Abstract
It is well acknowledged that microplastics are a major environmental problem and that the use of plastics, both petro- and bio- based, should be reduced. Nevertheless, it is also a necessity to reduce the amount of the already spread plastics. These cannot be easily degraded in the nature and accumulate in the food supply chain with major danger for animals and human life. It has been shown in the literature that advanced oxidation processes (AOPs) modify the surface of polylactic acid (PLA) materials in a way that bacteria more efficiently dock on their surface and eventually degrade them. In the present work we investigated the influence of different AOPs (ultrasounds, ultraviolet irradiation, and their combination) on the biodegradability of PLA films treated for different times between 1 and 6 h. The pre-treated samples have been degraded using a home model compost as well as a cocktail of commercial enzymes at mesophilic temperatures (37 °C and 42 °C, respectively). Degradation degree has been measured and degradation products have been identified. Excellent degradation of PLA films has been achieved with enzyme cocktail containing commercial alkaline proteases and lipases of up to 90% weight loss. For the first time, we also report valorization of PLA into bacterial nanocellulose after enzymatic hydrolysis of the samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fossil-based polymers are still widely used due to their versatility, light weight, durability, and low production cost, leading to their uncontrolled disposal in the environment. Currently, these polymers represent the most common pollutants, accounting for 54% of all human generated waste material (Hoellein et al. 2014; Venkatesh et al. 2021).
Current methods of managing polymer materials are landfilling, cement clinker co-processing, recycling by matrix degradation (i.e., use of microorganisms, electrolytes, solvents), mechanical recycling through matrix decomposition, high voltage fragmentation and microwaves (Mativenga et al. 2017; Wong et al. 2017). Bioplastics are seen as suitable alternative for a while now, but their participation in plastic market is still below 2% (Mos Moshood et al. 2022; Babu et al. 2013). Bioplastics help pave the way to circular economy of plastic materials, since they are produced from renewable resources and offer more end-of-life options (Jeremic et al. 2020). Landfilling should be avoided in the future as it terminates the opportunities to gain more value through circular thinking (i.e., recycle, reuse, reduce etc.) (Korniejenko et al. 2021; Levanen et al. 2021; Zorpas et al. 2021).
Due to its ability to be biosynthetic and biodegradable, PLA is considered the most promising environmentally friendly material for a sustainable bioeconomy (Xu et al. 2022). As the compost is further distributed for agricultural applications, the current PLA degradation strategy contributes to microplastics pollution in the environment that pose an even greater threat as they can concentrate pollutants and are easily spread across all ecological niches (Mato et al. 2001; Rochman 2018). On the other hand, complete degradation of PLA in nature can last even decades, while the degradation rate in seawater is even lower (Nampoothiri et al. 2010; Goswami et al. 2020; Upadhyay et al. 2020).
Both biodegradation and composting rely on depolymerization mediated by microorganisms and their enzymes. PLA degraders are amongst the least studied plastic degrading microorganisms. The depolymerisation of PLA and polymers in general cleaves their chemical backbone, resulting in into intermediate metabolites, such as monomers, dimers, and oligomers (Yasin et al. 2022; Qi et al. 2017). The most represented fungal PLA degraders are Ascomycota and Basidiomycota (Satti et al. 2017), while different families belonging to Actinobacteria showed PLA-degrading ability (Butbunchu and Pathom-Aree 2019; Kawai 2010), with Amycolatopsis and Actinomadura as the most potent genera (Pranamuda and Tokiwa 1999). Other PLA-degrading bacteria predominantly belong to Bacillaceae, in fact, the majority of the PLA-degrading enzymes, classified as alkaline proteases, are secreted from Bacillus species (Butbunchu and Pathom-Aree 2019; Oda et al. 2000a; Tokiwa and Calabia 2006). An increasing number of mesophilic PLA degraders are discovered in the last 10 years (Kim and Park 2010; Pattanasuttichonlakul et al. 2018; Decorosi et al. 2019). Recently, Richert and Dabrowska (2021) have tested different compost and activated sludge extracts and compared them with commercial enzymes showing that the first have the greatest impact on the biodegradation of PLA/PCL mixtures and pure PCL, while the commercially available microorganisms degrade PLA more efficiently. Morohoshi et al. (2018) have also shown the importance of the biofilm formation on the surface of biodegradable plastic for their degradation.
The structural homology of PLA with natural polymers permits its degradation by naturally occurring enzymes grouped as PLA depolymerases. According to primary substrate specificities, PLA depolymerases are classified as protease-type and lipase-type, the latter also including cutinases. Both types of PLA depolymerases are sharing serin hydrolase catalytic mechanisms, but significant differences in the stereochemistry of catalytic sites between them give a structural basis for different substrate specificities (Rosato et al. 2022; Kawai et al. 2011). Protease-type PLA depolymerases are strictly specific for PLA containing l-lactate, used in most studies, due to structural homology to proteins composed of L-aminoacids (Reeve et al. 1994). For a long time, it was thought that protease-type PLA depolymerases are more common and more efficient as comparative studies using PLLA as a substrate identified an abundance of commercial proteases, of both mammalian and microbial origin, capable of PLA degradation, as well as a general lack of commercial lipases for that purpose (Hoshino and Isono 2002; Masaki et al. 2005; Lim et al. 2005).
Among factors affecting the enzymatic polymer degradation rate crystallinity plays an important (Cui et al. 2022; Li and McCarthy 1999). The crystalline structure of PLA is an obstacle to enzymatic degradation at ambient conditions and poses a demand for material pretreatment, most commonly thermal pretreatment at temperatures close to PLA glass transition temperature (60 °C) (Karamanlioglu et al. 2017).
Enzymatic adsorption on the PLA surface was studied with Proteinase K as a model system and is an irreversible process in aqueous solutions, as degradation of PLA continues even after the enzyme solution replacement with a buffer (Yamashita et al. 2005; Bose et al. 2015). Amorphous regions of PLA are first subjected to enzymatic hydrolysis, so very often overall crystallinity increases during the initial enzymatic degradation and only later do crystalline regions begin to degrade (Kawai et al. 2011; Lee et al. 2014; Vasanthan and Gezer 2013). UV irradiation reduces the molar mass of PLA, which is known to have a positive effect on degradation rate, and therefore is a suitable alternative to thermal pretreatment (Pattanasuttichonlakul et al. 2018; Tsuji and Miyauchi 2001; Longieras et al. 2007).
Recently, we have shown that the surface chemistry and composition of PLA is improved towards bacterial degradation (Sourkouni et al. 2021; Kalogirou et al. 2022). Several other studies have shown that photodegradation yields positive effects on the biodegradation of polymers by deteriorating the polymer surface and increasing the surface availability for biodegradation (Yang et al. 2005; Longieras et al. 2007; Jeon and Kim 2013; Vimala and Mathew 2016). To enable the efficient docking of microorganisms and enable subsequent polymer degradation, a surface pretreatment may be necessary, especially to enrich surface with oxygen.
