Abstract
Potato dry rot is a global problem caused by Fusarium species. Symptoms of dry rot include wrinkled brown to black patches of tissue. Fusarium spp. infection in potato tubers results in the formation of mycotoxins. Fungi that cause dry rot are commonly Fusarium sambucinum and Fusarium solani. This article was made to determine the effect of the essential oils of the Beta vulagaris plant on Fusarium species, which is the causative agent of dry rot in potatoes. In the study, Beta vulgaris plant essential oil content was determined by Gas Chromatography (GC–MC). In addition, the amount of deoxynivalenol (DON) mycotoxin produced by Fusarium species was determined by enzyme-linked immunosorbent assay (ELISA) analyses. Inhibition rates of these essential oils (1, 5, 10, 20, 50, 100, 250, 500, 1000 µl) on Fusarium sambicunum (1.3, 3.0, 5.2, 7.3, 9.1, 12.7, 22.3, 27.1, 29.1%) were found. Similarly, inhibition rates on Fusarium solani (1.1, 2.8, 4.3, 6.7, 8.8, 10.5, 19.4, 24.7, 27.3%) were found. In addition, the results showed that the amount of deoxynivalenol DON in 25 potato tubers ranged from 44.1–172.6 ppb. Infections of potato tubers caused by certain Fusarium species are typically accompanied by mycotoxin production, thus posing a potential risk to human health and food safety. In this study, it was determined that the essential oils of Beta vulgaris plant were effective against Fusarium spp., which are the cause of dry rot disease that may occur in potatoes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potato dry rot disease is frequently observed in potatoes stored in humid conditions. It is known that dry rot disease is an important disease in potato tubers and has large negative economic value in the world (Tiwari et al. 2020). Pathogens causing dry rot have been reported in many studies. These pathogens can infect tubers, starting from injured tissues or rotten potato pieces. If the development of pathogens is not stopped, they begin to develop in tubers and cause dry rot at the injury points. Symptoms of dry rot disease on tubers appear as white, pink, blue-dark, crushed, wrinkled spots. Potato dry rot is characterized as a light to dark brown or black internal rot on potatoes.
Potato dry rot is an important fungal disease usually caused by Fusarium species (Khedher et al. 2021). Fusarium species are in the Ascomycota group and many species are generally found in soil and plants (Summerell et al. 2010). Fusarium sambucinum and Fusarium solani are pathogenic species associated with potato tuber dry rot (Zaker 2014; Mohammadi et al. 2020). They cause contamination in many food and feed products. In particular, toxic metabolites produced by these species show carcinogenic, mutagenic and teratogenic effects on the health of humans and animals (Bojanowski et al. 2013; Haque et al. 2020). Deoxynivalenol (DON) is an important Fusarium trichothecene mycotoxin commonly detected in foods and feeds (Luongo et al. 2010; Meybodi et al. 2011; Tian et al. 2022). DON is rapidly absorbed by cells and widely distributed in many organs. Animals and humans are highly sensitive to DON and detoxification is minimal. Generally, toxicological studies provide important information for the prevention and control of DON contamination (Sun et al. 2022) (Fig. 1).
Beta vulgaris is a member of the Amaranthaceae family. The leaves of this plant contain red and purple colored betacyanins (Kaur and Kapoor 2002; Olvera et al. 2008; El-Ghffar et al. 2019). Researchers have observed that betavulgarin and antifungal proteins (intercellular washing fluid, IWF) isolated from the leaves of this plant have stronger antifungal activity than well-known isoflavones such as genistein, biochanin A, and formononetin (Nielsen et al. 1996). Researchers have found that the leaves and root bark of the plant produce phenolic, flavonoid and protein-based molecules that are antifungal against fungi. In addition, these compounds and proteins associated with pathogenesis have been classified (Thabti et al. 2014).
This study was carried out to determine the fungal species that cause dry rot in potatoes and the mycotoxins produced by these species, as well as the effectiveness of different doses of B. vulgaris essential oils on these fungi.
