Abstract
The mechanical behavior of the white matter is important for estimating the damage of the spinal cord during accidents. In this study, we conducted uniaxial tension testing in vitro of bovine spinal cord white matter under extremely high strain rate conditions (up to 100 s−1). A visco-hyperelastic constitutive law for modeling the strain rate-dependent behavior of the bovine spinal cord white matter was developed. A set of material constants was obtained using a Levenberg–Marquardt fitting algorithm to match the uniaxial tension experimental data with various strain rates. Our experimental data confirmed that the modulus and tensile strength increased when the strain rate is higher. For the extremely high strain rate condition (100 s−1), we found that both the modulus and failure stress significantly increased compared with the low strain rate case. These new data in terms of mechanical response at high strain rate provide insight into the spine injury mechanism caused by high-speed impact. Moreover, the developed constitutive model will allow researchers to perform more realistic finite element modeling and simulation of spinal cord injury damage under various complicated conditions.
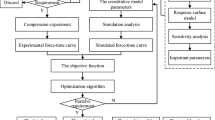
Graphical Abstract










Similar content being viewed by others
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- \(\lambda\) :
-
Stretch
- \({\varvec{\sigma}}\) :
-
Cauchy stress tensor
- \({{\varvec{\sigma}}}_{{\varvec{h}}}\) :
-
Cauchy stress tensor of hyperelastic unit
- \({{\varvec{\sigma}}}_{{\varvec{v}}}\) :
-
Cauchy stress tensor of viscoelastic unit
- \(W\) :
-
Strain energy potential function
- \({\varvec{F}}\) :
-
Deformation gradient tensor
- \({\varvec{X}}\) :
-
Initial position
- \({\varvec{x}}\) :
-
Current position
- \({u}_{i}\) :
-
The ith component of the displacement
- \({\varvec{C}}\) :
-
Right Cauchy–Green deformation tensor
- \({\varvec{E}}\) :
-
Green–Lagrangian strain tensor
- \({\varvec{I}}\) :
-
Identity matrix
- \({I}_{i}\) :
-
Invariants of \({\varvec{C}}\)
- \({\varvec{S}}\) :
-
The Second Piola–Kirchhoff stress tensor
- \({p}_{h}\) :
-
Hydrostatic pressure
- J :
-
Determinant of \({\varvec{C}}\)
- c ij :
-
Material constants
- \(\boldsymbol{\Omega }\) :
-
Tensor function describing the strain history effects on stress
- \({\sigma }_{ij}\) :
-
Components of \({\varvec{\sigma}}\)
- \(\tau\) :
-
Integral variable of time
- \(U\) :
-
Kernel function of deformation, time, and deformation rate
- \(m(t)\) :
-
Memory function
- \({m}_{i}\) :
-
Weight factors satisfying \({\sum }_{i=1}^{N}{m}_{i}=1\)
- \({\theta }_{i}\) :
-
Relaxation times
- \(\zeta\) :
-
History-dependent stretch
References
Sterba M, C-éric Aubin, Wagnac E, Fradet L, Arnoux P (2019) Effect of impact velocity and ligament mechanical properties on lumbar spine injuries in posterior-anterior impact loading conditions : a finite element study. Med Biol Eng Comput 57:1381
Zhang JG, Wang F, Zhou R, Xue Q (2011) A three-dimensional finite element model of the cervical spine: an investigation of whiplash injury. Med Biol Eng Comput 49:193–201. https://doi.org/10.1007/s11517-010-0708-9
Lin HM, Liu CL, Pan YN, Huang CH, Shih SL, Wei SH, Chen CS (2014) Biomechanical analysis and design of a dynamic spinal fixator using topology optimization: a finite element analysis. Med Biol Eng Comput 52:499–508. https://doi.org/10.1007/s11517-014-1154-x
Fradet L, Petit Y, Wagnac E, Aubin CE, Arnoux PJ (2014) Biomechanics of thoracolumbar junction vertebral fractures from various kinematic conditions. Med Biol Eng Comput 52:87–94. https://doi.org/10.1007/s11517-013-1124-8
Sokolis DP (2010) A passive strain-energy function for elastic and muscular arteries: correlation of material parameters with histological data. Med Biol Eng Comput 48:507–518. https://doi.org/10.1007/s11517-010-0598-x
Liu Q, Liu J, Guan F, Han X, Cao L, Shan K (2019) Identification of the visco-hyperelastic properties of brain white matter based on the combination of inverse method and experiment. Med Biol Eng Comput 57:1109–1120. https://doi.org/10.1007/s11517-018-1944-7