One can distinguish two types of polymers pre-treatment to enhance their degradability: thermal and non-thermal pre-treatment methods. It has been reported that not pre-treated PLA did not biodegrade under anaerobic conditions, while pre-treatment influenced the hydrolysis reaction rate, which increased the production of biogas during the biodegradation (Benn and Zitomer 2018; Wei and Zimmermann 2017). The non-thermal pretreatments can be categorized in (i) ultraviolet (UV) irradiation, (ii) high-power ultrasounds, (iii) grinding, (iv) plasma treatments and possible combinations of them (Yasin et al. 2022; Sourkouni et al. 2021; Kalogirou et al. 2022).
The objective of this study was to assess the impact of advanced oxidation processes as pretreatment technologies for PLA biodegradation at ambient to mesophilic conditions and to examine the effect on the enzymatic degradation of PLA materials as this methodology requires less energy and allows products of degradation to be further valorized. Overall, the present work aims to further contribute to a general understanding of the biodegradability of PLA under different environmental conditions which should be of primary importance in order to shift PLA from industrially compostable to fully biodegradable polymer.
Materials and methods
PLA films sample preparation
PLA used in this study was received from Nature works, Ingeo (4043D). The crystallinity of the pellet was 35.2%, the melting point 152.3 °C, and MFR, 6 g/10 min. MRF (Mass flow rate) is the measure of polymers flow behaviour. It measures the ease of flow of melted plastic, which is important to decide the pressing condition for making PLA films from pellets. It is expressed in a standard unit of g/10 min.
The melting point of PLA and the crystallinity of the PLA pellets and prepared films were measured based on the DSC analysis of the samples. The melting point of PLA polymer was measured at various times are and was found in the range of 152.3–153.6 °C.
Prior to processing the PLA pellets were dried for 50 °C for 5 h. Polymer pellets were pressed into films (ca. 1 mm thickness) using a Servitech Polystat 200 T compression press at 180 °C. The compression was held at 10 bar for 2 min, followed by 100 bar and 200 bar for 1 min each. The compressed sheet is crash cooled using tap water for 30 s and demoulded. The crystallinity of the prepared film was measured to be 1.9%.
PLA samples pretreated with ultraviolet (UV) waves for 6 h, ultrasonic waves (US) with a frequency of 20 kHz and 860 kHz, as well as the combinations of UV and US treatments, were cut into pieces (1 cm × 2 cm), weighted, rinsed with 70% (v/v) ethanol and air dried. Ethanol was used for disinfection of the sample surface to reduce the possibility of contamination, since it is rapidly bactericidal and evaporates fast at room temperature. This method of sterilizing samples prior to degradation experiments has been used in numerous studies (Syranidou et al. 2019; Mandic et al. 2019) with no significant effect on material performance or the course of the experiment. We certainly treated the control samples in the same way to ensure which changes are the result of the biodegradation itself.
All samples were prepared in duplicates, including non-treated control PLA sample. Details on the sample preparation as well as on the pre-treatment methods can be found in our previous publications (Sourkouni et al. 2021; Kalogirou et al. 2022).
Biodegradation in model compost
Biodegradation of control and pretreated PLA samples was performed in model compost according to the previously described protocol (Ponjavic et al. 2017a; Tomšič et al. 2022). Experiment was set up in glass Petri dishes (120 mm diameter, 30 mm height), in 150 g of compost per Petri dish. Samples were placed inside the compost at a depth of 1 cm. Petri dishes were incubated at 37 °C for 10 and 24 weeks, and the humidity of the compost was maintained around 50% by weight. At the end of experiment samples were rinsed with 70% (v/v) ethanol, air dried and weighted.
Microbial cell counts
In order to assess the number of viable microbial cells per gram of model compost before and after biodegradation experiment, colony forming units were determined by following standard protocols (Tomšič et al. 2022; Lee et al. 2021). Compost samples (1 g) were resuspended in distilled water, and serial dillutions prepared and plated on three types of growth media: Luria–Bertani agar (LA, Oxoid, UK) for heterotrophic bacteria; mannitol soy flower agar (MSF, Difco, UK) for sporulating aerobic bacteria and Sabouraud dextrose agar (SAB, Difco, UK) for fungi. Bacterial and fungi counts, determined 24 h and 72–96 h after plating, respectively, were expressed as colony forming units per gram of soil (CFU/g).
DSC analysis
DSC analysis was performed using PerkinElmer Pyris Diamond Scanning Calorimeter to understand the changes in the crystallinity among the powder, film, and pellet. The samples (3–5 mg), sealed in aluminum pans were used for the measurements. The samples are heated from − 100 to 2000 °C at the rate of 10 °C/min. The samples are crash cooled to − 100 °C to determine the enthalpy of cold crystallization. The percentage of crystallinity was calculated from the DSC data using the equation given below by considering the melting and cold crystallization enthalpy (Table 1).
where ΔΗm = Enthalpy of melting, ΔHcc = Enthalpy of cold crystallization, ΔH°m = Enthalpy of melting for 100% crystalline polymer. ΔH°m for PLA is 93 J/g.
Enzymatic biodegradation
Enzymatic hydrolysis of PLA samples was performed following standard protocols (Ponjavic et al. 2017b, 2018) by using enzyme mix of alcalase 2.4 L FG (Novozymes, batch PLN05554), savinase (Novozymes, GHSFS-1-02-1) and 3 lipases (Serowar PL; Sigma, cat.no. 54327 and Sigma, cat.no. 52001). Enzyme mix contained 5 mg/mL of alcalase and savinase, and 1.5 mg/mL of each of lipases in 20 mM Tris- HCl buffer (pH 8.5) and was stored at − 20 °C.
Film samples of approximately 50 mg were placed in glass vials and 6 mL of 20 mM Tris- HCl buffer (pH 8.5) was added. The reaction started by addition of 1 mL of enzyme mix. Control reactions contained no enzyme mix. Samples were incubated at 42 °C at 150 rpm for 16 weeks. Weekly, aliquots (1 mL) were taken and stored at -20 °C for analysis, while at the same time 1 mL aliquots of enzyme mix were added.
