Abstract
This study’s goal was to assess the catfish’s response to exposure to monoaromatic petroleum hydrocarbons (benzene, toluene, and xylene) and its recovery after exposure using oxidative stress, histopathological, and immunological changes as biomarkers. Four groups: one as control and other three exposed to benzene (0.762 ng/L), toluene (26.614 ng/L), and xylene (89.403 ng/L), respectively, for 30 days and then recovery period for 30 days. The levels of the cortisol, lipid peroxidation, and cytokines (IL-1β, IL-6) increased significantly (p < 0.05) after exposure to benzene and xylene compared to control. Superoxide dismutase (SOD), total antioxidant capacity (TAC), and acetylcholinstease (Ach) decreased significantly (p < 0.05) in fishes exposed to benzene only compared to control group. While glutathione-S-transferase (GST) did not show any change in different treatment groups compared to control group. The histopathological signs of liver exposed to benzene, toluene, and xylene displayed aggregation of melanomacrophages, congestion of sinusoids, vacuolar degeneration of hepatocytes, necrotic area with inflammatory cell infiltration, and thrombus of central vein. Kidney exposed to benzene, toluene, and xylene showed dilatation of Bowman’s space with atrophy of glomerular tuft, lyses of RBCs with mononuclear cell infiltration, multifocal area of hemopoietic tissue necrosis, organized thrombus with perivascular hemorrhage, focal inflammatory cellular reaction, renal tubular necrosis, and thrombus of blood vessels. Spleen exposed to benzene, toluene, and xylene showed hyperplasia of lymphoid follicles in white pulp in a mild degree. These lesions appeared to a mild degree or disappeared completely after recovery period to BTX except spleen. In conclusion, monocyclic aromatic hydrocarbons (BTX) are hazardous to fish and the toxicity level was as benzene > xylene > toluene even though after recovery period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The river Nile is utilized to transport a variety of commodities, including oil and oil-related products, as well as to supply water. Numerous industrial and oil distribution hubs can be found along its coasts, as well as additional sources of oil pollution such as tankers, barges, boats on rivers and canals, industrial waste, metallurgical industries, engineering projects, garages, and floating cruise ships (floating hotels) (El-Sheekh et al., 2000). The first study of oil and grease along the Nile was conducted by Moursy (1983), who found that the area around Lake Nasser port in Aswan was the most heavily polluted, followed by oil distribution hubs at ship-waiting areas in Assiut, boat docks, shipyards, an electric power plant in Cairo, and industries areas along Egypt-delta. According to a recent study by Moustafa and Shara (2009), oil contamination (PAHs) is detected in the river Nile water samples under study at varying amounts depending on the location and season.
Freshwater oil spills in particular have detrimental effects on the ecosystem and living things (Ewida, 2014). These adverse consequences are brought on by the release of numerous organic chemicals, the bulk of which are individual hydrocarbons, which give crude oil and oil distillates. In addition to carbon and hydrogen, other chemicals that can be found in crude oil and oil discharges include sulfur, nitrogen, and/or oxygen (Manoli et al., 2000). The fate of the oil spill and its impact on the ecosystem will be decided based on its properties. Occasionally, oil slicks form when light or less dense oil is spilt in surface water; the oil accumulates on top and scatters over a wide area. As a result, the oil has a greater resistance to natural dissipation processes, which prolongs its life in the environment (Irwin, 1997). There is mixture of substances that are differently soluble in water; some oil-based chemicals may partially dissolve in water, making them more bioavailable to aquatic organisms. The fish, which dwells in a contaminated environment, will continue to absorb some oil components into its body without excreting them; accordingly, the concentrations may exceed those of nearby waters by several orders of magnitude (Maskaoui & Hu, 2009).
It is estimated that from 18 to 59% of the composition of oil, gasoline, and its derivatives contain the volatile, monoaromatic hydrocarbons benzene, toluene, and xylene (BTX) (Silva et al., 2009). Plastics, rubbers, dyes, resins, insecticides, and solvents are all made using these hydrocarbons, which are readily detectable in the environment (Bolden et al., 2015). They are connected to oil or gasoline spills, industrial effluents, and atmospheric deposition in the aquatic environment (Gebara et al., 2013; Silva et al., 2009), which may threaten the quality of the water and the survival of vulnerable animals (Mendes et al., 2017).
There have been reports of some deleterious effects on aquatic organisms, including gill damage, delay in hatching, enzymatic alteration, circulation dysfunction, lipid peroxidation (LPO), DNA damage, and cortisol elevation (Achuba & Osakwe, 2003; Brown, 1993; Pacheco & Santos, 2001; Teuschler et al., 2005; Thomas et al., 1980; Vanzella et al., 2007; Zhang et al., 2003, 2004).
The immunological parameters, histopathological changes, and stress indicators are regarded as important biomarkers because xenobiotic exposure can cause significant changes in these parameters (Modesto & Martinez, 2010). They can be used to evaluate fish health and identify physio-pathologies in response to various stressors (Martinez & Souza, 2002; Nussey et al., 1995) and as an indicator of immune response (Tavares-Dias & de Moraes, 2004). When an animal’s defensive systems for eliminating reactive oxygen species (ROS) are ineffective or insufficient, oxidative damage develops, which is linked to a number of disease events, including DNA damage and lipid peroxidation (LPO) (Tripathy, 2016). These injuries are viewed as crucial indicators for determining how xenobiotic-exposed organisms react (Kurutas, 2016). The recovery evaluation is crucial for effect studies in order to confirm an organism’s ability to return to equilibrium after being under stress. Studies of xenobiotic impacts can use this type of information (Hasue et al., 2013).
This research examined the toxicological response in the tropical freshwater catfish, Clarias gariepinus, which are the most widely cultivated fishes, both within and outside of its native range of tropical and subtropical environments (Adewolu et al., 2008). In order to ascertain if the harm caused by monoaromatic petroleum hydrocarbons (BTX) was reversed, oxidative stress, histological alterations, and immunological changes as biomarkers were assessed in catfish following exposure and depuration periods.
2 Materials and Methods
2.1 Fish
The fish (Clarias gariepinus, weight of 300–350 g and length of 26–29 cm) were obtained from an aquaculture farm at Assiut University and transported to the Fish Biology and Pollution Laboratory, Assiut University. The fish were parasitic-free according to American Fisheries Society, Fish Health Section (AFS-FHS, 2017). Fish were acclimated for month in glass tanks (100 cm × 70 cm × 50 cm) under physicochemical conditions of the rearing water as conductivity 260.8 mM·cm−1, pH 7.4, dissolved oxygen 6.9 mg L−1, temperature 20.5 °C, photoperiod 12:12 h light:dark. During the acclimatization period, fish were fed commercial feed (30% protein) about 3% of their body weight. Fifty percent of the water was changed daily with re-dosing.