Ozawa H, Matsumoto T, Ohashi T, Sato M, Kokubun S (2001) Comparison of spinal cord gray matter and white matter softness: measurement by pipette aspiration method. J Neurosurg 95:221–224
Ichihara K, Taguchi T, Sakuramoto I, Kawano S, Kawai S (2009) Mechanism of the spinal cord injury and the cervical spondylotic myelopathy: new approach based on the mechanical features of the spinal cord white and gray matter. J Neurosurg Spine 99:278–285. https://doi.org/10.3171/spi.2003.99.3.0278
Ichihara K, Taguchi T, Shimada Y, Sakuramoto I, Kawano S, Kawai S (2001) Gray matter of the bovine cervical spinal cord is mechanically more rigid and fragile than the white matter. J Neurotrauma 18:361–367. https://doi.org/10.1089/08977150151071053
Bertram CD, Brodbelt AR, Stoodley MA (2005) The origins of syringomyelia: numerical models of fluid/structure interactions in the spinal cord. J Biomech Eng 127:1099–1109
Bilston LE, Thibault LE (1996) The mechanical properties of the human cervical spinal cord in vitro. Ann Biomed Eng 24:67–74
Cheng S, Bilston LE (2007) Unconfined compression of white matter. J Biomech 40:117–124. https://doi.org/10.1016/j.jbiomech.2005.11.004
Sparrey CJ, Keaveny TM (2011) Compression behavior of porcine spinal cord white matter. J Biomech 44:1078–1082. https://doi.org/10.1016/j.jbiomech.2011.01.035
Tunturi AR (1978) Elasticity of the spinal cord, pia, and denticulate ligament in the dog. J Neurosurg 48:975
Cheng S, Clarke EC, Bilston LE (2009) The effects of preconditioning strain on measured tissue properties. J Biomech 42:1360–1362. https://doi.org/10.1016/j.jbiomech.2009.03.023
Oakland RJ, Hall RM, Wilcox RK, Barton DC (2006) The biomechanical response of spinal cord tissue to uniaxial loading. 220:489–492. https://doi.org/10.1243/09544119JEIM135
Ramo NL, Troyer KL, Puttlitz CM (2018) Viscoelasticity of spinal cord and meningeal tissues. Acta Biomater 75:253–262. https://doi.org/10.1016/j.actbio.2018.05.045
Clarke EC (2010) Spinal cord mechanical properties. Springer, Berlin, Heidelberg, pp 25–40
Ramo NL, Shetye SS, Streijger F, Lee JHT, Troyer KL, Kwon BK, Cripton P, Puttlitz CM (2018) Comparison of in vivo and ex vivo viscoelastic behavior of the spinal cord. Acta Biomater 68:78–89. https://doi.org/10.1016/j.actbio.2017.12.024
Troyer KL, Estep DJ, Puttlitz CM (2012) Viscoelastic effects during loading play an integral role in soft tissue mechanics. Acta Biomater 8:234–243. https://doi.org/10.1016/j.actbio.2011.07.035
Hung T-K, Chang G-L, Lin H-S, Walter FR, Bunegin L (1981) Stress-strain relationship of the spinal cord of anesthetized cats. J Biomech 14:269–276. https://doi.org/10.1016/0021-9290(81)90072-5
Bilston LE, Thibault LE (1996) The mechanical properties of the human cervical spinal cord in vitro. Biomed Eng 24:67–74
Clarke EC, Cheng S, Ã LEB (2009) The mechanical properties of neonatal rat spinal cord in vitro , and comparisons with adult. 42:1397–1402. https://doi.org/10.1016/j.jbiomech.2009.04.008
Fiford RJ, Bilston LE (2006) The mechanical properties of rat spinal cord in vitro. 38:1509–1515. https://doi.org/10.1016/j.jbiomech.2004.07.009
Cheng S, Clarke EC, Bilston LE (2008) Rheological properties of the tissues of the central nervous system: a review. Med Eng Phys 30:1318–1337. https://doi.org/10.1016/J.MEDENGPHY.2008.06.003
Fung Y-C (1993) Biomechanics : mechanical properties of living tissues. Springer-Verlag, New York
Lucas SR, Bass CR, Salzar RS, Oyen ML, Planchak C, Ziemba A, Shender BS, Paskoff G (2008) Viscoelastic properties of the cervical spinal ligaments under fast strain-rate deformations. Acta Biomater 4:117–125. https://doi.org/10.1016/j.actbio.2007.08.003
Abramowitch SD, Woo SL-Y (2004) An Improved method to analyze the stress relaxation of ligaments following a finite ramp time based on the quasi-linear viscoelastic theory. J Biomech Eng 126:92–97
Provenzano PP, Lakes RS, Corr DT, Vanderby R (2002) Application of nonlinear viscoelastic models to describe ligament behavior. Biomech Model Mechanobiol 1:45–57. https://doi.org/10.1007/s10237-002-0004-1
Shetye SS, Troyer KL, Streijger F, Lee JHT, Kwon BK, Cripton PA, Puttlitz CM (2014) Nonlinear viscoelastic characterization of the porcine spinal cord. Acta Biomater 10:792–797. https://doi.org/10.1016/j.actbio.2013.10.038