HPLC analysis of PLA hydrolysates
Samples were prepared for HPLC analysis by adding 50 µL of 6 M HCl to 1 mL of the hydrolysates, mixed using vortex and centrifuged for 10 min at 12 000 × rpm (Eppendorf Centrifuge 5417 R, Germany). The supernatants were filtered through 0.2 µm syringe filters directly into clean vials. For the analysis an UltiMate 3000 HPLC (Thermo Fisher Scientific, USA) system equipped with a Eurospher II 100-3 C18A 150 × 4.6 mm (Knauer, Germany) column was used. The separation was performed under isocratic conditions at a flow rate of 1 mL/min and the mobile phase consisted of 95% (v/v) 20 mM NH4H2PO4 in ultrapure water (adjusted to pH 2 with H3PO4) and 5% (v/v) acetonitrile. The degradation products were detected at 210 nm according to De Baere et al. (2013) and compared to standard lactic acid.
Bacterial nanocellulose production
PLA hydrolysates from enzymatic biodegradation were used as substrate for nanocellulose production using Komagataeibacter medellinensis ID13488 (Jeremic et al. 2019). Komagataeibacter medellinensis strain transforms glucose to glucose-6-phosphate, glucose-1-phosphate, uridine diphosphate (UDP)-glucose, and finally to unbranched β-1,4-D-glucan i.e., bacterial nanocellulose, in the presence of glucokinase, phosphoglucomutase and uridine triphosphate (UTP)-glucose-1-phosphate uridylyltransferase, with the aid of cellulose synthases. Although glucose is considered the main source, all substrates that can be transformed to glucose are theoretically available for nanocellulose production (Wang et al. 2018). It is expected that during PLA degradation lactic acid is transformed to fructose through pyruvaldehyde and glyceraldehyde intermediers, and then isomerized to glucose, which is used by bacteria for nanocellulose production.
In order to prepare inoculum, K. medellinensis strain was cultivated in a standard Hestrin–Schramm medium pH 4.5 (HS) containing 20 g/L glucose, 5 g/L peptone, 5 g/L yeast extract, 2.5 g/L Na2HPO4and 1.15 g/L citric acid, under static conditions at 28 °C. Volume of 2 mL of PLA hydrolysates was poured to the glass tubes, pH was adjusted to 4.5 using concentrated HCl, and inoculum of 200 µL of bacterial culture was added (10% v/v, inoculum). All samples were incubated under static conditions at 28 °C for 7 days. After incubation, nanocellulose discs were treated with 5% KOH aqueous solution and extensively washed with distilled water until a pH of 7.0 was reached, air dried and weighted. Nanocellulose production was presented as g/L of medium obtained after 7 days.
Results and discussion
Degradation in compost
Biodegradation of control and pretreated PLA films in model compost showed small morphological changes in the shape and coloration of the samples treated for 24 weeks as can be seen in Figure S1.
Weight loss of only 0.2% and 0.6% after 10 and 24 weeks, respectively, was detected in the PLA samples pretreated using ultrasonic waves (US) with a frequency of 860 kHz. As already reported in our previous paper (Kalogirou et al. 2022) this is most probably due to the chemical interaction of the samples treated with 860 kHz leading to a shift of the C–O and C=O bonds to C–C bonds but also to changes of the PLA surface due to the strong oscillatory behavior of the cavitation bubbles at 860 kHz. Although the detected weight loss seems modest, it must be considered that promising results reported in the literature come from composting PLA under industrial conditions, at elevated temperatures (45–60 °C) (Kulikowska et al. 2020; Kalita et al. 2021a, 2021b), while in this study the composting was performed at 37 °C. Wilfred and coworkers showed similar weight loss of PLA films, 0.7%, after two-week incubation at 45 °C (Wilfred et al. 2018), while weight loss of up to 90% is characteristic for composting at close to 60 ºC (Kawashima et al. 2021; Boonmee et al. 2016). Currently, temperatures achieved in home composts are lower than ASTM/ISO test temperatures, so that compostable PLA in a home pile is unlikely to complete the process of biodegradation within the time limits. Recent study of PLA degradation in home compost (Solano et al. 2022) showed that even after 31 weeks of incubation at room temperature no biodegradation was observed. However, this study highlights the possibility of optimizing PLA pretreatment towards improved composting in mild, home composting conditions, thus leading to reduced greenhouse gas (GHG) emissions and better waste management globally. Until proper PLA composting is established, not only in composting plants, but in households as well, it is somewhat pointless to use PLA and other biodegradable plastic products, since they will not contribute to waste reduction drastically (Kawashima et al. 2021).
The total number of viable microorganisms, represented as CFU/g, after 10 weeks of degradation in model compost showed slight changes in number, within the error range, in all three tested growth media, while after 24 weeks a moderate increase in CFU/g was observed. This suggest that PLA degradation in model compost did not significantly affect the total number (Table S1) and visually noticeable diversity of present microorganisms (Figure S2), which is correlating with similar studies (Esan et al. 2019). The only significant difference is reduction of microorganism counts by two orders of magnitude from T0 to after 10 weeks incubation (LA). Reduction of one order of magnitude is due to the incubation of fresh compost sample for prolonged period in closed glass petri plates, and it is considered expected. The phenomenon of increase in the number of bacteria in compost after degradation is also observed in several other studies (Orhan et al. 2004; Gautam and Kaur 2013), indicating that plastic degradation products may stimulate the growth of certain bacterial species. Even though it is shown that PLA composting had no significant effect on the bacterial count, it is necessary to perform a detailed microbial community analysis to determine variations in bacterial composition.
Characterization of the composted samples and comparison with reference samples
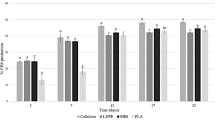
The pre-treated PLA samples have been weighed after exposition for 12 and 24 weeks to model compost (MC) and it has been found that depending on exposition time and pre-treatment method different degradation percentages result as summarized in Fig. 1.
Taking a closer look on the XPS (x-ray photoelectron spectroscopy) results of the pretreated PLA samples (Table S2) it is interesting to point out that the samples with the highest degradation rate (except the one at 12 weeks–20 kHz and 6 h treatment) contain nitrogen after the long compost contact time of 24 weeks (Kalogirou et al. 2022). That indicates the chemical involvement of nitrogen from ambient air in the degradation mechanism of pre-treated PLA. As the blind sample also contains nitrogen after 24 weeks the nitrogen involvement seems to be independent of the pre-treatment method.
From Fig. 2 it is obvious that during the compost exposition the degradation occurs over a mechanism that converts single bonded PLA oxygen to double bonded carboxylic oxygen following a mechanism as proposed by Song et al. (2016). Similarly, the strong increase in –CH3 percentage (Table S2) can be explained by the same mechanism, as –CH3 is obtained from both proposed reaction pathways.
It has been reported (Mofokeng et al. 2012), that in IR spectra the existence of the groups C=O and CH3 results in the appearance of peaks at wavelength 1747 cm−1 and 1455 cm−1 respectively. From the IR spectrum we get the information that the larger the peak, the lower the amount of the respective group in the sample as it is given in transmission. Based on that, the behavior found by XPS is confirmed also by IR-spectra of the samples degraded in compost for 12 and 24 weeks (Fig. 3).
