of dual-metal cations and incorporation of superionic conductor. J Mater Chem A 5:145–154. https://doi.org/10.1039/c6ta08891k
Kiziltas-Yavuz N, Yavuz M, Indris S, Bramnik NN, Knapp M, Dolotko O, Das B, Ehrenberg H, Bhaskar A (2016) Enhancement of electrochemical performance by simultaneous substitution of Ni and Mn with Fe in Ni-Mn spinel cathodes for Li-ion batteries. J Power Sources 327:507–518. https://doi.org/10.1016/j.jpowsour.2016.07.047
Li J, Li SF, Xu SJ, Huang S, Zhu JX (2017) Synthesis and electrochemical properties of LiNi0.5Mn1.5O4 cathode materials with Cr3+ and F- composite do** for lithium-ion batteries. Nanoscale Res Lett 12:414. https://doi.org/10.1186/s11671-017-2172-z
Guo LF, **e YL (2022) Na-doped LiNi1/3Co1/3Mn1/3O2 with enhanced rate performance as a cathode for Li-ion batteries. IONICS 28:2117–2123. https://doi.org/10.1007/s11581-022-04515-5
Mao J, Dai KH, Xuan MJ, Shao GS, Qiao RM, Yang WL, Battaglia VS, Liu G (2016) Effect of chromium and niobium do** on the morphology and electrochemical performance of high-voltage spinel LiNi0.5Mn1.5O4 cathode material. ACS Appl Mater Interfaces 8:9116–9124. https://doi.org/10.1021/acsami.6b00877
Zhang SM, Tang T, Ma ZH, Gu HT, Du WB, Gao MX, Liu YF, Jian DC, Pan H (2018) Tuning Li2MO3 phase abundance and suppressing migration of transition metal ions to improve the overall performance of Li- and Mn-rich layered oxide cathode. J Power Sources 380:1–11. https://doi.org/10.1016/j.jpowsour.2018.01.045
Gajraj V, Azmi R, Darma MSD, Indris S, Ehrenberg H, Mariappan CR (2021) Correlation between structural, electrical and electrochemical performance of Zn doped high voltage spinel LiNi0.5-xZnxMn1.5O4 porous microspheres as a cathode material for Li-Ion batteries. Ceram Int 47:35275–35286. https://doi.org/10.1016/j.ceramint.2021.09.070
Liu MH, Huang HT, Lin CM, Chen JM, Liao SC (2014) Mg gradient-doped LiNi0.5Mn1.5O4 as the cathode material for Li-ion batteries. Electrochim Acta 120:133–139. https://doi.org/10.1016/j.electacta.2013.12.065
Chen MZ, Hu Z, Wu ZG, Hua WB, Ozawa K, Gu QF, Kang YM, Guo XD, Chou SL, Dou SX (2016) Understanding performance differences from various synthesis methods: a case study of spinel LiCr0.2Ni0.4Mn1.4O4 cathode material. ACS Appl. Mater. Interfaces 8:26051–26057. https://doi.org/10.1021/acsami.6b08327
Mao J, Ma MZ, Liu PP, Hu JH, Shao GS, Battaglia V, Dai KH, Liu G (2016) The effect of cobalt do** on the morphology and electrochemical performance of high-voltage spinel LiNi0.5Mn1.5O4 cathode material. Solid State Ionics 292:70–74. https://doi.org/10.1016/j.ssi.2016.05.008
Liu SS, Zhao HY, Tan M, Hu YZ, Shu XH, Zhang ML, Chen B, Liu XQ (2017) Er-doped LiNi0.5Mn1.5O4 cathode material with enhanced cycling stability for lithium-ion batteries. Mater 10:859. https://doi.org/10.3390/ma10080859
Arunkumar TA, Manthiram A (2005) Influence of chromium do** on the electrochemical performance of the 5V spinel cathode LiMn1.5Ni0.5O4. Electrochim Acta 50:5568–5572. https://doi.org/10.1016/j.electacta.2005.03.033