2.2 Experimental Design
Catfish (Clarias gariepinus) was randomly distributed after adaptation period into four groups. Each group consists of 60 samples (each replicate 20 fish) and was kept in glass containers having the same volume of water (100 L). The 1st group was control (fed on the control diet in clean water) and the other three groups were treated with water mixed with BTX as exposed to benzene (0.762 ng/L), toluene (26.614 ng/L), and xylene (89.403 ng/L) for sequential 30 days. The concentration of the tested chemicals was environmentally relevant and selected in accordance with the method of Sayed et al. (2023).
After exposure, all groups remain in the water tanks without any additions except water change daily with 50% dechlorinated water; fish were fed commercial feed (30% protein) about 3% of their body weight every day for 30 days as recovery period.
Fish from each group were selected after 30 days and 30-day recovery, and ice anesthesia was administered to reduce stress during processing (Hamed et al., 2019). Blood samples were collected from the caudal vein in vacuum tubes. To obtain the serum, the blood was centrifuged undercooling for immunological parameters, stress, and antioxidant biomarkers. Liver, kidney, and spleen tissues were used for histopathological studies. Experimental setup, guidelines, and fish handling were approved by the Research and Ethical Committee of the Molecular Biology Research & Studies Institute, Assiut University, Assuit, Egypt (MB-21–27-R).
2.3 Stress and Antioxidant Parameters, Lipid Peroxidation, and Immunological Parameters
Stanbio kits were used to assay serum acetylcholinesterase (AchE) in accordance with the procedure specified by Knedel and Böttger (1967). According to Foster and Dunn, cortisol levels were tested using ELISA (1974). The glutathione S-transferase activity was measured, according to Habig et al. (1974). According to Nishikimi et al. (1972), superoxide dismutase (SOD) was tested. Utilizing kits, total antioxidant capacity (TAC) was determined (Sigma-Aldrich, USA). Malondialdehyde (MDA) was measured using a thiobarbituric acid reaction (Ohkawa et al., 1979). Cytokines IL-1 and IL-6 were quantified according to Wang and Secombes (2009) and Hanington and Belosevic (2007), respectively, by ELISA kits (Human Ultrasensitive, Biosource International Inc.).
2.4 Histopathological Examination
Tissue samples (liver, kidney, and spleen) were dissected anatomically from each fish and washed by neutral saline. Then each tissue specimen was fixed in neutral-buffered formaldehyde, ethanol-dehydrated, cleared in methyl benzoate, wax-embedded, and 5 µ-sectioned (Horobin, 2019). Slides were dewaxed, rehydrated, and then stained with hematoxylin and eosin (H&E) (Feldman & Wolfe, 2014). The histopathological characteristics were examined under a microscope and photographed using an Olympus CH30 microscope (Horobin, 2019). Severity of pathology was evaluated according to Meydan et al.(2019).
2.5 Statistical Analysis
The SPSS software was used to analyze the data, and 0.05 was chosen as the level of significance. Then, the data was checked for normalcy (Shapiro–Wilk test). After the one-way analysis of variance, the homogeneity of variances was examined (Levene’s test) (ANOVA). In the event of variance equality, Fisher’s LSD post hoc test was employed to compare the treatment groups to the control group. In situations of variance inequality, the treated groups were compared to the control group using Dunnett’s post hoc test.
3 Results
3.1 Stress Indicators
In both the exposure and depuration periods, the level of acetyl cholinesterase significantly reduced (p < 0.05) in the benzene group only compared to the control (Table 1). In contrast, during the exposure period, cortisol levels significantly rose (p < 0.05) in the benzene group only compared to the control (Table 1).
3.2 Antioxidant Parameters and Lipid Peroxidation
In both the exposure and depuration periods, there was no significant difference (p > 0.05) in the activity of glutathione-S-transferase (GST) between any experimental groups and its corresponding control group (Table 1). When compared to the control, only in the benzene groups, superoxide dismutase (SOD) and total antioxidant capacity (TAC) activity significantly reduced (p < 0.05) after the exposure and recovery periods (Table 1). Malondialdehyde (MDA) levels significantly raised (p < 0.05) in the benzene group alone in comparison to the control during both the exposure and depuration phases (Table 1).
3.3 Immunological Parameters
In comparison to the control group, IL-1β and IL-6 levels significantly raised (p < 0.05) in the benzene and toluene groups after the exposure. Even after the recovery, the level of IL-6 remained significantly higher (p < 0.05) compared to control group, even though IL-1β had reverted to the regulated level (Table 1).
3.4 Histopathological Changes of Liver
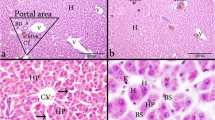
Our observed results of treated groups exhibited loss of normal cyto-architectural structure with complete degenerated hepatocytes accompanied with dilation and congestion of sinusoids which is clearly observed after xylene exposure (Fig. 1a and Table 2). Vacuolar degeneration appeared obviously after exposure and decreased significantly post-exposure to toluene (Fig. 1b and Table 2). Moderate vascular dilatations were noticed after exposure to BTX and decreased post-exposure. Multifocal cellular hepatic necrosis was clearly noticed after BTX exposure and decreased post-exposure especially after xylene and benzene accompanied with diffuse inflammatory cell infiltration (Fig. 1c and Table 2). Diffuse perivascular aggregations of melanomacrophages were recorded after BTX exposure and decreased obviously post-exposure. It was clear that congestion of hepatopancreas was noticed after benzene exposure but decreased and disappeared completely post-exposure to benzene and toluene. Heavy perivascular inflammatory cell infiltrations were observed with moderate necrotic changes of hepatocytes that decreased post-exposure (Fig. 1d and Table 2). Also, thrombus was observed clearly after recovery period to BTX especially benzene (Fig. 1e and Table 2).
Photomicrograph of catfish liver (H&E) staining after exposure and recovery periods of (BTX) showing a aggregation of melanomacrophages (MMc) and congestion of sinusoids (arrow). b Vacuolar degeneration of hepatocytes (VD). c Necrotic area with inflammatory cell infiltration (NA). d Perivascular inflammatory cell infiltration (arrow) and necrotic changes of hepatocytes (NC). e Thrombus formation inside central vein (star)
3.5 Histopathological Changes of Kidney
Kidney tissues of the catfish exposed to concentrations of benzene, toluene, and xylene displayed dilatation of Bowman’s space with severe atrophy of glomerular tuft especially after xylene exposure (Fig. 2a and Table 3). These lesions appeared to a mild degree or disappeared completely after recovery period to BTX. Diffuse dilatation of tubular lumen with hyaline cast and vacuolar degeneration of tubular epithelium were clearly observed after exposure to BTX and focally post-exposure (Fig. 2b and Table 3). Tubular epithelium was necrosed and separated from its basement membrane after BTX exposure with moderate inflammatory cell infiltrations (Fig. 2c and Table 3). Diffuse necrosis of hemobiotic tissue with melanomacrophage aggregation appeared obviously after exposure and decreased significantly post-exposure to toluene and xylene (Fig. 2d and Table 3). Focal perivascular inflammatory cellular reaction was observed. Severe degeneration of blood vessels wall with perivascular hemorrhages was clearly noticed after BTX and post-exposure to benzene only (Fig. 2e and Table 3). Also, thrombus was observed obviously post-exposure to benzene only (Fig. 2f and Table 3).