Enzymatic degradation of PLA films
Based on the vast literature data on enzymatic degradation of PLA (Zaaba and Jaafar 2020) and the fact that serine proteases as the main member of PLA-degrading proteases, but also some alkaline proteases, lipases and cutinases showed the activity, we have included commercial serine endo-peptidase active on variety of pH and temperatures as well as three lipases in the enzyme mix and exposed PLA films to their activity over prolonged period of time (up to 16-weeks) at 42 °C whereby high load of enzymes per amount of material was applied (15% w/w) and fresh aliquot of enzyme mix was supplied regularly.
In Fig. 4 the weight losses of selected PLA samples after 8 weeks of enzymatic degradation are presented along with the amount of produced lactic acid (monomer) in [%] of the corresponding weight loss for each pre-treatment method. It is obvious that ultrasounds at 860 kHz is the most effective method for activating the degradation of PLA and its combination with UV for 3 h to be the most effective pretreatment method. For the degradation of PLA in compost ultrasounds at 860 kHz exhibit also the highest degradation (Fig. 1).
After 8 weeks the conversion rate to the lactic acid monomer is highest in the 860 kHz pre-treated samples for 3 h. Ultrasounds have the most positive impact on the production of the pure lactic acid monomer as it leads to a conversion of the pre-treated PLA to lactic acid monomer of 40%. High frequency US shows high degradation rates and the conversion to monomer is increasing as the pre-treatment time increases from 1 to 3 h.
XPS results (Fig. 5) of the enzymatic degraded samples (here exemplarily the sample pre-treated with high frequency ultrasounds (HFUS) at 860 kHz for 6 h, which exhibited the highest degradation rate after 16 weeks) show that oxygen is used in the degradation reaction as it is diminished in the pre-treated sample.
IR spectra (Figure S1) confirm the existence of increased C=O concentration after 6 h at 860 kHz ultrasounds relative to C–O. This trend is pertained also after the enzymatic treatment of the samples as can be seen in Figure S4 in, meaning that oxygen is most probably needed only for the attachment of the enzymes and not involved in the degradation reaction. If oxygen is involved in the degradation reaction, then the high oxygen concentration in the degraded samples could be only explained if they have been continuously oxidized by the enzyme.
Considering the enzymatic degradation of all samples treated for 1 and 3 h after 8 and 16 weeks it was surprising that the untreated sample after 16 weeks exhibits an outstanding performance and competes with the pretreated samples (Fig. 6). It exhibits a similar performance as the samples treated with low frequency ultrasounds for 3 h. This is surprising at first glance, as ultrasounds at 20 kHz have a high impact on the surface morphology because of the high power of the US at this frequency, which leads to large cavitation bubbles. The explanation for this behavior lies on the fact, that the collapse of the cavities is so violent that after 3 h the surface is eroded and thus (at least partly) like the fresh one, as already reported (Sourkouni et al. 2021). Further these results indicate that once the right enzyme cocktails are found the degradation of PLA and most probably of any other plastic are possible without or with less polymer surface activation.
In Fig. 7 SEM micrographs are showing the surface changes and the bulk porosity induced to the samples (here the sample which was pre-treated at 860 kHz + UV for 6 h). The porosity is indirectly ascertained during drop contour measurements, where the drop flowed through the sample.
It has been recently reported that cutinases and esterases can degrade most plastics that are composed of polyester constituents. On the other hand, serine proteases such as proteinase K, trypsin, elastase, and chymotrypsin are able to depolymerise PLA into its dimers and monomers by a two-step hydrolysis reaction (Qi et al. 2017; Gan and Zhang 2019).
Proteinase K of fungal origin is the first enzyme ever reported to degrade PLA in 1981, with a temperature optimum of 50 °C (Williams 1981). Since then, none of the commercially available acid, neutral and alkaline proteases showed higher efficiency in PLA degradation in comparison to Proteinase K (Lim et al. 2005). Among 56 commercial proteases with industrial application, Savinase showed to be the second best in PLA degradation yet achieved only 50% of Proteinase K activity at 50 °C (Oda et al. 2000b). Generally, alkaline proteases showed to be the best, some neutral proteases showed to be active and acid proteases showed to be ineffective as PLA depolymerases. In 2005 cutinase from Cryptococcus sp. strain S-2 (CLE) with high-molecular-weight PLLA degradation 500 times more efficient in comparison to Proteinase K at 30 °C was discovered (Masaki et al. 2005).pH and temperature optimum for the majority of PLA degrading enzymes show that enzyme catalysis preferentially occurs at elevated temperatures above 37 °C and up to 70 °C in alkaline conditions, therefore activities of these enzymes are compatible with PLA melting and abiotic autocatalytic degradation processes (Sukkhum and Kitpreechavanich 2011). Comparative study of PLA degradation by Candida cylindracea lipase, pig liver esterase and Bacillus licheniformis alcalase at optimum conditions for each enzyme. Results showed that alcalase is the most potent enzyme that degraded PLA sample to microplastics, resulting in 25% weight loss after 21 days at pH 9.5 and 60 °C unlike in the case of lipase and esterase (1.4% weight loss) at pH 8 and 40 °C (Lee et al. 2014). Complete PLA degradation by Lipase PL was achieved after 20 days at 55 °C, pH 8.5 while the same sample remained intact at 37 °C, pH 7 throughout 100 days of incubation (Hoshino and Isono 2002). Poly (DL-lactide) (average molar mass Mw 1.0–1.8 × 104) powder was degraded by almost 40% by 50 µg of RPA1511 (protein from Rhodopseudomonas palustris), and 90% by 50 µg of ABO2449 (protein from Alcanivorax borkumensis) upon 0.1% surfactant addition after 36 h of incubation at 35 °C and pH 8.0. Both enzymes were inactive towards either form of enantiopure PLA (Hajighasemi et al. 2016). Proteinase K and CLE decreased the weight of PLLA and PDLA films (15 mm × 5 mm) after 4 days at 37 °C by 7% and 14%, respectively (Kawai et al. 2011). The first non-commercial protease-type PLA depolymerase was purified from Amycolatopsis sp. strains K104-1 and it degraded over 90% of PLA film at 37 °C and pH 8.6 after 48 h with extensive pH control achieved through dialysis of reaction to prevent enzyme inactivation by pH drop throughout reaction (Nakamura et al. 2001). Xu et al. provided an overview of enzymes, PLA substrates, degradation conditions and percent degradation reported in the literature from 1997 to 2016 (Xu et al. 2022). Report on PLA film weight reduction by 26% was achieved by purified lipase from Sphingobacterium sp. strain S2 at 37 °C and pH 8 after 72 h (Satti et al. 2019).
