Abstract
Seabirds are one of the most threatened avian groups. Viruses, including herpesvirus, represent considerable threats to marine avifauna. Herein, our goal was to survey herpesvirus in Procellariiformes that stranded in Brazil between June and July 2021. We analyzed 12 Cory's shearwaters (Calonectris borealis), two Great Shearwaters (Ardenna gravis, syn. Puffinus gravis) and one Yellow-nosed Albatross (Thalassarche chlororynchos) found in an unusual mortality event in Bahía state, northeastern Brazil. After necropsy, selected tissue samples were tested for herpesvirus using a broad-range nested PCR. Overall, 20% (3/15) of the birds were herpesvirus-positive, i.e., two Cory's Shearwaters and one Great Shearwater. One alphaherpesvirus sequence type was identified in each shearwater species, classified into the genus Mardivirus. This study describes two likely novel herpesviruses in shearwaters, contributing to the currently very scarce data regarding infectious agents in Procellariiformes. Further studies are necessary to evaluate the presence and characteristics of herpesvirus in Procellariiformes, and the presence (or not) of related disease in order to understand the epidemiology of this infectious agent and eventually contribute to the conservation of this endangered seabird group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seabirds are among the most threatened avian groups, also considered good indicators of marine ecosystems health (Croxall et al. 2012). Epidemiological surveillance provides crucial information regarding pathogens’ hosts and geographic distribution, shedding light into the ecology, epidemiology and potential impact of diseases, a crucial strategy in wildlife conservation. Viruses, including herpesvirus, represent considerable threats to marine avifauna and birds undergoing rehabilitation, as previously reported in mortality outbreaks worldwide (Niemeyer et al. 2017; Sebastiano et al. 2020; Ewbank et al. 2023).
Herpesviruses (order Herpesvirales) are linear, enveloped, double-stranded DNA viruses able to infect vertebrates and some invertebrate species (Davison et al. 2009). The members of the family Orthoherpesviridae, namely the subfamilies Alpha-, Beta- and Gammaherpesvirinae, are thought to have coevolved with their host species, generally showing low pathogenicity and establishing latent infections in their natural hosts (Davison et al. 2009). Less commonly, immune challenges (e.g., concomitant disease, captivity or intoxication by chemical pollutants) may trigger viral reactivation, leading to disease and even death of the natural hosts (Kaleta and Docherty 2007; Niemeyer et al. 2017). Additionally, cross-species infection (“spillovers”) may cause severe disease and high mortality, even leading to severe outbreaks (Kaleta and Docherty 2007).
To this date, alphaherpesvirus within the genera Iltovirus and Mardivirus have been described in a variety of avian orders (Davison et al. 2009; Niemeyer et al. 2017; de Francisco et al. 2023; Ewbank et al. 2023). In seabirds, several alphaherpesviruses have been described in Charadriiformes, Gaviiformes, Procellariiformes, Phaethontiformes, Sphenisciformes, and Suliformes (Quesada et al. 2011; Niemeyer et al. 2017; Verdugo et al. 2019; Sebastiano et al. 2020); however, little is known about herpesviruses in Procellariiformes, limited to the description of a herpesvirus in a Yellow-nosed Albatross (Thalassarche chlororynchos) (Niemeyer et al. 2017). Stranding events involving pelagic seabirds provide an opportunity to assess population threats, infectious agents and related stressors. Thus, our goal was to molecularly survey and characterize herpesviruses in migrating Procellariiformes stranded alive in northeastern Brazilian coast and admitted into a rehabilitation center.
Material and methods
Samples
Overall, 12 Cory's Shearwaters (Calonectris borealis, fam. Procellariidae; 5 males, 6 females, and 1 undetermined; 5 adults, 5 juveniles, and 2 undetermined), two Great Shearwaters (Ardenna gravis, syn. Puffinus gravis, family Procellariidae; 1 adult male and 1 adult of unknown age), and one Atlantic Yellow-nosed Albatross (fam. Diomedeidae; juvenile female) were found debilitated during an unusual mortality event in a coastal strip of Bahía state (13º0′36.216″ S -38º31′3.468″ W to 12º21′39.06″ S -37º51′43.164″ W), northeastern Brazil, between June and July 2021.