Photomicrograph of catfish kidney (H&E) staining after exposure and recovery periods of (BTX) showing a and b dilatation of Bowman’s space with atrophy of glomerular tuft (GA). c Necrosis of tubular epithelium (circle). d Multifocal area of hemobiotic tissue necrosis (HN), melanomacrophages (MMC), and mononuclear cell infiltrations (arrow). e Thrombus (star) and perivascular hemorrhage (H). f Thrombus (star) in blood vessels and necrosis of tubular epithelium (arrow) with aggregation of melanomacrophages (MMc)
3.6 Histopathological Changes of Spleen
Our results clearly showed mild congestion of red pulp of spleen that was observed after exposure to benzene, toluene, and xylene, but increased post-exposure to BTX (Fig. 3a). There is severe depletion of lymphocytes or necrosis in white pulp after exposure to xylene and post-exposure to benzene, and in mild degree after exposure and post-exposure to toluene (Fig. 3b and Table 4). Our observation also includes marked activation of melanomacrophage centers of spleen observed in a high degree after exposure to BTX and after recovery; high density of hemosedrine pigment was clearly observed after recovery of xylene. Blood vessels of spleen were congested clearly in all groups with inflammatory cell infiltration especially in case of benzene exposure (Fig. 3c and Table 4). Congestion of blood vessels with thickening of its wall due to hypertrophy of tunica media observed obviously post-exposure to benzene and xylene (Fig. 3d and Table 4). Also, degeneration of blood vessel wall was clearly observed after toluene and xylene (Fig. 3e and Table 4). Thrombosis appeared obviously after recovery of benzene, but in mild degree after recovery of xylene (Fig. 3f and Table 4).
Photomicrograph of catfish spleen (H&E) staining after exposure and recovery of (BTX) showing a lymphoid exhaustion of white pulp (LE) with depletion of lymphocytes (arrow) and severe congestion of red pulp (RC). b Severe necrosis in white pulp (star). c Blood vessels were congested, multiple activation of melanomacrophage centers (MMC). d Thickening of blood vessels wall (double head arrow) with perivascular inflammatory cell infiltration. e Congestion of blood vessels (star) with degeneration of its wall (arrow). f Thrombosis (TH), congestion of red pulp (RC), lymphoid necrosis in white pulp (LN), and thickening of blood vessels wall (arrow)
4 Discussion
Water pollution is the most serious problem facing the community of the ecosystem (Inyinbor Adejumoke et al., 2018). There are three monocyclic aromatic hydrocarbons in the world: benzene, toluene, and xylene. They are used in household and industrial applications in both their individual and mixed forms. Cortisol levels significantly rose in the benzene group only compared to the control group during the exposure period. Our results are in line with those of Alkindi et al. (1996), who reported that Pleuronectes flesus exposed to a water-soluble portion of crude oil had a significantly higher plasma cortisol level. Accordingly, Stephens et al. (1997) found that turbot larvae exposed to the water-soluble fraction (WSF) of crude oil had considerably higher whole-body cortisol concentration. Also, our cortisol findings are consistent with research by Simonato et al. (2013) that discovered neotropical freshwater fish (Prochilodus lineatus) exposed to WSFG for 96 h had significantly higher plasma cortisol levels. Likewise, Reddam et al. (2017) found a significant increase in cortisol level in response to exposing Gulf toadfish (Opsanus beta) to PAHs. Those results were inconsistent with certain earlier results such as the investigation on turbot where Stephens et al. (2000) discovered no appreciable rise in the fish’s total body cortisol after 6 weeks of chronic exposure to 100,000 and 10,000 diluted produced waters (PW). Also, after 6 days of exposure to gasoline water-soluble fraction (GWSF) and diesel oil water-soluble fraction (DWSF), plasma cortisol levels in European eels (Anguilla anguilla L.) considerably decreased (Pacheco & Santos, 2001). Intermittent exposure of juvenile Atlantic cod to 100 ppm and 200 ppm PW did not negatively affect their development or cause stress, according to Pérez-Casanova et al. (2010). It has been proposed in the past that persistent exposure to pollutants may cause the ACTH receptors to become desensitized or downregulated, which would inhibit the cortisol response to a specific stressor (Gesto et al., 2008). Additionally, it has been hypothesized that corticosteroid elimination under prolonged stress may rise with exposure time (Barton et al., 1986).
In both the exposure and depuration periods, the level of acetyl cholinesterase substantially reduced in the benzene group only compared to the control group. Additionally, Akaishi et al. (2004) found that the WSF of crude oil decreased the AChE activity utilizing Astyanax altiparanae. The metals in fuel oil and other petrochemical combinations may be a factor in these products’ anticholinesterasic effects according to Vieira et al. (2008). Additionally, Esteban-Sánchez et al. (2021) found that both brief and prolonged exposures to chemically dispersed crude oil resulted in a considerable AChE inhibition. On the other hand, Anwar (2021) discovered that exposing fish to WSF of crude oil considerably enhanced the level of acetylcholinesterase (AChE), which is in contradiction to the results of our study. Additionally, WSFG 1.5% had no effect on A. altiparanae brain or muscle AChE activity (Bettim et al., 2016).
Glutathione-S-transferase (GST) activity did not differ between experimental and control groups throughout the exposure and depuration periods in this study. Similar outcomes were discovered by Sun et al. (2006) for Carassius auratus after exposure to phenantrene (0.05 mg L−1) and a depuration period. Compared to controls, crude oil, benzene, toluene, and xylene dramatically reduced GST activity in the gills and livers of fish (Clarias gariepinus) (Otitoloju & Olagoke, 2011). Delunardo et al. (2015) found that fish (Hippocampus reidi) exposed for 96 h to various crude oil concentrations had lower glutathione-S-transferase levels. Conversion of PAHs in crude oil has been problematic due to an inability to effectively conjugate them via the glutathione pathway or alternate pathways, such as UDP-GT and sulfotransferase, which indicated by the inhibition of GST activity (Silva et al., 2013). As reduced glutathione, or GSH which can conjugate electrophilic intermediates with substrates under GST’s catalytic activity, another reason for our results is that PAH chemicals hindered this enzyme (Habig et al., 1974). During the decay of contamination (DC) group, hepatic A. altiparanae GST activity decreased below the respective controls, whereas the activity reverted back to equal to the respective controls in the water-soluble fraction of gasoline (WSFG) 96 h (Bettim et al., 2016). A significant reduction in SOD and TAC activity was observed only in the benzene group compared to the control group during exposure and depuration. Similarly, Yan and Zhou (2011) discovered that at 50.0 and 100 mg L−1 toluene and ethylbenzene, SOD activity reduced in comparison to that in the control. It shows that greater levels of toluene and ethylbenzene caused more O2 to be created, resulting in oxidative stress, and that too much O2 might cause the SOD to become inactive. Also, with the exception of 50.0 mg L−1, all doses of SOD in Hydrilla verticillata reduced its activity. One rationale was that xylene, as opposed to toluene and ethylbenzene, induced more severe oxidative damage to SOD. To protect against oxidative damage in such a situation, O2 may be lowered by alternative antioxidant defense mechanisms, such as nonenzymatic antioxidants such ascorbate and glutathione (Fatima and Ahmad, 2005). Additionally, fish exposed to all of the hydrocarbon chemicals studied had decreased SOD activity in their liver and gill tissues (Otitoloju & Olagoke, 2011). In contrast, SOD increased in Cyprinus carpio and Hyphothalmichtys molitrix exposed to oil fraction (Deér et al., 2010). Anwar (2021) stated that there was no induction of the biotransformation enzymes, and it is likely that this ROS creation was not the consequence of metabolic processes.
