Abstract
Purpose
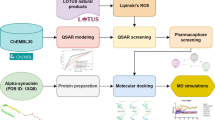
Previous studies from our lab utilized an ultra-high throughput screening method to identify compound 1 as a small molecule that binds to alpha-synuclein (α-synuclein) fibrils. The goal of the current study was to conduct a similarity search of 1 to identify structural analogs having improved in vitro binding properties for this target that could be labeled with radionuclides for both in vitro and in vivo studies for measuring α-synuclein aggregates.
Methods
Using 1 as a lead compound in a similarity search, isoxazole derivative 15 was identified to bind to α-synuclein fibrils with high affinity in competition binding assays. A photocrosslinkable version was used to confirm binding site preference. Derivative 21, the iodo-analog of 15, was synthesized, and subsequently radiolabeled isotopologs [125I]21 and [11C]21 were successfully synthesized for use in in vitro and in vivo studies, respectively. [125I]21 was used in radioligand binding studies in post-mortem Parkinson’s disease (PD) and Alzheimer’s disease (AD) brain homogenates. In vivo imaging of an α-synuclein mouse model and non-human primates was performed with [11C]21.
Results
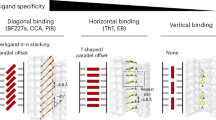
In silico molecular docking and molecular dynamic simulation studies for a panel of compounds identified through a similarity search, were shown to correlate with Ki values obtained from in vitro binding studies. Improved affinity of isoxazole derivative 15 for α-synuclein binding site 9 was indicated by photocrosslinking studies with CLX10. Design and successful (radio)synthesis of iodo-analog 21 of isoxazole derivative 15 enabled further in vitro and in vivo evaluation. Kd values obtained in vitro with [125I]21 for α-synuclein and Aβ42 fibrils were 0.48 ± 0.08 nM and 2.47 ± 1.30 nM, respectively. [125I]21 showed higher binding in human postmortem PD brain tissue compared with AD tissue, and low binding in control brain tissue. Lastly, in vivo preclinical PET imaging showed elevated retention of [11C]21 in PFF-injected mouse brain. However, in PBS-injected control mouse brain, slow washout of the tracer indicates high non-specific binding. [11C]21 showed high initial brain uptake in a healthy non-human primate, followed by fast washout that may be caused by rapid metabolic rate (21% intact [11C]21 in blood at 5 min p.i.).
Conclusion
Through a relatively simple ligand-based similarity search, we identified a new radioligand that binds with high affinity (<10 nM) to α-synuclein fibrils and PD tissue. Although the radioligand has suboptimal selectivity for α-synuclein towards Aβ and high non-specific binding, we show here that a simple in silico approach is a promising strategy to identify novel ligands for target proteins in the CNS with the potential to be radiolabeled for PET neuroimaging studies.








Similar content being viewed by others
Data Availability
The data needed to evaluate the conclusions of the paper are present in the paper and/or the Supplementary Materials.