Based on all these data, we made a cocktail of commercially available enzymes including Alcalase FG, Savinase and 3 lipases including Lipase PL and optimized enzymatic process to achieve 90% weight-loss at 42 °C and at mild alkaline conditions (pH 8.5) over 16 weeks (Figs. 4, 5, 6, 7). The reported weight loss was achieved only in a combination of pretreatment and enzymatic degradation, not pretreatment alone. There is room for further optimization of the bioprocess, but these results are a huge step in biotechnological enhancement of the PLA depolymerisation and subsequent valorization of the hydrolysis products.
Nanocellulose production from enzymatic PLA hydrolysate
Acidified enzymatic PLA hydrolysate successfully supported growth and nanocellulose production by K. medellinensis within 3 days of incubation (Figure S4). After 7 days of incubation, dry nanocellulose was weighted and the results are presented in Table 2 and the dried nanocellulose in Fig. 8. Obtained nanocellulose yields were comparable with nanocellulose obtained from standard HS medium supplemented with 2% glucose. Slightly higher nanocellulose production was supported by hydrolysates of PLA pre-treated with US860kHz and US860kHz + UV during 6 h (Table 1).
The results in Table 1 indicate that the monomer amount depends on the pre-treatment method and the treatment time. For example, despite the high nanocellulose yield using 20 kHz ultrasounds and UV (Fig. 4), their combination most probably degrades also the monomer, and it is no more available for the nanocellulose production leading to low yields (1.8 g/L with US20kHz + UV-6 h).
Previously, increased bacterial nanocellulose production was obtained in the presence of lactic acid by Acetobacter sp. V6 (Jung et al. 2010).
It has been shown in the recent literature that the nanocellulose produced by bacteria is nano-dimensional (Ludwicka et al. 2020; Sharma et al. 2019; Trache et al. 2020). There are generally three types of nanocelluloses: cellulose nanocrystals, cellulose nanofibrils and bacterial cellulose. During nanocellulose production by bacteria, numerous polymerized β-1,4 glucan chains are secreted outside the bacterial cell through a linear array of 3.5 nm pores on the outer membrane. Once out, the β-1,4 glucan chains are assembled in a precise and hierarchical process. First, sub-fibrils form, consisting of 10 to 15 chains of nascent glucans. Then, these sub-fibrils assemble and crystallize to form fibrils, which then combine to form a cellulose nanofiber and microfiber comprised of about 1000 individual glucan chains (30–100 nm diameter).
Conclusions
This study aimed to assess the effectiveness of different advanced oxidation processes (AOPs) for PLA pre-treatment on improving its enzymatic degradability and compostability under mesophilic conditions. Since AOPs modify the surface of PLA towards enhancing bacterial docking, the action of bacterial enzymes and degradation itself is facilitated. Obtained results corroborated assumption, with excellent PLA degradation—up to 90% weight loss—achieved using US/UV pre-treatment and enzyme hydrolysis. The established experimental setup will enable study extension on other pre-treatments and their impact on the PLA biodegradability. Degradation of pre-treated PLA samples in model compost under mild conditions was also proven in this study, emphasizing the possibility of home composting of PLA materials in the future.
In this respect a commercial enzyme mix containing serine endo-peptidases and lipases was found to be highly efficient in PLA hydrolysis. For the first time, we report valorization of PLA into bacterial nanocellulose after enzymatic hydrolysis of the samples. It has been found that the monomer amount depends on the pre-treatment method and the treatment time. Depending on the pre-treatment method between 1.8 and 4.3 g/L nanocellulose have been produced.
In this study for the first-time products of PLA degradation were valorized into bacterial nanocellulose, proving thus the possibility of a circular economy concept for PLA. Future studies will be focused on bioprocess optimization towards higher nanocellulose yields and characterization of such obtained material.
Data availability
Most of the raw data can be found in the publication itself (XPS, IR spectra) and in the supplemental information file of the present publication. Additional raw data cannot be published at present as it is an onoing European Project. Nevertheless data can be made available to the community upon request.
References
Arias-Nava EH, Sullivan BP, Valles-Rosales DJ (2021) Biopolymer degradation analysis: accelerated life testing study to characterize polylactic acid durability. Materials 14:5730
Babu RP, O’Connor K, Seeram R (2013) Current progress on bio-based polymers and their future trends. Prog Biomater 2:8. https://doi.org/10.1186/2194-0517-2-8
Bandopadhyay S, Martin-Closas L, Pelacho AM, DeBruyn JM (2018) Biodegradable plastic mulch films: impacts on soil microbial communities and ecosystem functions. Front Microbiol 9:819
Benn N, Zitomer D (2018) Pretreatment and anaerobic co-digestion of selected PHB and PLA bioplastics. Front Environ Sci 5:1–9. https://doi.org/10.3389/fenvs.2017.00093
Boonmee C, Kositanont C, Leejarkpai T (2016) Degradation of poly (lactic acid) under simulated landfill conditions. Environ Nat Res J 14:1–9
Bose S, Keller SS, Boisen A, Almdal K (2015) Microcantilever sensors for fast analysis of enzymatic degradation of poly (D, L-lactide). Polym Degrad Stab 1(119):1–8
Butbunchu N, Pathom-Aree W (2019) Actinobacteria as promising candidate for polylactic acid type bioplastic degradation. Front Microbiol 10:2834. https://doi.org/10.3389/fmicb.2019.02834.PMID:31921021;PMCID:PMC6930877
Cui L, Wang X, Szarka G, Hegyesi N, Wang Y, Sui X et al (2022) Quantitative analysis of factors determining the enzymatic degradation of poly(lactic acid). Int J Biol Macromol 1(209):1703–1709
De Baere S, Eeckhaut V, Steppe M, De Maesschalck C, De Backer P, Van Immerseel F, Croubels S (2013) Development of a HPLC–UV method for the quantitative determination of four short-chain fatty acids and lactic acid produced by intestinal bacteria during in vitro fermentation. J Pharm Biomed Anal 80(2013):107–115. https://doi.org/10.1016/j.jpba.2013.02.032