Rescue and postmortem examinations were performed by the Instituto Mamíferos Aquáticos (IMA), a rehabilitation center in the same state. All animals died, either during transport (n = 2) or while undergoing rehabilitation (n = 13), being necropsied following standard procedures (Hocken 2002). The body condition was classified into good, moderate, poor, or cachectic, based on pectoral muscle development and presence (or absence) of internal and subcutaneous fat deposits. Age class and sex were determined based on plumage pattern and upon visualization of the gonads. Tissue samples (i.e., lungs, brain, liver, spleen, and kidneys) were collected and fixed in 10% buffered formalin and frozen at -20/-80 °C until processed.
Molecular techniques
Total DNA of selected frozen tissue samples (brain, lung and kidney) was extracted using the DNeasy Blood and Tissue kit, according to the manufacturer's guidelines. No swabs were available. Subsequently, DNA was tested with a broad-range nested PCR protocol for herpesviruses, with an annealing temperature of 46 ºC for both PCRs, able to amplify a fragment of approximately 215–315 bp of the DNA polymerase gene of different alpha, beta- and gammaherpesviruses (VanDevanter et al. 1996). In herpesvirus-positive animals, other available frozen tissue samples (spleen and liver) were also tested following the same methodology described above. A previously confirmed cetacean gammaherpesvirus positive sample was used as positive and DPEC water was used as no template control. All amplicons of the expected size were purified with ExoSAP-IT and directionally sequenced. The obtained consensus sequences were analyzed for further phylogenetic classification using Mega 7.0. Subsequently, a DNA polymerase amino acid maximum likelihood phylogram was constructed including the obtained sequences, representative sequences of the genera Mardivirus and Iltovirus, and Human gammaherpesvirus 8 as an outgroup, using Mega 7.0.
Histopathology
Light microscopy histopathological evaluation was performed in the HV-PCR-positive individuals, on formalin-fixed paraffin-embedded tissues sectioned at 5 μm and stained with hematoxylin and eosin.
Results
Molecular findings
Three animals were herpesvirus-positive; two tested Cory's Shearwaters (IMA4473 and IMA4486; 2/12; 16.7%), and one Great Shearwater (IMA4476; 1/2; 50%). The Yellow-nosed Albatross was herpesvirus-negative (Table 1).
In the Cory's Shearwaters, case IMA4473 was herpesvirus-positive in the spleen and the kidney, and negative in brain, lung and liver; while IMA4486 was only herpesvirus-positive in the lung and brain, and negative in spleen, liver and kidney. Both Cory's Shearwater herpesviral sequences were identical between them, and presented the highest nucleotide (68.9%) and amino acid identities (67.8%) to different Columbid alphaherpesvirus 1 sequences (e.g., GenBank accession nº NC_034266, MW625939, MW625940, detected in Rock Pigeons (Columba livia) in China in 2013, and in Slovenia in 2019).
Regarding the Great Shearwater (IMA4476), lung, spleen, liver and kidney samples were herpesvirus-positive, while the brain was negative. The same herpesviral consensus sequence was obtained in all of this individual’s positive samples, with the highest nucleotide identity (63.5%) to an alphaherpesvirus identified in a Neotropical river otter (Lontra longicaudis, GenBank accession nº OQ980217) in Brazil in 2017—although our nucleotide sequence is slightly longer (212 vs. 178), followed by a herpesvirus (60.3%) identified in Common Buzzards (Buteo buteo, GenBank accession nº MW533125-MW533127) in Slovenia, in 2018. The highest amino acid (71.6%) identity was to Equid alphaherpesvirus 1 sequences (GenBank accession nº KP689589, KX101098) detected in African elephants (Loxodonta africana) in Kenya in 2015 and in a plain zebra (Equus quagga boehmi) in Germany in 2014, followed by alphaherpesvirus sequences (67.1%) found in Ural Owls (Strix uralensis, GenBank accession nº MH084654, MW315868) and Long-eared Owl (Asio otus, GenBank accession nº MH084655, MW533133) of Slovenia identified between 1995 and 2019, and by a Thalassarchid herpesvirus 1 (64.3%) sequence (GenBank accession nº KR092313), detected in the oral swab of an Atlantic Yellow-Nosed Albatross (Thalassarche chlororhynchos) in Brazil, in 2013. The sequences obtained in this study clustered within herpesviral sequences of genus Mardivirus (Fig. 1).