Nie X, Zhong BH, Chen MZ, Yin K, Li L, Liu H, Guo XD (2013) Synthesis of LiCr0.2Ni0.4Mn1.4O4 with superior electrochemical performance via a two-step thermo polymerization technique. Electrochim Acta 97:184–191. https://doi.org/10.1016/j.electacta.2013.01.124
Gong JJ, Yan SP, Lang YQ, Zhang Y, Fu SX, Guo JL, Wang L, Liang GC (2021) Effect of Cr3+ do** on morphology evolution and electrochemical performance of LiNi0.5Mn1.5O4 material for Li-ion battery. J Alloys Compd 859:157885. https://doi.org/10.1016/j.jallcom.2020.157885
Zhong GB, Wang YY, Yu YQ, Chen CH (2012) Electrochemical investigations of the LiNi0.45M0.10Mn1.45O4 (M = Fe Co, Cr) 5 V cathode materials for lithium ion batteries. J Power Sources 205:385–393. https://doi.org/10.1016/j.jpowsour.2011.12.037
Wang H, Li J, Yang S, Zhang B, ** on crystal structure and electrochemical performances of LiNi0.5Mn1.5O4. Mater Technol 30:A75–A78. https://doi.org/10.1179/17535557A15Y.000000008
Kim MC, Lee YW, Pham TK, Sohn JI, Park KW (2020) Chemical valence electron-engineered LiNi0.4Mn1.5MtO.4 (Mt = Co and Fe) cathode materials with high-performance electrochemical properties. Appl Surf Sci 504:144514. https://doi.org/10.1016/j.apsusc.2019.144514
Hu XH, Ai XP, Yang HX, Li SX (1998) A study of LiMn2O4 synthesized from Li2CO3 and MnCO2. J Power Sources 74:240–243. https://doi.org/10.1016/S0378-7753(98)00049-4
Berbenni V, Marini A (2003) Solid state synthesis of lithiated manganese oxides from mechanically activated Li2CO3-Mn3O4 mixtures. J Anal Appl Pyrolysis 70:437–456. https://doi.org/10.1016/S0165-2370(03)00003-2
Rim H, Park HR, Song MY (2013) Synthesis of LiNi0.9Co0.1O2 from Li2CO3, NiO or NiCO3, and CoCO3 or Co3O4 and their electrochemical properties. Ceram Int 39:7297–7303. https://doi.org/10.1016/j.ceramint.2013.02.068
Pasero D, Reeves N, Pralong V, West AR (2008) Oxygen nonstoichiometry and phase transitions in LiMn1.5Ni0.5O4−δ. J Electrochem Soc 155:A282–A291. https://doi.org/10.1149/1.2832650
Yang YX, Yang HL, Cao HB, Wang ZH, Liu CW, Sun Y, Zhao H, Zhang Y, Sun Z (2019) Direct preparation of efficient catalyst for oxygen evolution reaction and high-purity Li2CO3 from spent LiNi0.5Mn03Co02O2 batteries. J Cleaner Prod 236:117576. https://doi.org/10.1016/j.jclepro.2019.07.051.
Tang YQ, Chen SJ (2021) Enhanced electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 for lithium-ion batteries co-modified by lanthanum and aluminum. IONICS 27:935–948. https://doi.org/10.1007/s11581-020-03888-9
Huang ZD, Zhang K, Zhang TT, Liu RQ, Lin XJ, Li Y, Masese T, Liu XM, **M Feng, Ma YW (2016) High rate and thermally stable Mn-rich concentration-gradient layered oxide microsphere cathodes for lithium-ion batteries. Energy Storage Mater 5:205–213. https://doi.org/10.1016/j.ensm.2016.08.001
Michael M, Thackeray, (1997) Manganese oxides for lithium batteries. Prog Solid State Chem 25:1–71. https://doi.org/10.1016/S0079-6786(97)81003-5
Liu YH, Tsai TY (2021) Improving electrochemical performance of lithium ion batteries using a binder-free carbon fiber-based LiNi0.5(1-x)Mn1.5(1-x/3)CrxO4 cathode with a conventional electrolyte. J Power Sources 484:229262. https://doi.org/10.1016/j.jpowsour.2020.229262.