Decorosi F, Exana ML, Pini F, Adessi A, Messini A, Giovannetti L, Viti C (2019) The degradative capabilities of new amycolatopsis isolates on polylactic acid. Microorganisms. https://doi.org/10.3390/microorganisms7120590
DeStefano V, Khan S, Tabada A (2020) Applications of PLA in modern medicine. Eng Regen 1:76–87
Esan EO, Abbey L, Yurgel S (2019) Exploring the long-term effect of plastic on compost microbiome. PLoS ONE 25:e0214376
Gan Z, Zhang H (2019) Original article PMBD: a comprehensive plastics microbial biodegradation. Database. https://doi.org/10.1093/database/baz119
Gautam N, Kaur I (2013) Soil burial biodegradation studies of starch grafted polyethylene and identification of Rhizobium meliloti therefrom. J Environ Chem Ecotoxicol 5:147–158
Goswami P, Vinithkumar NV, Dharani G (2020) First evidence of microplastics bioaccumulation by marine organisms in the Port Blair Bay, Andaman Islands. Mar Pollut Bull 155:111163
Hajighasemi M, Nocek BP, Tchigvintsev A, Brown G, Flick R, Xu X et al (2016) Biochemical and structural insights into enzymatic depolymerization of polylactic acid and other polyesters by microbial carboxylesterases. Biomacromol 17(6):2027–2039
Hoellein T, Rojas M, Pink A, Gasior J, Kelly J (2014) Anthropogenic litter in urban freshwater ecosystems: distribution and microbial interactions. PLoS ONE 9:e98485
Hoshino A, Isono Y (2002) Degradation of aliphatic polyester films by commercially available lipases with special reference to rapid and complete degradation of poly(L-lactide) film by lipase PL derived from Alcaligenes sp. Biodegradation 13(2):141–147
https://www.statista.com. Accessed Jul 2022
Jeon HJ, Kim MN (2013) Biodegradation of poly(L-lactide) (PLA) exposed to UV irradiation by a mesophilic bacterium. Int Biodeterior Biodegrad 85:289–293
Jeremic S, Djokic L, Ajdačić V, Božinović N, Pavlovic V, Manojlović DD, Babu R, Senthamaraikannan R, Rojas O, Opsenica I, Nikodinovic-Runic J (2019) Production of bacterial nanocellulose (BNC) and its application as a solid support in transition metal catalysed cross-coupling reactions. Int J Biol Macromol 129:351–360
Jeremic S, Radivojevic J, Mojicevic M, Skaro Bogojevic S, Nikodinovic-Runic J (2020) Understanding bioplastic materials —Current state and trends. J Serb Chem Soc 85:51–51
Jung HJ, Lee OM, Jeong JH, Jeon YD, Park KH, Kim HS, An WG, Son HJ (2010) Production and characterization of cellulose by Acetobacter sp. V6 using a cost-effective molasses-corn steep liquor medium. Appl Biochem Biotechnol 162:486–497. https://doi.org/10.1007/s12010-009-8759-9
Kalita NK, Damare NA, Hazarika D, Bhagabati P, Kalamdhad A, Katiyar V (2021a) Biodegradation and characterization study of compostable PLA bioplastic containing algae biomass as potential degradation accelerator. Environ Chall 3:100067
Kalita NK, Sarmah A, Bhasney SM, Kalamdhad A, Katiyar V (2021b) Demonstrating an ideal compostable plastic using biodegradability kinetics of poly (lactic acid) (PLA) based green biocomposite films under aerobic composting conditions. Environ Chall 3:100030
Kalogirou Ch, Höfft O, Gödde A, Papadimitriou N, Pandis PK, Argirusis C, Sourkouni G (2022) Assessing the time dependence of AOPs on the surface properties of polylactic acid. J Poly Environ. https://doi.org/10.1007/s10924-022-02608-w
Karamanlioglu M, Preziosi R, Robson GD (2017) Abiotic and biotic environmental degradation of the bioplastic polymer poly(lactic acid): a review. Polym Degrad Stab 1(137):122–130
Kawai F (2010) Polylactic acid (PLA)-degrading microorganisms and PLA depolymerases. Green Polym Chem 1043:405–414
Kawai F, Nakadai K, Nishioka E, Nakajima H, Ohara H, Masaki K et al (2011) Different enantioselectivity of two types of poly(lactic acid) depolymerases toward poly(L-lactic acid) and poly(D-lactic acid). Polym Degrad Stab 96(7):1342–1348
Kawashima N, Yagi T, Kojima K (2021) Pilot-scale composting test of polylactic acid for social implementation. Sustainability 13:1654
Kim M, Park S (2010) Degradation of poly(L-lactide) by a mesophilic bacterium. J Appl Polym Sci 117:67–74
Korniejenko K, Kozub B, Bąk A, Balamurugan P, Uthayakumar M, Furtos G (2021) Tackling the circular economy challenges—Composites recycling: Used tyres, wind turbine blades, and solar panels. J Compos Sci. https://doi.org/10.3390/JCS5090243
Kulikowska D, Bernat K, Wojnowska-Baryła I, Pasieczna-Patkowska S, Jabłoński R (2020) Composting as a disposal route of PLA materials: kinetics of the aerobic biodegradation. Desalin Water Treat 206:153–164
Lee SH, Kim IY, Song WS (2014) Biodegradation of polylactic acid (PLA) fibers using different enzymes. Macromol Res 22(6):657–663
Lee J, Kim HS, Jo HY, Kwon MK (2021) Revisiting soil bacterial counting methods: optimal soil storage and pretreatment methods and comparison of culture-dependent and -independent methods. PLoS ONE 16(2):e0246142. https://doi.org/10.1371/journal.pone.0246142
Levanen J, Uusitalo V, Harri A, Kareinen E, Linnanen L (2021) Innovative recycling or extended use? Comparing the global warming potential of different ownership and end-of-life scenarios for textiles. Environ Res Lett. https://doi.org/10.1088/1748-9326/abfac3
Li S, McCarthy S (1999) Influence of crystallinity and stereochemistry on the enzymatic degradation of Poly(lactide)s. Macromolecules 32(13):4454–4456
Lim HA, Raku T, Tokiwa Y (2005) Hydrolysis of polyesters by serine proteases. Biotechnol Lett 27(7):459–464
Longieras A, Tanchette J-B, Erre D, Braud C, Copinet A (2007) Compostability of poly(lactide): degradation in an inert solid medium. J Polym Environ 15:200–206
Ludwicka K, Kaczmarek M, Białkowska A (2020) Bacterial nanocellulose—A biobased polymer for active and intelligent food packaging applications: recent advances and developments. Polymers 12(10):2209. https://doi.org/10.3390/polym12102209
Mandic M, Spasic J, Ponjavic M, Nikolic MS, Cosovic VR, O’Connor KE, Nikodinovic-Runic J, Djokic L, Jeremic S (2019) Biodegradation of poly(ε-caprolactone) (PCL) and medium chain length polyhydroxyalkanoate (mcl-PHA) using whole cells and cell free protein preparations of Pseudomonas and Streptomyces strains grown on waste cooking oil. Polym Degrad Stab 162(2019):160–168. https://doi.org/10.1016/j.polymdegradstab.2019.02.012