As soon as 0.05 mg L−1 of WSF of diesel oil was added to C. auratus, the SOD activity increased after 4 days, then progressively declined, and the activity was comparable with that of the control group after 15 days. It was only after depuration that the enzyme activity recovered. The kidney demonstrated greater SOD activity in the WSFG 96 h and DC groups compared to the corresponding control groups, supporting the idea that the WSFG 1.5% causes the generation of ROS (Zhang et al., 2004). Additionally, during depuration intervals, SOD activity restored to normal levels. Similar to this, the production of ROS by WSFG 5% boosted the activity of antioxidant enzymes in P. lineatus, and greater SOD activity was also seen for cells exposed to other WSF (Nogueira et al., 2011; Zhang et al., 2004). According to Neuparth et al. (2014), a significant rise in LPO levels was discovered in male’s Gammarus locusta exposed to greater p-xylene concentrations, indicating the production of ROS.
This outcome is consistent with research by Achuba and Osakwe (2003) and Avci et al. (2005), which found that fish tissues exposed to petroleum hydrocarbons had higher levels of LPO. Furthermore, Hatlen et al. (2009) found that after 36 days of exposure to the water-soluble component of crude oil, the levels of malondialdehyde (MDA) in the tissue of Arctic sea ice (the amphipod Gammarus wilkitzkii) considerably rose. Similar research was conducted by Kayode et al. (2014), who found that the liver of Clarias gariepinus exposed to Nigerian crude oil had considerably higher levels of MDA. The Nile tilapia (Oreochromis niloticus) liver MDA activities were dramatically elevated by exposing fish to sublethal amounts of crude oil for 96 h, according to Gad (2011), who discovered a similar pattern in our work. Similar to this found that goldfish (Carassius auratus) exposed to crude oil-contaminated soil had considerably higher MDA and superoxide dismutase SOD activity in their liver tissues. Additionally, Wegwu and Omeodu (2010) noted that exposure to an aqueous extract of Nigerian crude oil dramatically raised the MDA in the liver of Clarias gariepinus. The most toxic test compound in this study was benzene, which was then followed by toluene and xylene.
The level of IL-6 remained high even though IL-1β had reverted to the regulated level after the depuration phase. Furthermore, juvenile Atlantic cod (Gadus morhua L.) exposed to generated water showed a considerable up-regulation of interleukins-1b and 8 (Pérez-Casanova et al., 2010). Similarly, heavy oil exposure elevated the expression of IL-8 in juvenile Japanese flounder (Paralichthys olivaceus) (Nakayama et al., 2008). This increase in the interleukins was primarily generated by blood monocytes and tissue macrophages as a response to tissue damage, illness, and other immunological responses (Dinarello, 1997; Kany et al., 2019).
Histopathology gives helpful information for the mechanisms of injuries (Khoshnood et al., 2010). Liver is the major detoxification organ which can metabolize or accumulate greater concentrations of toxicants in it, causing morphological and physiological alterations. A particular toxicant’s toxic potential determines how severe the damage is (Au, 2004). Kulkarni and Kaware (2013) also suggested that during exposure to toxicants, morphological changes in liver tissues through rapid metabolism and excretion lead to possible irreversible damages, to make animal rids themselves of toxic compounds in their liver rapidly. Current liver histopathological findings confirm the mentioned fact, where catfish exposed to and post-exposed to BTX revealed loss of normal cytoarchitectural structure with complete degenerated hepatocytes accompanied with thickening of central vein and congestion of sinusoids, thrombosis, and multifocal hepatic necrosis accompanied with diffuse inflammatory cell infiltration. One of the largest lymphoid and immunocompetent organs in fish is the head kidney (Zapata et al., 2006). In teleost fish, these two organs typically undergo physiological and structural changes under environmental stress (Xu et al., 2018). In our study catfish suffered from renal dysfunctions which were in the form of cloudy swelling and vacuolar degeneration of tubular epithelium, hemopoietic necrosis, thrombus formation, and interstitial inflammatory cell infiltration after 15 days of exposure. Our observation includes severe lymphocytic necrosis and marked activation of melanomacrophage centers of spleen observed in a high degree after exposure and post-exposure to BTX. Kaewamatawong et al. (2013) reported similar alterations in kidney, spleen, and liver after exposure of O. niloticus to mercury. Our observed results are closely related to the results of Ayanda et al. (2015) that examine the effect of glyphosate and paraquat, on liver of Clarias gariepinus juveniles for 8 weeks and of Meydan et al. (2019) who observed that after 15 days of toluene injection in albino rats there were sinusoid dilation, hemorrhage, vacuolization, and necrosis. Clearly, toluene exhibits hepatotoxic effects. Evidence of liver damage was reported by Persis and Kalaiarasi (2001) in freshwater catfish exposed to an organophosphate pesticide which is closely related to our observed results. Elias et al. (2018) reported the effects of CITRON®, 50EC on the African catfish (Clarias gariepinus) for 3, 9, and 15 days that showed vacuolar degeneration, necrobiotic changes in hepatocytes, dissociated hepatocytes, and few melanomacrophage cell infiltration in the portal area, which were similar to our results. Also, like our results, Li et al. (2015) cleared structural and functional impairment of various organs after exposure to toluene and renal dysfunction in workers exposed to a mixture of toluene and xylene (Akgül et al., 2011). Roy and Bhattacharya (2006) also reported similar results in arsenic-intoxicated fish. Translocation of toluene metabolites from the liver to the kidney via general circulation produces kidney injury. Ortiz et al. (2003) showed tubular necrosis and vacuolization of renal tubular epithelium of fishes exposed to linden. Kronevi et al. (1979) showed that epicutaneous administration of toluene in guinea pigs caused histopathological damage in kidney. Wei et al. (2021) reported pathological damage to the mouse bone marrow, thymus, and spleen after exposure to benzene. As a result of these studies, benzene was shown to have significant immune-toxic properties that caused direct damage to immune organs and suppressed their functions physiologically. Ahmad et al. (2003) described high levels of hepatic vacuolation as a sign of degeneration which indicates metabolic damage. Underwood (1992) reported that hydropic degeneration characterized by cell swelling after hypoxia, chemical poisoning, and contaminated water exposure. Uy et al. (2017) reported that intimal thickening and medial hypertrophy of splenic blood vessels formed as a result of Splenic Vein Aneurysm (SVA) that leads to elevated portal pressure and subsequent fibrosis and aneurysm formation (Ma et al., 2012; Torres et al., 1999), whereas thrombosis occurs after compression of the vessel (Shimoda et al., 2003; Tolgonay et al., 1998).