References
Henderson MX, Trojanowski JQ, Lee VM (2019) α-Synuclein pathology in Parkinson’s disease and related α-synucleinopathies. Neurosci Lett 709:134316
Seibyl JP (2022) Alpha-synuclein PET and Parkinson disease therapeutic trials: ever the twain shall meet? J Nucl Med 63(10):1463–1466
Pancoe SX et al (2022) Effects of mutations and post-translational modifications on α-synuclein in vitro aggregation. J Mol Biol 434(23):167859
Gibb W, Lees A (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51(6):745–752
Gibb W, Lees A (1989) The significance of the Lewy body in the diagnosis of idiopathic Parkinson’s disease. Neuropathol Appl Neurobiol 15(1):27–44
Jellinger KA, Seppi K, Wenning GK (2005) Grading of neuropathology in multiple system atrophy: proposal for a novel scale. Mov Disord Off J Mov Disord Soc 20(S12):S29–S36
Dickson D et al (1999) Widespread alterations of α-synuclein in multiple system atrophy. Am J Pathol 155(4):1241–1251
Cykowski MD et al (2015) Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain 138(8):2293–2309
Prasad EM, Hung SY (2021) Current therapies in clinical trials of Parkinson’s disease: a 2021 update. Pharmaceuticals (Basel) 14(8):717
Smit JW et al (2022) Phase 1/1b Studies of UCB0599, an Oral Inhibitor of α-Synuclein Misfolding, Including a Randomized Study in Parkinson’s Disease. Mov Disord 37(10):2045–2056
Buddhala C et al (2015) Dopaminergic, serotonergic, and noradrenergic deficits in Parkinson disease. Ann Clin Transl Neurol 2(10):949–959
Covell DJ et al (2017) Novel conformation-selective alpha-synuclein antibodies raised against different in vitro fibril forms show distinct patterns of Lewy pathology in Parkinson’s disease. Neuropathol Appl Neurobiol 43(7):604–620
Li Y et al (2018) Amyloid fibril structure of alpha-synuclein determined by cryo-electron microscopy. Cell Res 28(9):897–903
Kotzbauer PT, Tu Z, Mach RH (2017) Current status of the development of PET radiotracers for imaging alpha synuclein aggregates in Lewy bodies and Lewy neurites. Clin Transl Imaging 5(1):3–14
Ferrie JJ et al (2020) Identification of a nanomolar affinity α-synuclein fibril imaging probe by ultra-high throughput in silico screening. Chem Sci 11(47):12746–12754
Johnson DK, Karanicolas J (2016) Ultra-High-Throughput Structure-Based Virtual Screening for Small-Molecule Inhibitors of Protein-Protein Interactions. J Chem Inf Model 56(2):399–411
Brundin P, Dave KD, Kordower JH (2017) Therapeutic approaches to target alpha-synuclein pathology. Exp Neurol 298(Pt B):225–235
Hsieh CJ et al (2018) Alpha Synuclein Fibrils Contain Multiple Binding Sites for Small Molecules. ACS Chem Neurosci 9(11):2521–2527
Lengyel-Zhand Z et al (2020) Synthesis and characterization of high affinity fluorogenic alpha-synuclein probes. Chem Commun (Camb) 56(24):3567–3570
Sonustun B et al (2022) Pathological relevance of post-translationally modified alpha-synuclein (pSer87, pSer129, nTyr39) in idiopathic Parkinson’s disease and multiple system atrophy. Cells 11(5):906
Schmid AW et al (2013) Alpha-synuclein post-translational modifications as potential biomarkers for Parkinson disease and other synucleinopathies. Mol Cell Proteomics 12(12):3543–58
Fauvet B, Lashuel HA (2016) Semisynthesis and enzymatic preparation of post-translationally modified α-synuclein. Methods Mol Biol 1345:3–20
Kaide S, Watanabe H, Shimizu Y, Iikuni S, Nakamoto Y, Hasegawa M, Itoh K, Ono M (2020) Identification and evaluation of bisquinoline scaffold as a new candidate for α-synuclein-PET imaging. ACS Chem Neurosci 11(24):4254-4261
Pan B et al (2021) Chemoenzymatic semi-synthesis enables efficient production of isotopically labeled α-synuclein with site-specific tyrosine phosphorylation. ChemBioChem 22(8):1440–1447
Pan B, Rhoades E, Petersson EJ (2020) Chemoenzymatic semisynthesis of phosphorylated α-synuclein enables identification of a bidirectional effect on fibril formation. ACS Chem Biol 15(3):640–645
Zhao K et al (2020) Parkinson’s disease-related phosphorylation at Tyr39 rearranges α-synuclein amyloid fibril structure revealed by cryo-EM. Proc Natl Acad Sci 117(33):20305–20315
Zhang S et al (2023) Post-translational modifications of soluble α-synuclein regulate the amplification of pathological α-synuclein. Nat Neurosci 26:213–225
Morris GM et al (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30(16):2785–2791