Chladil L, Kunický D, Kazda T, Vanýsek P, Cech O, Baca P (2021) In-situ XRD study of a chromium doped LiNi0.5Mn1.5O4 cathode for Li-ion battery. J Energy Storage 41:102907. https://doi.org/10.1016/j.est.2021.102907
Biesinger MC, Payne BP, Grosvenor AP, Lau LWM, Gerson AR, Smart RS (2011) Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl Surf Sci 257:2717–2730. https://doi.org/10.1016/j.apsusc.2010.10.051
Guo J, Li YJ, Chen YX, Deng SY, Zhu J, Wang SL, Zhang JP, Chang SH, Zhang DW, ** XM (2019) Stable interface Co3O4-coated LiNi0.5Mn1.5O4 for lithium-ion batteries. J Alloys Compd 811:152031. https://doi.org/10.1016/j.jallcom.2019.152031
Zhang PP, Wang Y, Lei WX, Zou YL, Jiang WJ, Ma ZS, Lu CH (2019) Enhancement effects of Co do** on interfacial properties of Sn electrode-collector: a first-principles study. ACS Appl Mater Inter 11:24648–24658. https://doi.org/10.1021/acsami.9b01418
**ao J, Chen XL, Sushko PV, Sushko ML, Kovarik L, Feng JJ, Deng ZQ, Zheng JM, Graff GL, Nie ZM, Choi DW, Liu J, Zhang JG, Whittingharm MS (2012) High-performance LiNi0.5Mn1.5O4 spinel controlled by Mn3+ concentration and site disorder. Adv Mater 24:2109–2116. https://doi.org/10.1002/adma.201104767
He T, Chen L, Su YF, Lu Y, Bao LY, Chen G, Zhang QY, Chen S, Wu F (2019) The effects of alkali metal ions with different ionic radii substituting in Li sites on the electrochemical properties of Ni-rich cathode materials. J Power Sources 441:227195. https://doi.org/10.1016/j.jpowsour.2019.227195
Yang H, Savory CN, Morgan BJ, Scanlon DO, Skelton JM, Walsh A (2020) Chemical trends in the lattice thermal conductivity of Li(Ni, Mn, Co)O2 (NMC) Battery Cathodes. Chem Mater 32:7542–7550. https://doi.org/10.1021/acs.chemmater.0c02908
Fei L, Ma JN, Lin JY, Zhang XH, Yu H, Yang GC (2020) Exploring the origin of electrochemical performance of Cr-doped LiNi0.5Mn1.5O4. Phys Chem Chem Phys 22:3831–3838. https://doi.org/10.1039/c9cp06545h
Luo Y, Lu TL, Zhang YX, Yan LQ, Mao SS, ** with improved high-rate cyclability. J Alloys Compd 703:289–297. https://doi.org/10.1016/j.jallcom.2017.01.248
Zhong GB, Wang YY, Zhao XJ, Wang QS, Yu Y, Chen CH (2012) Structural, electrochemical and thermal stability investigations on LiNi0.5-xAl2xMn1.5-xO4 (0<=2x<=1.0) as 5 V cathode materials. J Power Sources 216:368–375. https://doi.org/10.1016/j.jpowsour.2012.05.108
Chu CT, Mondal A, Kosova NV, Lin JY (2020) Improved high-temperature cycliability of AlF3 modified spinel LiNi0.5Mn1.5O4 cathode for lithium-ion batteries. Appl Surf Sci 530:147169. https://doi.org/10.1016/j.apsusc.2020.147169
Qiu XY, Zhuang QC, Zhang QQ, Cao R, Qiang YH, Ying PZ, Sun SG (2013) Reprint of ‘“Investigation of layered LiNi1/3Co1/3Mn1/3O2 cathode of lithium-ion battery by electrochemical impedance spectroscopy.”’ J Electroanal Chem 688:393–402. https://doi.org/10.1016/j.jelechem.2013.02.009
** GC, Jiao X, Peng QM, Zeng TB (2021) Anchored CoCO3 on peeled graphite sheets toward high-capacity lithium-ion battery anode. J Mater Sci 56:10510–10522. https://doi.org/10.1007/s10853-021-05933-y
Funding
This study is financially supported by the Key R&D Plan of Shaanxi Province-General Industrial Project (No.2022GY-160), the National Natural Science Foundation of China (No.51504181), the Nature Science Foundational of Shaanxi Province (No. 2020JQ-679), Key Laboratory Project of Education Department of Shaanxi Province (No.20JS064), the National Innovation and Entrepreneurship Training program for college students in 2021, and the third high-level innovative and entrepreneurial talents (team) project of Sanmenxia city (No. 2020709).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, J., Cui, Y., Li, Q. et al. Cr3+ and Co2+ do** modification on electrochemical performance of LiNi0.5Mn1.5O4 for Li-ion battery. Ionics 29, 973–982 (2023). https://doi.org/10.1007/s11581-023-04886-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-04886-3