Cytologically, melanomacrophage centers (MMCs) are so commonly increased at sites of tissue injury (Agius, 1985; Stoskopf, 1993). Diffuse activation of melanomacrophage centers appeared deep in color and increased in its number after exposure to BTX and turned to bale coloration and its number decreased obviously post-exposure to BTX. MMCs were most abundant in spleen and kidney, and less common in liver tissue; hepatic MMCs were closely embedded in the wall of blood vessels or in necrotic areas. These results are in agreement with the results of others (Dang et al., 2019; Sayed & Younes, 2017; Sayed et al., 2019; Suresh, 2009).
Increasing MMCs were linked to histopathological alterations, suggesting that oxidative stress causes lymphocytes to aggregate, which indicates an immune response (Kranz, 1989). Researchers recorded similar findings in other studies, which were in agreement with the findings of the present study (Agius, 1985; Stoskopf, 1993). Wolke et al. (1985) observed that increases in the numbers and size of MMCs are cleared after environmental pollution or environmental stress (Micale & Perdichizzi, 1990).
This outcome is consistent with Irwing (1971) research, which claimed that benzene’s water-soluble component was more harmful than other monocyclic aromatic chemicals. Additionally, when tested against C. gariepinus, benzene was discovered to be the least dangerous test substance, followed by toluene, xylene, and crude oil (Otitoloju & Olagoke, 2011).
5 Conclusion
The monocyclic aromatic chemicals (BTX) were more hazardous to fish than crude oil. The toxicity level was as benzene > xylene > toluene even though after recovery period. Benzene is more stable and has a longer lifespan than toluene and xylene; it is more easily accessible to the exposed animals compared to the more volatile portions. The recovery period made an improvement in most of the oxidative and immunological biomarkers but still not as control.
Data availability
All data generated or analyzed during this study are included in the research article.
References
Achuba, F. I., & Osakwe, S. A. (2003). Petroleum-induced free radical toxicity in African catfish (Clarias gariepinus). Fish Physiology and Biochemistry, 29, 97–103.
Adewolu, M., Ogunsanmi, A. O., & Yunusa, A. (2008). Studies on growth performance and feed utilization of two Clariid catfish and their hybrid reared under different culture systems. European Journal of Scientific Research, 23, 252–260.
AFS-FHS. (2003). Suggested procedures for the detection and identification of certain finfish and shellfish pathogens. Bethesda, Maryland, USA: American Fisheries Society.
Agius, C. (1985). The melano-macrophage centres of fish: A review. In M. J. Manning & M. F. Tatner (Eds.), Fish Immunology (pp. 85–105). Academic Press.
Ahmad, I., Pacheco, M., & Santos, M. A. (2003). Naphthalene-induced differential tissue damage association with circulating fish phagocyte induction. Ecotoxicology and Environmental Safety, 54, 7–15.
Akaishi, F. M., de Assis, H. C., Jakobi, S. C., Eiras-Stofella, D. R., St-Jean, S. D., Courtenay, S. C., Lima, E. F., Wagener, A. L., Scofield, A. L., & Ribeiro, C. A. (2004). Morphological and neurotoxicological findings in tropical freshwater fish (Astyanax sp.) after waterborne and acute exposure to water soluble fraction (WSF) of crude oil. Archives of Environmental Contamination and Toxicology, 46, 244–253.
Akgül, T., Huri, E., Yagmurdur, H., Ayyıldız, A., Ustün, H., & Germiyanoğlu, C. (2011). Phosphodiesterase 5 inhibitors attenuate renal tubular apoptosis after partial unilateral ureteral obstruction: An experimental study. Kaohsiung Journal of Medical Sciences, 27, 15–19.
Alkindi, A. Y. A., Brown, J. A., Waring, C. P., & Collins, J. E. (1996). Endocrine, osmoregulatory, respiratory and haematological parameters in flounder exposed to the water soluble fraction of crude oil. Journal of Fish Biology, 49, 1291–1305.
Anwar, A. Y. (2021). Dietary antioxidant supplementation ameliorates the physiological toxicity induced by kurdistan crude oil in common carp Cyprinus carpio. University of Plymouth–UK, 2013.
Au, D. W. (2004). The application of histo-cytopathological biomarkers in marine pollution monitoring: A review. Marine Pollution Bulletin, 48, 817–834.
Avci, A., Kaçmaz, M., & Durak, I. (2005). Peroxidation in muscle and liver tissues from fish in a contaminated river due to a petroleum refinery industry. Ecotoxicology and Environmental Safety, 60, 101–105.
Ayanda, O. I., Oniye, S. J., Auta, J., & Ajibola, V. O. (2015). Acute toxicity of glyphosate and paraquat to the African catfish (Clarias gariepinus, Teugels 1986) using some biochemical indicators. Tropical Zoology, 28, 152–162.
Barton, B. A., Schreck, C. B., & Barton, L. D. (1987). Effects of chronic cortisol administration and daily acute stress on growth, physiological conditions, and stress responses in juvenile rainbow trout. Diseases of Aquatic Organisms, 2(3), 173–185.
Bettim, F. L., Galvan, G. L., Cestari, M. M., Yamamoto, C. I., & de Assis, H. C. S. (2016). Biochemical responses in freshwater fish after exposure to water-soluble fraction of gasoline. Chemosphere, 144, 1467–1474.
Bolden, A. L., Kwiatkowski, C. F., & Colborn, T. (2015). New look at BTEX: Are ambient levels a problem? Environmental Science & Technology, 49(9), 5261–5276.
Brown, J. A. (1993). Endocrine responses to environmental pollutants. In J. C. Rankin & F. B. Jensen (Eds.), Fish Ecophysiology (pp. 276–296). Springer.
Dang, M., Nowell, C., Nguyen, T., Bach, L., Sonne, C., Nørregaard, R., Stride, M., & Nowak, B. (2019). Characterisation and 3D structure of melanomacrophage centers in shorthorn sculpins (Myoxocephalus scorpius). Tissue and Cell, 57, 34–41.
Deér, A. K., Henczová, M., Banka, L., Varanka, Z., & Nemcsók, J. (2010). Effects of crude oil and oil fractions on the liver P450-dependent monooxygenase activities and antioxidant defence system of different freshwater fish species. Acta Biologica Hungarica, 61, 262–273.
Delunardo, F. A., de Carvalho, L. R., da Silva, B. F., Galão, M., Val, A. L., & Chippari-Gomes, A. R. (2015). Seahorse (Hippocampus reidi) as a bioindicator of crude oil exposure. Ecotoxicology and Environmental Safety, 117, 28–33.
Dinarello, C. A. (1997). Interleukin-1. Cytokine & Growth Factor Reviews, 8, 253–265.