Materials and Methods
Preparation of Plant Essential Oils
Beta vulgaris used in the study were collected from Sivas city of Turkey. Samples were brought to the laboratory on the same day. Samples were dried for one week at room temperature. Essential oils were essential oiled from the chopped leaves (100 g) by hydro-distillation using the Clevenger instrument. Essential oilion was carried out in 1 L of distilled water for 5 h. The resulting essential oil was stored at 4 °C until next use. Different concentrations of plant essential oils were prepared to be used in the experiments.
Gas Chromatography (GC–MC) Analyses
Samples were analyzed by Gas Chromatography (Shimadzu GC 17). For sample preparation, 5 g of plant leaf material was homogenized in diethyl ether 1:100 w/v (Merck). Chromatography was performed with a capillary column (25 m long and 0.25 mm in diameter, Permabound 25, Machery-Nagel, Germany) using helium (flow rate 0.8 ml/min) as carrier gas. The injection block and detector temperature were set to 250 °C. Standard and sample mixtures were analyzed under the same conditions.
Enzyme-Linked Immunosorbent Assay (ELISA) Analyses
Twenty-five potato tubers with dry rot disease were collected for the study. Five gram was taken from the diseased parts. 10 ml of dH2O was added to the samples. The tubes were centrifuged at 4000 rpm for 5 min (Fig. 2). The supernatant was transferred to a new tube and re-centrifuged. Afterwards, the ready samples were used in ELISA analysis. An ELISA test kit (MBS283277 96-Strip-Wells) was used in this study. In this test, the anti-DON antibody on the microplate binds to the mycotoxins in the samples and creates a blue color. Then the microplate was read with an ELISA reader and the amount of mycotoxin was determined (Wang et al. 2016).
The ELISA assay kit contained 96 wells. The kit consisted of standard DON solution (0 ppb, 3 ppb, 9 ppb, 27 ppb, 81 ppb, 243 ppb), redissolving solution, antibody, HRP-Conjugate, wash buffer, substrate A, Substrate B and stop solution. It can be stored for up to 6 months at 2–8 °C. First, 50 µl of antibody solution was placed in the wells at room temperature. Then, 50 µl of samples or DON standards was added. Micropilate was mixed gently and incubated at 37 °C for 30 min. The liquid was poured and washed 3 times with 250 µl wash buffer (15–20 s). One hundred microlitres of HRP-Conjugate was added to each well and incubated at 37 °C for 30 min. The liquid was poured and washed 3 times with 250 µl wash buffer (15–20 s). Substrate A and B solutions were added to each well and incubated at 37 °C for 15 min. Then, 50 µl of stop solution was added to each well and color changes were observed. Microplatin was measured with an ELISA reader at 450 nm.
Quantitative Determination
Microplate was measured with an ELISA reader at 450 nm. The absorbance of the test solution was measured according to the optical density, and the absorbance of the standard solution was also measured according to the optical density, and the percent absorbance value was calculated according to the following formula. The mean relative absorbance values were obtained from the average optical density value (B) of the sample and from the average optical density value of the standard solution (B0 standard) by dividing B by B0 and subsequently multiplying by 100%, that is: percentage of absorbance value = (B/B0) × 100%. Using the professional software of the ELISA kit, accurate and fast analysis of a large number of samples has been made was possible.
Preparation of Fusarium Species
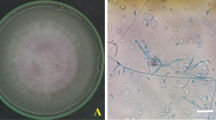
Potato tubers were thoroughly washed with water and surface sterilized with 1% sodium hypochlorite for 1–2 min. Diseased tissue was cultured on potato dextrose agar containing ampicillin. All fungi were identified using morphological features of colonies and conidia, including morphological structures (Leslie and Summerell 2008) (Fig. 3).
Antifungal Effect Analyses
Nine different doses of plant leaf essential oil (1 µl, 5 µl, 10 µl, 20 µl, 50 µl, 100 µl, 250 µl, 500 µl, 1000 µl) were added to sterilized potato agar medium (PDA). PDA medium was shaken well and was poured into petri dishes. Then, disc-shaped fungi were added to the center of the petri dishes. The prepared samples and control groups were incubated at 25 °C. The diameters of fungal colonies were continuously measured to assess inhibition rates. The inhibition percentages were calculated according to the formula below (Deans and Svoboda 1990). Differences between doses in essential oil treatments were determined using ANOVA statistical test.