Maximum likelihood phylogram of the alignment of the herpesviral DNA polymerase sequences (1) obtained in this study (blue dots), and (2) previously detected in birds, including those classified into the genus Mardivirus (yellow square) and Iltovirus (blue square). Human gammaherpesvirus 8 was selected as outgroup. The phylogram was based on the gamma distributed Le and Gascuel model with Invariant sites and Gamma distribution (LG + I + G). The reliability of the phylogram was tested by a 1000 bootstrap analysis. Bootstrap values lower than 70 were omitted
Gross and histopathological findings
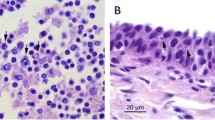
Overall, tissue samples of herpesvirus-positive individuals were compromised due to advanced autolysis. Nevertheless, in two of the positive birds (Cory's Shearwater 4473 and the herpesvirus-positive Great Shearwater) pulmonary edema was observed. Additionally, mononuclear adrenalitis and moderate mixed celomitis were present in the Great Shearwater (Table 1).
Discussion
Herein we found two likely novel alphaherpesvirus sequence types, reported for the first time in Cory’s and Great Shearwaters, and in the family Procellariidae. To the authors’ knowledge, these are the second and third descriptions of herpesviruses in Procellariiformes worldwide, following the report of Thalassarchid herpesvirus 1 in one of the 12 Yellow-nosed Albatross under rehabilitation in Rio Grande do Sul State, southern Brazil (Niemeyer et al. 2017). Procellariiformes is a large avian group comprised of long-lived, pelagic species that spend most of their life cycle at sea (Stidworthy and Denk 2018). Due to these particular life history traits, procellariforms are amongst the most endangered threatened taxonomic groups (Croxall et al. 2012), considered highly sensitive to anthropogenic activities (e.g., fisheries interaction, habitat degradation, climate change, invasive and non-native species) (Tavares et al. 2019; Rodríguez et al. 2022). Therefore, data on the infectious agents affecting Procellariiformes is pivotal for the conservation of this group.
The obtained sequence types are highly divergent when compared to the closest from GenBank/DDBJ/EMBL database, with more than 30% of difference. This fact, along with their detection in novel host species, support their classification as novel herpesviral sequences. Nevertheless, further studies detecting more Cory’s and the Great Shearwater individuals infected with these likely novel herpesviruses are necessary in order to establish if these birds are their natural hosts. According to our phylogenetic tree, both our sequence types are classified within the genus Mardivirus, which includes relevant avian herpesviruses, such as Gallid alphaherpesvirus 2 (Marek’s disease) (Davison et al. 2009).
In our study, no associated lesions were observed in any of the herpesvirus-positive individuals, although the advanced autolysis precluded a detailed histopathological examination in some tissue samples. Herpesviruses generally show low virulence in their natural host, which could be the case here (Davison et al. 2009). The herpesvirus-positive species -Cory’s Shearwater and the Great Shearwater- breed, respectively, in Portugal and Canary Islands (Spain), and in the Tristan da Cunha archipelago (United Kingdom) and Malvinas/Falkland Islands, spending their non-breeding season in the Atlantic (Birdlife International 2023a, b). We hypothesize that the stress associated with the long migration of these species from their breeding territories to Brazil likely triggered the reactivation of the detected herpesviruses (Niemeyer et al. 2017), which were detected in several tissue samples.