Elias, M., Mudege, N., Lopez, D. E., Najjar, D., Kandiwa, V., Luis, J., Yila, J., Tegbaru, A., Ibrahim, G., Badstue, L., Njuguna-Mungai, E., & Bentaibi, A. (2018). Gendered aspirations and occupations among rural youth, in agriculture and beyond: A cross-regional perspective. Journal of Gender, Agriculture and Food Security, 3, 82–107.
El-Sheekh, M. M., El-Naggar, A. H., Osman, M. E. H., & Haieder, A. (2000). Comparative studies on the green algae Chlorella homosphaera and Chlorella vulgaris with respect to oil pollution in the River Nile. Water, Air, and Soil Pollution, 124, 187–204.
Esteban-Sánchez, A., Johann, S., Bilbao, D., Prieto, A., Hollert, H., Seiler, T.-B., & Orbea, A. (2021). Multilevel responses of adult zebrafish to crude and chemically dispersed oil exposure. Environmental Sciences Europe, 33, 106.
Ewida, A. (2014). Oil spills: Impact on water quality and microbial community of the Nile River. Egypt. International Journal of Environment, 3, 192–198.
Feldman, A. T., & Wolfe, D. (2014). Tissue processing and hematoxylin and eosin staining. In C. E. Day (Ed.), Histopathology: Methods and Protocols (pp. 31–43). Springer.
Gad, N. S. (2011). Oxidative stress and antioxidant enzymes in Oreochromis niloticus as biomarkers of exposure to crude oil pollution. International Journal of Environmental Science and Engineering, 1, 49–58.
Gebara, S. S., Re-Poppi, N., Nascimento, A. L. C. S., & Raposo Junior, J. L. (2013). Methods for analysis of PAH and BTEX in groundwater from gas stations: A case study in Campo Grande, MS, Brazil. Quimica Nova, 36, 1030–1037.
Gesto, M., Soengas, J. L., & Míguez, J. M. (2008). Acute and prolonged stress responses of brain monoaminergic activity and plasma cortisol levels in rainbow trout are modified by PAHs (naphthalene, β-naphthoflavone and benzo(a)pyrene) treatment. Aquatic Toxicology, 86, 341–351.
Habig, W. H., Pabst, M. J., & Jakoby, W. B. (1974). Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry, 249, 7130–7139.
Hamed, M., Soliman, H. A. M., Osman, A. G. M., & Sayed, A.E.-D.H. (2019). Assessment the effect of exposure to microplastics in Nile tilapia (Oreochromis niloticus) early juvenile: I. Blood Biomarkers. Chemosphere, 228, 345–350.
Hanington, P. C., & Belosevic, M. (2007). Interleukin-6 family cytokine M17 induces differentiation and nitric oxide response of goldfish (Carassius auratus L.) macrophages. Developmental and Comparative Immunology, 31, 817–829.
Hasue, F., Passos, M., Santos, T., Rocha, A., Vignardi, C., Sartorio, P., Gomes, V., & Ngan, P. (2013). Assessment of genotoxicity and depuration of anthracene in the juvenile coastal fish Trachinotus carolinus using the comet assay. Brazilian Journal of Oceanography, 61, 215–222.
Hatlen, K., Camus, L., Berge, J., Olsen, G. H., & Baussant, T. (2009). Biological effects of water soluble fraction of crude oil on the Arctic sea ice amphipod Gammarus wilkitzkii. Chemistry and Ecology, 25, 151–162.
Horobin, R. W. (2018). Theory of histological staining. Bancroft’s theory and practice of histological techniques (8th ed. E-Book, pp. 114–123). China: Elsevier Health Sciences.
Inyinbor Adejumoke, A., Adebesin Babatunde, O., Oluyori Abimbola, P., Adelani Akande Tabitha, A., Dada Adewumi, O., & Oreofe Toyin, A. (2018). Water pollution: Effects, prevention, and climatic impact. Water Challenges of an Urbanizing World, 33, 33–47.
Irwin, R. J., Specialists, S. A. C., Van Mouwerik, M. A. R. K., Stevens, L., Seese, M. D., & Basham, W. (1997). Environmental contaminants encyclopedia: Oil spills entry. National Park Service-Water Resources Divisions, Water Operations Branch.
Irwing, R. J. (1971). Environmental contaminants encyclopedia entry for BTEX and BTEX compounds (p. 36). Colorado: National Park Service.
Kaewamatawong, T., Rattanapinyopituk, K., Ponpornpisit, A., Pirarat, N., Ruangwises, S., & Rungsipipat, A. (2013). Short-term exposure of Nile tilapia (Oreochromis niloticus) to mercury: Histopathological changes, mercury bioaccumulation, and protective role of metallothioneins in different exposure routes. Toxicologic Pathology, 41, 470–479.
Kany, S., Vollrath, J. T., & Relja, B. (2019). Cytokines in inflammatory disease. International Journal of Molecular Sciences, 20(23), 6008.
Kayode, S. J., Chidimma, U. I., & Alwell, E. E. (2014). Response of some antioxidant parameters in post juveniles of Clarias gariepinus after exposure to Nigerian crude oil (Forcados, Bonny Light and Qua-Iboe). Pakistan Journal of Biological Sciences, 17, 1225–1230.
Khoshnood, Z., Mokhlesi, A., & Khoshnood, R. (2010). Bioaccumulation of some heavy metals and histopathological alterations in liver of Euryglossa orientalis and Psettodes erumei along North Coast of the Persian Gulf. African Journal of Biotechnology, 9, 6966–6972.
Knedel, M., & Böttger, R. (1967). A kinetic method for determination of the activity of pseudocholinesterase (acylcholine acyl-hydrolase 3.1.1.8.). Klinische Wochenschrift, 45, 325–327.
Kranz, H. (1989). Changes in splenic melano-macrophage centres of dab Limanda limanda during and after infection with ulcer disease. Diseases of Aquatic Organisms, 6(3), 167–173.
Kronevi, T., Wahlberg, J., & Holmberg, B. (1979). Histopathology of skin, liver, and kidney after epicutaneous administration of five industrial solvents to guinea pigs. Environmental Research, 19, 56–69.
Kulkarni, S. J., & Kaware, J. P. (2013). Review on research for removal of phenol from wastewater. International Journal of Scientific and Research Publications, 3, 1–5.
Kurutas, E. B. (2016). The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutrition Journal, 15, 016–0186.
Li, T., Hasegawa, T., Yin, X., Zhu, Y., Boote, K., Adam, M., Bregaglio, S., Buis, S., Confalonieri, R., Fumoto, T., Gaydon, D., Marcaida III, M., Nakagawa, H., Oriol, P., Ruane, A.C., Ruget, F., Singh, B.-., Singh, U., Tang, L., Tao, F., Wilkens, P., Yoshida, H., Zhang, Z., Bouman, B., 2015. Uncertainties in predicting rice yield by current crop models under a wide range of climatic conditions. 21, 1328–1341.