Masaki K, Kamini NR, Ikeda H, Iefuji H (2005) Cutinase-like enzyme from the yeast Cryptococcus sp. Strain S-2 hydrolyzes polylactic acid and other biodegradable plastics. Appl Environ Microbiol 71(11):7548–7550
Mativenga PT, Sultan AAM, Agwa-Ejon J, Mbohwa C (2017) Composites in a circular economy: a study of United Kingdom and South Africa. Procedia CIRP 61:691–696. https://doi.org/10.1016/j.procir.2016.11.270
Mato Y, Isobe T, Takada H, Kanehiro H, Ohtake C, Kaminuma T (2001) Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ Sci Technol 35:318–324
Mofokeng JP, Luyt AS, Tábi T, Kovács J (2012) Comparison of injection moulded, natural fibre-reinforced composites with PP and PLA as matrices. J Thermoplast Compos Mater 25(8):927–948. https://doi.org/10.1177/0892705711423291
Morohoshi T, Oi T, Aiso H, Suzuki T, Okura T, Sato S (2018) Biofilm formation and degradation of commercially available biodegradable plastic films by bacterial consortiums in freshwater environments. Microbes Environ 33:332–335. https://doi.org/10.1264/jsme2.ME18033
Mos Moshood TD, Nawanir G, Mahmud F, Mohamad F, Ahmad MH, AbdulGhani A (2022) Sustainability of biodegradable plastics: new problem or solution to solve the global plastic pollution? Curr Res Green Sustain Chem 5:100273
Nakamura K, Tomita T, Abe N, Kamio Y (2001) Purification and characterization of an extracellular poly(L-lactic acid) depolymerase from a soil isolate, Amycolatopsis sp. strain K104–1. Appl Environ Microbiol 67(1):345–353
Nampoothiri KM, Nair NR, John RP (2010) An overview of the recent developments in polylactide (PLA) research. Bioresour Technol 101:8493–8501. https://doi.org/10.1016/j.biortech.2010.05.092
Ncube LK, Ude AU, Ogunmuyiwa EN, Zulkifli R, Beas IN (2020) environmental impact of food packaging materials: a review of contemporary development from conventional plastics to polylactic acid based materials. Materials 13:4994
Oda Y, Yonetsu A, Urakami T, Tonomura K (2000a) Degradation of polylactide by commercial proteases. J Polym Environ 8:29–32
Oda Y, Yonetsu A, Urakami T, Tonomura K (2000b) Degradation of polylactide by commercial proteases. J Polym Environ 8(1):29–32
Orhan Y, Hrenović J, Büyükgüngör H (2004) Biodegradation of plastic compost bags under controlled soil conditions. Acta Chim Slov 51:579–588
Pattanasuttichonlakul W, Sombatsompop N, Prapagdee B (2018) Accelerating biodegradation of PLA using microbial consortium from dairy wastewater sludge combined with PLA-degrading bacterium. Int Biodeterior Biodegradation 132:74–83
Pattanasuttichonlakul W, Sombatsompop N, Prapagdee B (2018) Accelerating biodegradation of PLA using microbial consortium from dairy wastewater sludge combined with PLA-degrading bacterium. Int Biodeterior Biodegrad 1(132):74–83
Ponjavic M, Nikolic MS, Nikodinovic-Runic J, Jeremic S, Stevanovic S, Djonlagic J (2017a) Degradation behaviour of PCL/PEO/PCL and PCL/PEO block copolymers under controlled hydrolytic, enzymatic and composting conditions. Polym Testing 57:67–77
Ponjavic M, Nikolic MS, Nikodinovic-Runic J, Jeremic S, Stevanovic S, Djonlagic J (2017b) Degradation behaviour of PCL/PEO/PCL and PCL/PEO block copolymers under controlled hydrolytic, enzymatic and composting conditions. Polym Testing 57(2017):67–77. https://doi.org/10.1016/j.polymertesting.2016.11.018
Ponjavic M, Nikolic MS, Jeremic S, Djokic L, Nikodinovic-Runic J, Cosovic VR, Djonlagic J (2018) Influence of short central PEO segment on hydrolytic and enzymatic degradation of triblock PCL copolymers. J Polym Environ 26:2346–2359. https://doi.org/10.1007/s10924-017-1130-2
Pranamuda H, Tokiwa Y (1999) Degradation of poly(L-lactide) by strains belonging to genus Amycolatopsis. Biotech Lett 21:901–905
Qi X, Ren Y, Wang X (2017) New advances in the biodegradation of Poly(lactic) acid. Int Biodeterior Biodegrad 117:215–223. https://doi.org/10.1016/j.ibiod.2017.01.010
Reeve MS, McCarthy SP, Downey MJ, Gross RA (1994) Polylactide stereochemistry: effect on enzymic degradability. Macromolecules 27(3):825–831
Reeve MS, McCarthy SP, Downey MJ, Gross RA (1994) Polylactide stereochemistry: effect on enzymic degradability. Macromolecules 27:825–831
Richert A, Dąbrowska GB (2021) Enzymatic degradation and biofilm formation during biodegradation of polylactide and polycaprolactone polymers in various environments. Int J Biol Macromol 176:226–232
Rochman CM (2018) Microplastics research-from sink to source. Science 360:28–29
Rosato A, Romano A, Totaro G, Celli A, Fava F, Zanaroli G et al (2022) Enzymatic degradation of the most common aliphatic bio-polyesters and evaluation of the mechanisms involved: an extended study. Polymers 14(9):1850
Satti SM, Shah AA, Auras R, Marsh TL (2017) Isolation and characterization of bacteria capable of degrading poly(lactic acid) at ambient temperature. Polym Degrad Stab 144:392–400
Satti SM, Abbasi AM, Salahuddin, Rana QuA, Marsh TL, Auras R, Hasan F, Badshah M, Farman M, Shah AA et al (2019) Statistical optimization of lipase production from Sphingobacterium sp. strain S2 and evaluation of enzymatic depolymerization of Poly(lactic acid) at mesophilic temperature. Polym Degrad Stab 160:1–13
Sharma C, Bhardwaj K, Nishi K (2019) Bacterial nanocellulose: present status, biomedical applications and future perspectives. Mater Sci Eng C 104:109963. https://doi.org/10.1016/j.msec.2019.109963
Solano G, Rojas-Gätjens D, Rojas-Jimenez K, Chavarría M, Romero MRM (2022) Biodegradation of plastics at home composting conditions. Environ Chall 7:100500
Song AY, Oh YA, Roh SH, Kim JH, Min SC (2016) Cold oxygen plasma treatments for the improvement of the physicochemical and biodegradable properties of polylactic acid films for food packaging. J Food Sci 81(1):E86–E96. https://doi.org/10.1111/1750-3841.13172
Sourkouni G, Kalogirou Ch, Moritz Ph, Gödde A, Pandis PK, Höfft O, Vouyiouka S, Zorpas AA, Argirusis C (2021) Study on the influence of advanced treatment processes on the surface properties of polylactic acid for a bio-based circular economy for plastics. Ultrason Sonochem 76:105627