- I:
-
% inhibition rate
- C:
-
the diameters of control colonies
- P:
-
the diameters of colonies in petri dishes containing essential oil
Results
GC–MC Analysis Results
As a result of the study, 17 chemical contents were determined in the essential oil of B. vulgaris plant. The total determined essential oil content was 78.4%. These oils were 1,3-dimethylbenzene, α-pinene, β-caryophyllene, neophytadiene, 1-ethyl-3-methyl benzene, 1-methyl-3-propylbenzene, 1-ethyl-3,5-dimethyl benzene, phytol, γ-irone, δ-cadinene, α-muurolene, tridecane, neophytadiene, α-bulnesene, γ-cadinene, α-cadinol, and spathulelol. In the essential oil content of B. vulgaris, 24.8% γ-irone major oil and 0.09% 1-methyl-3-propylbenzene minor oil were determined (Table 1).
ELISA Results
In the study, 25 potato tubers with dry rot disease were examined and DON mycotoxin produced by Fusarium was found. The results showed that the amount of DON in 25 potato tubers ranged from 44.1 to 172.6 ppb. The average DON amount in all potato tubers was determined as 79.58 ppb. Standard errors in all measurements were calculated between 0.6 and 2.2. The results determined by the ELISA kit are shown in the Table 2.
Antifungal Analysis Results
In this study, F. sambucinum and F. solani species were found to be sensitive to plant leaf essential oils. In applications which were performed with B. vulgaris essential oil (1, 5, 10, 20, 50, 100, 250, 500, 1000 µl), F. sambucinum inhibition rates were found to be 1.3, 3.0, 5.2, 7.3, 9.1, 12.7, 22.3, 27.1, 29.1%, respectively. Similarly, B. vulgaris essential oil (1, 5, 10, 20, 50, 100, 250, 500, 1000 µl), F. solani inhibition rates were found to be 1.1, 2.8, 4.3, 6.7, 8.8, 10.5, 19.4, 24.7, 27.3%, respectively. It was observed and statistically (p < 0.05) proven that antifungal effects increased as doses of plant essential oils increased (Fig. 4, Table 3). A one-way ANOVA on the inhibition of Fusarium species (F. sambucinum and F. solani) revealed a statistically significant difference between the nine groups. F. sambucinum (F(8, 1816) = [1163.85], p = [0.019]), F. solani (F(8, 1963) = [1614.91], p = [0.027]).
Discussion
Many microorganisms that harm plants, animals and human health develop resistance due to careless use of antibiotics. Therefore, researchers need to investigate plant components that are natural medicine sources. However, it is difficult to associate a particular chemical molecule with antifungal activities due to the complexity and variability of plant essential oils (Gibbons 2005). Fusarium species are the most important cause of dry rot infection in potato tubers. There are different disease agents within Fusarium spp. and their distributions indicate the importance of develo** management and control strategies for dry rot with rapid and precise identification techniques (Peters et al. 2008). In one study, 17 F. solani isolates were isolated. The results show a strong correlation between pathogenicity and DON produced by F. solani (cf. Almasoodi and Naser 2022). Similarly, F. sambucinum and F. solani strains isolated from various potato crops in Poland were noted to be the main cause of dry rot in potato tubers (Lenc et al. 2008). This has resulted in the growing importance of research aimed at finding natural methods to control plant pathogens.