Ma, R., Levard, C., Marinakos, S. M., Cheng, Y., Liu, J., Michel, F. M., Brown, G. E., Jr., & Lowry, G. V. (2012). Size-controlled dissolution of organic-coated silver nanoparticles. Environmental Science & Technology, 46, 752–759.
Manoli, E., Samara, C., Konstantinou, I., & Albanis, T. (2000). Polycyclic aromatic hydrocarbons in the bulk precipitation and surface waters of Northern Greece. Chemosphere, 41, 1845–1855.
Martinez, C. B., & Souza, M. M. (2002). Acute effects of nitrite on ion regulation in two neotropical fish species. Comparative Biochemistry and Physiology Part a: Molecular & Integrative Physiology, 133, 151–160.
Maskaoui, K., & Hu, Z. (2009). Contamination and ecotoxicology risks of polycyclic aromatic hydrocarbons in Shantou coastal waters, China. Bulletin of Environment Contamination and Toxicology, 82, 172–178.
Mendes, M. P., Salomão, A. L. S., Niemeyer, J. C., & Marques, M. (2017). Ecological risk assessment in a tropical wetland contaminated with gasoline: Tier 1. Human and Ecological Risk Assessment: An International Journal, 23, 992–1007.
Meydan, S., Esrefoglu, M., Selek, S., Akbas Tosunoglu, E., Ozturk, O., Kurbetli, N., Bayındır, N., Bulut, H., & Meral, I. (2019). Protective effects of caffeic acid phenethyl ester and thymoquinone on toluene induced liver toxicity. Biotechnic & Histochemistry, 94, 277–282.
Micale, V., & Perdichizzi, F. (1990). A quantitative and histochemical study on melano-macrophage centres in the spleen of the teleost fish Diplodus annularis L. Journal of Fish Biology, 37, 191–197.
Modesto, K. A., & Martinez, C. B. (2010). Effects of Roundup Transorb on fish: Hematology, antioxidant defenses and acetylcholinesterase activity. Chemosphere, 81, 781–787.
Moursy, A. S. (1983). Oil pollution studies in the Nile River. I. Survey of oil and grease in Nile water. Environment International, 9, 107–111.
Moustafa, Y. M., & Shara, S. I. (2009). Studies of seasonal variations on polynuclear aromatic hydrocarbons along the Nile River, Egypt. Journal of Applied Sciences Research, 5, 2349–2356.
Nakayama, K., Kitamura, S., Murakami, Y., Song, J. Y., Jung, S. J., Oh, M. J., Iwata, H., & Tanabe, S. (2008). Toxicogenomic analysis of immune system-related genes in Japanese flounder (Paralichthys olivaceus) exposed to heavy oil. Marine Pollution Bulletin, 57, 445–452.
Neuparth, T., Capela, R., Pereira, S. P., Moreira, S. M., Santos, M. M., & Reis-Henriques, M. A. (2014). Toxicity effects of hazardous and noxious substances (HNS) to marine organisms: Acute and chronic toxicity of p-xylene to the amphipod Gammarus locusta. Journal of Toxicology and Environmental Health. Part A, 77, 1210–1221.
Nishikimi, M., Rao, N. A., & Yagi, K. (1972). The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochemical and Biophysical Research Communications, 46, 849–854.
Nogueira, L., Rodrigues, A. C., Trídico, C. P., Fossa, C. E., & de Almeida, E. A. (2011). Oxidative stress in Nile tilapia (Oreochromis niloticus) and armored catfish (Pterygoplichthys anisitsi) exposed to diesel oil. Environmental Monitoring and Assessment, 180, 243–255.
Nussey, G., Van Vuren, J. H. J., & du Preez, H. H. (1995). Effect of copper on the haematology and osmoregulation of the Mozambique tilapia, Oreochromis mossambicus (Cichlidae). Comparative Biochemistry and Physiology Part c: Pharmacology, Toxicology and Endocrinology, 111, 369–380.
Ohkawa, H., Ohishi, N., & Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 95, 351–358.
Ortiz, I., Revah, S., & Auria, R. (2003). Effects of packing material on the biofiltration of benzene, toluene and xylene vapours. Environmental Technology, 24, 265–275.
Otitoloju, A., & Olagoke, O. (2011). Lipid peroxidation and antioxidant defense enzymes in Clarias gariepinus as useful biomarkers for monitoring exposure to polycyclic aromatic hydrocarbons. Environmental Monitoring and Assessment, 182, 205–213.
Pacheco, M., & Santos, M. A. (2001). Biotransformation, endocrine, and genetic responses of Anguilla anguilla L. to petroleum distillate products and environmentally contaminated waters. Ecotoxicology and Environmental Safety, 49, 64–75.
Pérez-Casanova, J. C., Hamoutene, D., Samuelson, S., Burt, K., King, T. L., & Lee, K. (2010). The immune response of juvenile Atlantic cod (Gadus morhua L.) to chronic exposure to produced water. Marine Environment Research, 70, 26–34.
Persis, V. T., & Kalaiarasi, J. M. V. (2001). Histopathological responses of Mystus vittatus to chronic sublethal and acute lethal toxicity of an organophosphate pesticide. J Exp Zool India, 4, 103–108.
Reddam, A., Mager, E. M., Grosell, M., & McDonald, M. D. (2017). The impact of acute PAH exposure on the toadfish glucocorticoid stress response. Aquatic Toxicology, 192, 89–96.
Roy, S., & Bhattacharya, S. (2006). Arsenic-induced histopathology and synthesis of stress proteins in liver and kidney of Channa punctatus. Ecotoxicology and Environmental Safety, 65, 218–229.
Sayed, A. H., & Younes, H. A. M. (2017). Melanomacrophage centers in Clarias gariepinus as an immunological biomarker for toxicity of silver nanoparticles. Journal of Microscopy and Ultrastructure, 5, 97–104.
Sayed, A.E.-D.H., Abd-Elkareem, M., & Abou Khalil, N. S. (2019). Immunotoxic effects of 4-nonylphenol on Clarias gariepinus: Cytopathological changes in hepatic melanomacrophages. Aquatic Toxicology, 207, 83–90.
Sayed, A. E. D. H., Idriss, S. K., Abdel-Ghaffar, SKh., & Hussein, A. A. A. (2023). Haemato-biochemical mutagenic and histopathological changes in Oreochromis niloticus exposed to BTX. Environmental Science Pollution Research, 30(21), 59301–59315. https://doi.org/10.1007/s11356-023-26604-2
Shimoda, M., Kubota, K., Sakuma, A., Hogami, T., Yamaguchi, H., & Tagaya, N. (2003). Intra-abdominal hemorrhage due to rupture of a splenic vein aneurysm: A case report. Journal of Gastrointestinal Surgery, 7, 683–686.
Silva, F. L. D. N., Santos Jr, J. R. D., Moita Neto, J. M., Silva, R. L. G., Flumignan, D. L., & Oliveira, J. E. D. (2009). Determination of benzene, toluene, ethylbenzene and xylenes in commercial gasoline from Piaui state. Quimica Nova, 32, 56–60.