Steiner T, Zhang Y, Möller JN, Agarwal S, Löder MGJ, Greiner A, Laforsch C, Freitag R (2022) Municipal biowaste treatment plants contribute to the contamination of the environment with residues of biodegradable plastics with putative higher persistence potential. Sci Rep 12:9021
Sukkhum S, Kitpreechavanich V (2011) New insight into biodegradation of poly (L-lactide), enzyme production and characterization progress in molecular and environmental bioengineering. Progress in Molecular and Environmental Bioengineering-From Analysis and Modeling to Technology Applications. IntechOpen, London. https://doi.org/10.5772/19469
Sun C, Wei S, Tan H, Huang Y, Zhang Y (2022) Progress in upcycling polylactic acid waste as an alternative carbon source: a review. Chem Eng J 446:136881
Syranidou E, Karkanorachaki K, Amorotti F, Avgeropoulos A, Kolvenbach B, Zhou N-Y, Fava F, Corvini P-X, Kalogerakis N (2019) Biodegradation of mixture of plastic films by tailored marine consortia. J Hazardous Mater 375:33–42. https://doi.org/10.1016/j.jhazmat.2019.04.078
Tokiwa Y, Calabia BP (2006) Biodegradability and biodegradation of poly(lactide). Appl Microbiol Biotechnol 72:244–251
Tomšič B, Marković D, Janković V, Simončič B, Nikodinovic-Runic J, Ilic-Tomic T, Radetić M (2022) Biodegradation of cellulose fibers functionalized with CuO/Cu2O nanoparticles in combination with polycarboxylic acids. Cellulose 29:287–302. https://doi.org/10.1007/s10570-021-04296-6
Trache D, Tarchoun AF, Derradji M, Hamidon TS, Masruchin N, Brosse N, Hussin MH (2020) Nanocellulose: from fundamentals to advanced applications. Front Chem Polymer Chem. https://doi.org/10.3389/fchem.2020.00392
Tsuji H, Miyauchi S (2001) Enzymatic hydrolysis of poly(lactide)s: effects of molecular weight, L-lactide content, and enantiomeric and diastereoisomeric polymer blending. Biomacromol 2(2):597–604
Upadhyay RK, Mishra AK, Kumar A (2020) Mechanical degradation of 3D printed PLA in simulated marine environment. Surf Interfaces 21:100778
Vasanthan N, Gezer H (2013) Thermally induced crystallization and enzymatic degradation studies of poly (L-lactic acid) films. J Appl Polym Sci 127(6):4395–4401
Venkatesh S, Mahboob S, Govindarajan M, Al-Ghanim KA, Ahmed Z, Al-Mulhm N, Gayathri R, Vijayalakshmi S (2021) Microbial degradation of plastics: sustainable approach to tackling environmental threats facing big cities of the future. J King Saud University Sci 33:101362
Vimala PP, Mathew L (2016) Biodegradation of polyethylene using Bacillus subtilis. Procedia Technol 24:232–239
Wang SS, Han YH, Chen JL, Zhang DC, Shi XX, Ye YX, Chen DL, Li M (2018) Insights into bacterial cellulose biosynthesis from different carbon sources and the associated biochemical transformation pathways in Komagataeibacter sp. W1. Polymers 10(9):963. https://doi.org/10.3390/polym10090963
Wei R, Zimmermann W (2017) Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: how far are we? Microb Biotechnol 10(6):1308–1322. https://doi.org/10.1111/1751-7915.12710
Wilfred O, Tai H, Marriott R, Liu Q, Tverezovskiy V, Curling S, Fan Z, Wang W (2018) Biodegradation of polyactic acid and starch composites in compost and soil. Int J Nano Res 1:1–11
Williams DF (1981) Enzymic hydrolysis of polylactic acid. Eng Med 10(1):5–7
Wong K, Rudd C, Pickering S, Liu X (2017) Composites recycling solutions for the aviation industry. Sci China Technol Sci 60:1–10. https://doi.org/10.1007/s11431-016-9028-7
Xu B, Chen Y, He J, Cao S, Liu J, Xue R, **n F, Qian X, Zhou J, Dong W, Jiang M (2021) New insights into the biodegradation of polylactic acid: from degradation to upcycling. Environ Rev 30:30–38
Xu B, Chen Y, He J, Cao S, Liu J, Xue R et al (2022) New insights into the biodegradation of polylactic acid: from degradation to upcycling. Environ Rev 30(1):30–38
Yamashita K, Kikkawa Y, Kurokawa K, Doi Y (2005) Enzymatic degradation of poly(l-lactide) film by proteinase K: quartz crystal microbalance and atomic force microscopy study. Biomacromol 6(2):850–857
Yang H-S, Yoon J-S, Kim M-N (2005) Dependence of biodegradability of plastics in compost on the shape of specimens. Polym Degrad Stab 87:131–135
Yasin NM, Akkermans S, Van Imp JFM (2022) Enhancing the biodegradation of (bio)plastic through pretreatments: a critical review. Waste Manage 150:1–12
Zaaba NF& Jaafar M, (2020) A review on degradation mechanisms of polylactic acid:Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym Eng Sci 60:2061–2075. https://doi.org/10.1002/pen.25511
Zorpas AA, Doula MK, Jeguirim M (2021) Waste strategies development in the framework of circular economy. Sustainability 13:13467. https://doi.org/10.3390/su132313467
Acknowledgements
We are indebted to Assoc. Professor Matina Vougiouka (School of Chemical Engineering, National Technical University of Athens, Greece) for providing part of the PLA foils and to Dr. René Gustus (SEAL—Surface Engineering and Analysis Laboratories of the CZM, TU Clausthal) for his help with the HR-SEM micrographs. We acknowledge Novozymes (https://www.novozymes.com/en) for providing enzymes used in this study.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors declare that this study has been funded from the European Union´s Horizon 2020 research and innovation program under grant agreement number 870292 (BioICEP) and by the corresponding National Natural Science Foundation of China (Nos. 31961133016, 31961133015, and 31961133014).
Author information
Authors and Affiliations
Contributions
GS, JNR, and CA contributed to the study conception and design. Material preparation, data collection and analysis were performed by CK, GS, SJ, MN, VJ, OH, DR, RP, and PP. Quality control of the data and analyses were performed by GS, JNR, SJ, and CA. The first draft of the manuscript was written by GSA and CA and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sourkouni, G., Jeremić, S., Kalogirou, C. et al. Study of PLA pre-treatment, enzymatic and model-compost degradation, and valorization of degradation products to bacterial nanocellulose. World J Microbiol Biotechnol 39, 161 (2023). https://doi.org/10.1007/s11274-023-03605-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03605-4