In our study, nine different plant essential oil doses were used, and samples were compared with control plates. As a result, it was observed that there was a significant decrease in the growth diameters of fungi in petri dishes containing plant essential oils. It was observed that plant essential oils prevented the growth of F. sambucinum and F. solani. In particular, when the dose of plant leaf essential oils was increased, more inhibition was observed in petri dishes. However, the inhibition rates found in this study should be evaluated together with other studies. One of them is the use of essential oils. Thanks to their rich chemical composition, essential oils have many antimicrobial activities. The presence of Fusarium and its secondary metabolites DON in food products is a serious problem affecting food quality of agricultural produce. A study investigated the effects of plant essential oils on the growth of Fusarium species and the biosynthesis of mycotoxins in maize seeds. As a result of the study, it was reported that a significant decrease in DON concentrations was achieved in all plant essential oils (90.69–100%) applications. It has also been shown that essential oils have great potential to reduce the DON concentration in the seed as well as inhibit the growth of Fusarium species (Perczak et al. 2019). Essential oils from aromatic plants are natural products with great therapeutic potential and natural preservatives. One study investigated the chemical composition and antifungal effects of Origanum vulgare essential oils. The chemical analysis in this study was done by GC–MS techniques. In vitro antifungal activity was evaluated against Fusarium species isolated from dry rot of potato tubers by disc diffusion, agar dilution, and spore germination inhibition methods. As a result, it was stated that the essential oil exhibited great antifungal activity. It was also stated that essential oils inhibit the growth of spore germination effectively in all tested Fusarium species. The results of our study were supported by the above-mentioned studies, but there was no previous study on the antifungal properties of the essential oils of the B. vulgaris plant that was used in our study. In conclusion, plant essential oils have antifungal effects and reduce the production of DON mycotoxins, especially produced by Fusarium species.
Conclusion
Infections of potato tubers caused by certain Fusarium species are typically accompanied by mycotoxin production, thus posing a potential risk to human health and food safety. Ingestion of low to moderate amounts of Fusarium mycotoxins is common and does not usually result in obvious poisoning. However, these low amounts can impair intestinal health and immune function in humans and animals. Therefore, care should be taken not to have dry rot disease, especially of consumed potatoes. In this regard, it is necessary to control the potato in the processes from production to consumption.
References
Almasoodi IH, Naser NK (2022) Investigation of the correlation between the pathogenicity and toxins produced by Fusarium solani. In IOP Conf Ser: Earth Environ Sci 1060(1):012104. https://doi.org/10.1088/1755-1315/1060/1/012104
Bojanowski A, Avis TJ, Pelletier S, Tweddell RJ (2013) Management of potato dry rot. Postharvest Biol Technol 84:99–109. https://doi.org/10.1016/j.postharvbio.2013.04.008
Deans SG, Svoboda KP (1990) The antimicrobial properties of marjoram (Origanum majorana L.) volatile oil. Flavour Fragr J 5(3):187–190. https://doi.org/10.1002/ffj.2730050311
El-Ghffar EAA, Hegazi NM, Saad HH, Soliman MM, El-Raey MA et al (2019) HPLC-ESI-MS/MS analysis of beet (Beta vulgaris) leaves and its beneficial properties in type 1 diabetic rats. Biomed Pharmacother 120:109541. https://doi.org/10.1016/j.biopha.2019.109541
Gibbons S (2005) Plants as a source of bacterial resistance modulators and anti-infective agents. Phytochem Rev 4:63–78. https://doi.org/10.1007/s11101-005-2494-9
Haque MA, Wang Y, Shen Z, Li X, Saleemi MK et al (2020) Mycotoxin contamination and control strategy in human, domestic animal and poultry: a review. Microb Pathog 142:104095. https://doi.org/10.1016/j.micpath.2020.104095