Galle B, Ouyang H, Shi R, Nauman E (2010) A transversely isotropic constitutive model of excised guinea pig spinal cord white matter. J Biomech 43:2839–2843. https://doi.org/10.1016/j.jbiomech.2010.06.014
Karimi A, Shojaei A, Tehrani P (2017) Mechanical properties of the human spinal cord under the compressive loading. J Chem Neuroanat 86:15–18. https://doi.org/10.1016/j.jchemneu.2017.07.004
Garcia-Gonzalez D, Jerusalem A (2019) Energy based mechano-electrophysiological model of CNS damage at the tissue scale. J Mech Phys Solids 125:22–37
Liu H, Holzapfel GA, Skallerud BH, Prot V (2019) Anisotropic finite strain viscoelasticity: constitutive modeling and finite element implementation. J Mech Phys Solids 124:172–188. https://doi.org/10.1016/j.jmps.2018.09.014
Bischoff JE, Arruda EM, Grosh K (2004) A rheological network model for the continuum anisotropic and viscoelastic behavior of soft tissue. Biomech Model Mechanobiol 3:56–65
Chui C, Kobayashi E, Chen X, Hisada T, Sakuma I (2007) Transversely isotropic properties of porcine liver tissue: experiments and constitutive modelling. Med Biol Eng Comput 45:99–106
Mendis KK, Stalnaker RL, Advani SH (1995) A constitutive relationship for large deformation finite element modeling of brain tissue. J Biomech Eng 117:279–285. https://doi.org/10.1115/1.2794182
Miller K (1999) Constitutive model of brain tissue suitable for finite element analysis of surgical procedures. J Biomech 32:531–537. https://doi.org/10.1016/S0021-9290(99)00010-X
Samadi-Dooki A, Voyiadjis GZ, Stout RW (2018) A combined experimental, modeling, and computational approach to interpret the viscoelastic response of the white matter brain tissue during indentation. J Mech Behav Biomed Mater 77:24–33. https://doi.org/10.1016/j.jmbbm.2017.08.037
Holzapfel GA (2000) Nonlinear solid mechanics: a continuum approach for engineering. Wiley
Avril S (2017) Hyperelasticity of soft tissues and related inverse problems. Springer, Cham, pp 37–66
Lockett FJ (1972) Nonlinear viscoelastic solids. Academic Press
Yang LM, Shim VPW, Lim CT (2000) A visco-hyperelastic approach to modelling the constitutive behaviour of rubber. Int J Impact Eng 24:545–560. https://doi.org/10.1016/S0734-743X(99)00044-5
Anani Y, Alizadeh Y (2011) Visco-hyperelastic constitutive law for modeling of foam’s behavior. Mater Des 32:2940–2948. https://doi.org/10.1016/j.matdes.2010.11.010
Wang L-L, Huang D, Gan S (1996) Nonlinear viscoelastic constitutive relations and nonlinear viscoelastic wave propagation for polymers at high strain rates. Constitutive relation in high/very high strain rates. Springer, Tokyo Japan, pp 137–146
Strutz, T. (2011) Data fitting and uncertainty: A practical introduction to weighted least squares and beyond. Germany: Vieweg+ Teubner, Wiesbaden
Ogden R (1997) Non-linear elastic deformations. Dover Publications, New York
Hung T-K, Chang G-L, Chang J-L, Albin MS (1981) Stress-strain relationship and neurological sequelae of uniaxial elongation of the spinal cord of cats. Surg Neurol 15:471–476. https://doi.org/10.1016/S0090-3019(81)80043-2
Hung T-K, Chang G-L (1981) Biomechanical and neurological response of the spinal cord of a puppy to uniaxial tension. J Biomech Eng 103:43–47
Cooney GM, Moerman KM, Takaza M, Winter DC, Simms CK (2015) Uniaxial and biaxial mechanical properties of porcine linea alba. J Mech Behav Biomed Mater 41:68–82. https://doi.org/10.1016/j.jmbbm.2014.09.026
Funding
This study was supported by a Grant-in-Aid for Scientific Research (C) from the JSPS (grant 21560107).
Author information
Authors and Affiliations
Contributions
Fei Jiang wrote and prepared the manuscript. Itsuo Sakuramoto and Junji Ohgi conducted the experiment. Fei Jiang and Yoshikatsu Onomoto developed the constitutive model. Norihiro Nishida and **an Chen gave advice. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, F., Sakuramoto, I., Nishida, N. et al. The mechanical behavior of bovine spinal cord white matter under various strain rate conditions: tensile testing and visco-hyperelastic constitutive modeling. Med Biol Eng Comput 61, 1381–1394 (2023). https://doi.org/10.1007/s11517-023-02787-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-023-02787-1