Large interannual coastal strandings of seabirds (alive and/or dead) have been reported worldwide (Bugoni et al. 2007; Haman et al. 2023; Simeone et al. 2021). The main causes of such events include pollution, weather events (storms and hurricanes, El Niño Southern Oscillation (ENSO), bycatch, starvation (Tavares et al. 2019), and juvenile inexperience (Riotte-Lambert and Weimerskirch 2013). Unfortunately, the causes of the stranding event studied herein were not clear; there were no abnormal or extreme associated weather conditions, the mixed presence of adults and juveniles does not indicate inexperience, and the advanced tissue autolysis prevented a more informative histopathological evaluation.
In summary, this study contributes to the currently very scarce data regarding infectious agents in Procellariiformes by describing two likely novel herpesvirus species in shearwaters. Further studies are necessary to evaluate the presence and characteristics of herpesvirus in Procellariiformes and the presence (or not) of related disease in order to understand the epidemiology of this infectious agent and contribute to the conservation of this endangered seabird group.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files]. The alphaherpesvirus sequences obtained in Cory’s and Great Shearwaters were deposited in GenBank under accession numbers PP449022 and PP449023, respectively.
References
BirdLife International (2023a) Species factsheet: Calonectris borealis. BirdLife International. http://datazone.birdlife.org/species/factsheet/corys-shearwater-calonectris-borealis. Accessed 12 Mar 2023
BirdLife International (2023b) Species factsheet: Ardenna gravis. BirdLife International. http://datazone.birdlife.org/species/factsheet/great-shearwater-ardenna-gravis. Accessed 12 Mar 2023
Bugoni L, Sander M, Costa ES (2007) Effects of the first southern Atlantic hurricane on Atlantic petrels (Pterodroma incerta). Wilson J Ornithol 119:725–729
Croxall JP, Butchart SHM, Lascelles B, Stattersfield AJ, Sullivan B, Symes A, Taylor P (2012) Seabird conservation status, threats and priority actions: a global assessment. Bird Conserv Int 22:1–34
Davison AJ, Eberle R, Ehlers B, Hayward GS, McGeoch DJ, Minson AC, Pellett PE, Roizman B, Studdert MJ, Thiry E (2009) The order Herpesvirales. Arch Virol 154:171–177
de Francisco ON, Sacristán I, Ewbank AC, Velarde R, Afonso I, Garcia-Ferré D, Martín-Maldonado B, Esperón F, Iglesias I, de la Torre A, Margalida A, Sacristán C (2023) First detection of herpesvirus and hemosporidians in the endangered Pyrenean Capercaillie (Tetrao urogallus aquitanicus). Sci Rep 13:21936
Ewbank AC, Duarte-Benvenuto A, Ramblas RZ, Gattamorta MA, Godoy SN, Gravinatti ML, Brandão PE, Catão-Dias JL, Sacristán C (2023) Herpesvirus, flavivirus, and coronavirus surveillance in magnificent frigatebirds (Fregata magnificens), alcatrazes archipelago, southeastern Brazil. J Wildl Dis 59:353–358
Haman KH, Norton TM, Ronconi RA, Nemeth NM, Thomas AC, Courchesne SJ, Segars A, Keel MK (2023) Great shearwater (Puffinus gravis) mortality events along the eastern coast of the United States. J Wildl Dis 49:235–245
Hocken AG (2002) Post-mortem examination of penguins, DOC Science Internal Series 65. New Zealand Department of Conservation, Wellington
Kaleta EF, Docherty DE (2007) Avian Herpesviruses. In: Thomas NJ, Hunter DB, Atkinson CT (eds) Infectious diseases of wild birds. Wiley, New Jersey, pp 63–86
Niemeyer C, Favero CM, Shivaprasad HL, Uhart M, Musso CM, Rago MV, Silva-Filho RP, Canabarro PL, Craig MI, Olivera V, Pereda A (2017) Genetically diverse herpesviruses in south American Atlantic coast seabirds. PLoS One 12:e0178811
Quesada RJ, Heard DJ, Aitken-Palmer C, Hall N, Conley KJ, Childress AL, Wellehan Jr JF (2011) Detection and phylogenetic characterization of a novel herpesvirus from the trachea of two stranded Common Loons (Gavia immer). J Wildl Dis 47:233–239