Kaur C, Kapoor HC (2002) Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol 37(2):153–161. https://doi.org/10.1046/j.1365-2621.2002.00552.x
Khedher SB, Mejdoub-Trabelsi B, Tounsi S (2021) Biological potential of Bacillus subtilis V26 for the control of Fusarium wilt and tuber dry rot on potato caused by Fusarium species and the promotion of plant growth. Biol Control 152:104444. https://doi.org/10.1016/j.biocontrol.2020.104444
Lenc L, Łukanowski A, Sadowski C (2008) The use of PCR amplification in determining the toxigenic potential of F. sambucinum and F. solani isolated from potato tubers with symptoms of dry rot. Phytopathol Pol 48:13–23
Leslie JF, Summerell BA (2008) The Fusarium laboratory manual. John Wiley & Sons
Luongo D, Severino L, Bergamo P, D’arienzo R, Rossi M (2010) Trichothecenes NIV and DON modulate the maturation of murine dendritic cells. Toxicon 55(1):73–80. https://doi.org/10.1016/j.toxicon.2009.06.039
Meybodi DE, Torkamaneh D, Danaee M, Hashemi M (2011) The correlation of genetic diversity and geographic distribution of Fusarium graminearum in North part of Iran. Pak J Biol Sci 14(17):831–837. https://doi.org/10.3923/pjbs.2011.831.837
Mohammadi S, Taghizadeh Z, Alaie H, Mohamadi R (2020) In vitro investigation of antagonistic potential of Talaromyces flavus against Fusarium solani the causal agent of Fusarium dry rot of potato. Bioagrica 1(1):1–8
Nielsen KK, Nielsen JE, Madrid SM, Mikkelsen JD (1996) New antifungal proteins from sugar beet (Beta vulgaris L.) showing homology to non-specific lipid transfer proteins. Plant Mol Biol 31:539–552. https://doi.org/10.1007/BF00042227
Olvera HF, Smets E, Vrijdaghs A (2008) Floral and inflorescence morphology and ontogeny in Beta vulgaris, with special emphasis on the ovary position. Ann Bot 102(4):643–651. https://doi.org/10.1093/aob/mcn140
Perczak A, Gwiazdowska D, Gwiazdowski R, Juś K, Marchwińska K et al (2019) The inhibitory potential of selected essential oils on Fusarium spp. growth and mycotoxins biosynthesis in maize seeds. Pathogens 9(1):23. https://doi.org/10.3390/pathogens9010023
Peters JC, Lees AK, Cullen DW, Sullivan L, Stroud GP et al (2008) Characterization of Fusarium spp. responsible for causing dry rot of potato in Great Britain. Plant Pathology 57(2):262–271. https://doi.org/10.1111/j.1365-3059.2007.01777.x
Summerell BA, Laurence MH, Liew EC, Leslie JF (2010) Biogeography and phylogeography of Fusarium: a review. Fungal Diversity 44(1):3–13. https://doi.org/10.1007/s13225-010-0060-2
Sun Y, Jiang J, Mu P, Lin R, Wen J et al (2022) Toxicokinetics and metabolism of deoxynivalenol in animals and humans. Arch Toxicol 96(10):2639–2654. https://doi.org/10.1007/s00204-022-03337-8
Thabti I, Elfalleh W, Tlili N, Ziadi M, Campos MG et al (2014) Phenols, flavonoids, and antioxidant and antibacterial activity of leaves and stem bark of Morus species. Int J Food Prop 17(4):842–854. https://doi.org/10.1080/10942912.2012.660722
Tian Y, Zhang D, Cai P, Lin H, Ying H et al (2022) Elimination of Fusarium mycotoxin deoxynivalenol (DON) via microbial and enzymatic strategies: current status and future perspectives. Trends Food Sci Technol 124:96–107. https://doi.org/10.1016/j.tifs.2022.04.002
Tiwari RK, Kumar R, Sharma S, Sagar V, Aggarwal R et al (2020) Potato dry rot disease: current status, pathogenomics and management. 3 Biotech 10:1–18. https://doi.org/10.1007/s13205-020-02496-8
Wang XC, Bao M, Li FH, Fan HX et al (2016) Development of a sensitive, competitive, indirect ELISA for the detection of fumonisin B1 in corn originating from Anhui province, China. J Environ Sci Health 51(2):107–112. https://doi.org/10.1080/03601234.2015.1092829
Zaker M (2014) Antifungal evaluation of some plant essential oils in controlling Fusarium solani, the causal agent of potato dry rot in vitro and in vivo. Int J Agric Biosci 3(4):190–195
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zöngür, A. Efficacy of Beta vulgaris Essential Oils Against Potato Dry Rot Disease and Deoxynivalenol (DON) Mycotoxin. Potato Res. (2024). https://doi.org/10.1007/s11540-024-09742-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11540-024-09742-z