Phillips JC et al (2005) Scalable molecular dynamics with NAMD. J Comput Chem 26(16):1781–1802
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14(1):33–38
Riad A et al (2020) The sigma-2 receptor/TMEM97, PGRMC1, and LDL receptor complex are responsible for the cellular uptake of Aβ42 and its protein aggregates. Mol Neurobiol 57:3803–3813
Klunk WE et al (2001) Uncharged thioflavin-T derivatives bind to amyloid-beta protein with high affinity and readily enter the brain. Life Sci 69(13):1471–1484
Lougee MG et al (2022) Harnessing the intrinsic photochemistry of isoxazoles for the development of chemoproteomic crosslinking methods. Chem Commun 58(65):9116–9119
Montine TJ et al (2012) National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123:1–11
McKeith IG et al (2017) Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 89(1):88–100
Bagchi DP et al (2013) Binding of the radioligand SIL23 to α-synuclein fibrils in Parkinson disease brain tissue establishes feasibility and screening approaches for develo** a Parkinson disease imaging agent. PloS one 8(2):e55031
Klunk WE et al (2003) The binding of 2-(4′-methylaminophenyl) benzothiazole to postmortem brain homogenates is dominated by the amyloid component. J Neurosci 23(6):2086–2092
Luk KC et al (2012) Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338(6109):949–953
Mirrione MM et al (2007) A novel approach for imaging brain–behavior relationships in mice reveals unexpected metabolic patterns during seizures in the absence of tissue plasminogen activator. Neuroimage 38(1):34–42
Karp JS et al (2003) Performance of a brain PET camera based on anger-logic gadolinium oxyorthosilicate detectors. J Nucl Med 44(8):1340–1349
DePierro A (1989) On some nonlinear iterative relaxation methods in remote sensing. Mat Apl Comput 8:153–166
Browne J, De Pierro A (1996) A row-action alternative to the EM algorithm for maximizing likelihood in emission tomography. IEEE Trans Med Imaging 15(5):687–699
Daube-Witherspoon ME, Matej S, Karp JS (2001) Assessment of image quality with a fast fully 3D reconstruction algorithm. 2001 IEEE Nuclear Science Symposium Conference Record (Cat. No. 01CH37310), San Diego, pp 2238–2242 vol. 4
Maurer A et al (2020) 11C radiolabeling of anle253b: a putative PET tracer for Parkinson’s disease that binds to α-synuclein fibrils in vitro and crosses the blood-brain barrier. Chem Med Chem 15(5):411–415
Cheng Y, Prusoff WH (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22(23):3099–3108
Pang A et al (2003) Interdomain dynamics and ligand binding: molecular dynamics simulations of glutamine binding protein. FEBS Lett 550(1–3):168–74
Brettschneider J et al (2018) Converging Patterns of alpha-Synuclein Pathology in Multiple System Atrophy. J Neuropathol Exp Neurol 77(11):1005–1016
Zarranz JJ et al (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55(2):164–173
Chu W et al (2015) Design, synthesis, and characterization of 3-(Benzylidene)indolin-2-one derivatives as ligands for alpha-synuclein fibrils. J Med Chem 58(15):6002–17
McKeith IG et al (1996) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 47(5):1113–1124
Kikuchi A et al (2010) In vivo visualization of alpha-synuclein deposition by carbon-11-labelled 2-[2-(2-dimethylaminothiazol-5-yl)ethenyl]-6-[2-(fluoro)ethoxy]benzoxazole positron emission tomography in multiple system atrophy. Brain 133(Pt 6):1772–8
Irwin DJ, Lee VM-Y, Trojanowski JQ (2013) Parkinson’s disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci 14(9):626–636
Tuttle MD et al (2016) Solid-state NMR structure of a pathogenic fibril of full-length human alpha-synuclein. Nat Struct Mol Biol 23(5):409–415
Acknowledgments
This research was funded by the Michael J. Fox Foundation and NIH grant U19-NS110456. M.G.L. was supported by an Age Related Neurodegenerative Disease Training Grant fellowship (NIH T32-AG000255). H.J.K. was supported by an NIH Predoctoral Fellowship (NIH F31-AG069390).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Janssen, B., Tian, G., Lengyel-Zhand, Z. et al. Identification of a Putative α-synuclein Radioligand Using an in silico Similarity Search. Mol Imaging Biol 25, 704–719 (2023). https://doi.org/10.1007/s11307-023-01814-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-023-01814-9