Silva, C., Oliveira, C., Gravato, C., & Almeida, J. R. (2013). Behaviour and biomarkers as tools to assess the acute toxicity of benzo(a)pyrene in the common prawn Palaemon serratus. Marine Environment Research, 90, 39–46.
Simonato, J., Fernandes, M., & Martinez, C. (2013). Physiological effects of gasoline on the freshwater fish Prochilodus lineatus (Characiformes: Prochilodontidae). Neotropical Ichthyology, 11, 683–691.
Stephens, S. M., Alkindi, A. Y. A., Waring, C. P., & Brown, J. A. (1997). Corticosteroid and thyroid responses of larval and juvenile turbot exposed to the water-soluble fraction of crude oil. Journal of Fish Biology, 50, 953–964.
Stephens, S. M., Frankling, S. C., Stagg, R. M., & Brown, J. A. (2000). Sub-lethal effects of exposure of juvenile turbot to oil produced water. Marine Pollution Bulletin, 40, 928–937.
Stoskopf, M. K. (1993). Fish medicine. W.B. Saunders Co.
Sun, Y., Yu, H., Zhang, J., Yin, Y., Shi, H., & Wang, X. (2006). Bioaccumulation, depuration and oxidative stress in fish Carassius auratus under phenanthrene exposure. Chemosphere, 63, 1319–1327.
Suresh, N. (2009). Effect of cadmium chloride on liver, spleen and kidney melano macrophage centres in Tilapia mossambica. Journal of Environmental Biology, 30, 505–508.
Tavares, M., & Moraes, F. (2004). Hematologia de peixes teleósteos. Ribeirão Preto: Villimpress Complexo Gráfico.
Teuschler, L. K., Gennings, C., Hartley, W. R., Carter, H., Thiyagarajah, A., Schoeny, R., & Cubbison, C. (2005). The interaction effects of binary mixtures of benzene and toluene on the develo** heart of medaka (Oryzias latipes). Chemosphere, 58, 1283–1291.
Thomas, P., Woodin, B. R., & Neff, J. M. (1980). Biochemical responses of the striped mullet Mugil cephalus to oil exposure I. Acute responses—Interrenal activations and secondary stress responses. Marine Biology, 59, 141–149.
Tolgonay, G., Ozbek, S. S., Oniz, H., Süzer, E., & Yurdakul, L. O. (1998). Regression of splenic vein aneurysm following resolution of splenomegaly. Journal of Clinical Ultrasound, 26, 98–102.
Torres, R., Turner, D. R., Silva, N., & Rutllant, J. (1999). High short-term variability of CO2 fluxes during an upwelling event off the Chilean coast at 30°S. Deep Sea Research Part i: Oceanographic Research Papers, 46, 1161–1179.
Tripathy, A. (2016). Oxidative stress, reactive oxygen species (ROS) and antioxidative defense system, with special reference to fish. International Journal of Current Research in Biosciences and Plant Biology, 3(10), 79–89.
Underwood, A. J. (1992). Beyond BACI: The detection of environmental impacts on populations in the real, but variable, world. Journal of Experimental Marine Biology and Ecology, 161, 145–178.
Uy, P. P. D., Francisco, D. M., Trivedi, A., O’Loughlin, M., & Wu, G. Y. (2017). Vascular diseases of the spleen: A review. Journal of Clinical and Translational Hepatology, 5, 152–164.
Vanzella, T. P., Martinez, C. B., & Cólus, I. M. (2007). Genotoxic and mutagenic effects of diesel oil water soluble fraction on a neotropical fish species. Mutation Research, 631, 36–43.
Vieira, L. R., Sousa, A., Frasco, M. F., Lima, I., Morgado, F., & Guilhermino, L. (2008). Acute effects of benzo[a]pyrene, anthracene and a fuel oil on biomarkers of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Science of the Total Environment, 395, 87–100.
Wang, T., & Secombes, C. J. (2009). Identification and expression analysis of two fish-specific IL-6 cytokine family members, the ciliary neurotrophic factor (CNTF)-like and M17 genes, in rainbow trout Oncorhynchus mykiss. Molecular Immunology, 46, 2290–2298.
Wegwu, M. O., & Omeodu, S. I. (2010). Evaluation of selected biochemical indices in Clarias gariepinus exposed to aqueous extract of Nigerian Crude oil (Bonny Light). Journal of Applied Sciences and Environmental Management, 14(1).
Wei, H., **e, H., Qu, J., **e, A., **e, S., Huang, H., Li, J., Fang, C., Shi, F., Qiu, H., Qi, Y., Tian, X., Yang, Q., & Huang, J. (2021). TLR7 modulating B-cell immune responses in the spleen of C57BL/6 mice infected with Schistosoma japonicum. PLoS Neglected Tropical Diseases, 15, e0009943.
Wolke, R. E., Murchelano, R. A., Dickstein, C. D., & George, C. J. (1985). Preliminary evaluation of the use of macrophage aggregates (MA) as fish health monitors. Bulletin of Environmental Contamination and Toxicology, 35, 222–227.
Xu, Z., Li, M.-H., Zimmermann, N. E., Li, S.-P., Li, H., Ren, H., Sun, H., Han, X., Jiang, Y., & Jiang, L. (2018). Plant Functional Diversity Modulates Global Environmental Change Effects on Grassland Productivity., 106, 1941–1951.
Yan, S., & Zhou, Q. (2011). Toxic effects of Hydrilla verticillata exposed to toluene, ethylbenzene and xylene and safety assessment for protecting aquatic macrophytes. Chemosphere, 85, 1088–1094.
Zapata, A., Diez, B., Cejalvo, T., Gutiérrez-de Frías, C., & Cortés, A. (2006). Ontogeny of the immune system of fish. Fish & Shellfish Immunology, 20, 126–136.
Zhang, J. F., Shen, H., Xu, T. L., Wang, X. R., Li, W. M., & Gu, Y. F. (2003). Effects of long-term exposure of low-level diesel oil on the antioxidant defense system of fish. Bulletin of Environment Contamination and Toxicology, 71, 234–239.
Zhang, J. F., Wang, X. R., Guo, H. Y., Wu, J. C., & Xue, Y. Q. (2004). Effects of water-soluble fractions of diesel oil on the antioxidant defenses of the goldfish, Carassius auratus. Ecotoxicology and Environmental Safety, 58, 110–116.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Experimental design: Alaa El-Din H. Sayed, Sary Kh. Abdel-Ghaffar, Asmaa A. A. Hussein. Experiment and analysis: Alaa El-Din H. Sayed. Data interpretation: Alaa El-Din H. Sayed. Writing and revision: all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Studies were approved by the Research Ethics Committee of the Molecular Biology Research and Studies Institute (MB-21–27-R), Assiut University, Assiut, Egypt.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sayed, A.ED.H., Soliman, H.A.M., Idriss, S.K. et al. Oxidative Stress and Immunopathological Alterations of Clarias gariepinus Exposed to Monocyclic Aromatic Hydrocarbons (BTX). Water Air Soil Pollut 234, 354 (2023). https://doi.org/10.1007/s11270-023-06343-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06343-3