Riotte-Lambert L, Weimerskirch H (2013) Do naive juvenile seabirds forage differently from adults? Proc Biol Sci 280:20131434
Rodríguez B, Rodríguez A, Siverio F, Martínez JM, Sacramento E, Acosta Y (2022) Introduced predators and nest competitors shape distribution and breeding performance of seabirds: feral pigeons as a new threat. Biol Invasions 24:1561–1573
Sebastiano M, Canestrelli D, Bisconti R, Lavergne A, Pineau K, Chastel O, Lacoste V, Costantini D (2020) Detection and phylogenetic characterization of a novel herpesvirus in sooty terns Onychoprion fuscatus. Front Vet Sci 7:567
Simeone A, Anguita C, Daigre M, Arce P, Vega R, Luna-Jorquera G, Portflitt-Toro M, Suazo CG, Miranda-Urbina D, Ulloa M (2021) Spatial and temporal patterns of beached seabirds along the Chilean coast: Linking mortalities with commercial fisheries. Biol Conserv 256:109026
Stidworthy MF, Denk D (2018) Sphenisciformes, gaviiformes, podicipediformes, procellariiformes, and pelecaniformes. In: Terio KA, McAloose D, St. Leger J (eds) Pathology of wildlife and zoo animals. Academic Press, Cambridge, pp 653-686
Tavares DC, Moura JF, Merico A, Siciliano S (2019) Mortality of seabirds migrating across the tropical Atlantic in relation to oceanographic processes. Anim Conserv 23:307–319
VanDevanter DR, Warrener P, Bennett L, Schultz ER, Coulter S, Garber RL, Rose TM (1996) Detection and analysis of diverse herpesviral species by consensus primer PCR. J Clin Microbiol 34:1666–1671
Verdugo C, Pinto A, Ariyama N, Moroni M, Hernandez C (2019) Molecular identification of avian viruses in neotropic cormorants (Phalacrocorax brasilianus) in Chile. J Wildl Dis 55:105–112
Acknowledgements
We thank CAPES, CNPq, FAPESP, Agencia Estatal de Investigación/Ministerio de Ciencia, Innovación y Universidades and the staff of Instituto de Mamíferos Aquáticos.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. We thank CAPES, CNPQ and FAPESP. We also thank Juan de la Cierva incorporación (IJC2020-046019-I) and formación (JDC2022-048632-I) fellowships, funded by Agencia Estatal de Investigación/Ministerio de Ciencia, Innovación y Universidades). JLCD is a recipient of a professorship from CNPq (#304999/2018–0 and 304106/2022–4). AD-B was recipient of a PhD grant from CNPq (#141868/2019–8). CS and ACE were recipients of Postdoctoral and PhD grants by FAPESP (2019/26794–0 and 2018/20956–0, respectively).
Author information
Authors and Affiliations
Contributions
Perform experiments A.C.E., A.D-B., P.H-N., R.Z-R., J.L.C-D., C.S. Analyze experiments A.C.E., A.D-B., P.H-N., R.Z-R., L.B.C., J.I., J.L.C-D., C.S. Design the study A.C.E., J.L.C-D., C.S. Wrote the paper A.C.E., and C.S. with the contribution of all coauthors. Supervise the study F.E., A.M., and C.S. All authors reviewed and approved the final the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All study samples were collected in full compliance with specific federal permits issued by the Brazil Ministry of Environment (MMA) and the Chico Mendes Institute for Biodiversity Conservation (ICMBio), and under the Biodiversity Information and Authorization System (SISBIO authorization no. 76020) and National System of Genetic Resource Management and Associated Traditional Knowledge (SisGen: A831F74). All procedures were approved by the Ethical Committee of the School of Veterinary Medicine and Animal Sciences, University of São Paulo (Ceuavet n◦ 1753110716).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sacristán, C., Duarte-Benvenuto, A., Navas-Suárez, P.E. et al. Herpesviruses in migrating procellariforms, northeastern Brazil. Vet Res Commun (2024). https://doi.org/10.1007/s11259-024-10434-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11259-024-10434-9