Abstract
Objective
This study is to investigate the safety and efficacy of ureteroscope-assisted laparoscopic ureteroplasty in treating ureteral stricture after pelvic surgery.
Methods
A retrospective analysis of the clinical data of 95 patients treated for ureteral stricture at Ganzhou People's Hospital from June 2017 to March 2023 after pelvic surgery. In this group, 49 patients underwent ureteroscope and laparoscopic ureteroplasty under lithotomy position. The control group consisted of 46 patients who underwent simple laparoscopic ureteroplasty in a supine position. Postoperative data from both groups were collected and compared, including operation time, amount of blood loss during surgery, postoperative hospital stay, incidence of complications, success rate of ureteroplasty, and effectiveness of the operation.
Results
The success rate of end-to-end ureteral anastomosis in the observation group was 93.88%, and the operation effectiveness rate was 100%. The success rate in the control group was 78.26% and the operation effectiveness rate was 89.1%.The average operation time and intraoperative blood loss in the observation group were (121.3 ± 44.6) min and (137.5 ± 34.2) ml, respectively, while in the control group they were (151.2 ± 52.3) min and (165.6 ± 45.8) ml, the difference were statistically significant (P < 0.05). The incidence of perioperative complications in the observation group was 2%, significantly lower than that in the control group (19.6%) (P < 0.05).
Conclusion
Ureteroscope-assisted laparoscopic ureteroplasty for ureteral stricture after pelvic surgery has the advantages of shortened operation time, increased success rate, and reduced incidence of complications, making it an optional surgical scheme in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
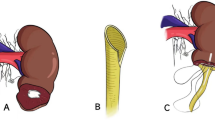
With the rising incidence of tumoral diseases in the pelvis, the frequency of pelvic surgical interventions is also increasing. Consequently, postoperative ureteral stenosis following pelvic surgery has become more prevalent in clinical practice [1]. In addition, as the proportion of minimally invasive surgical interventions in urology is constantly increasing, holmium laser and other high-energy lithotripsy tools have become routine surgical instruments in urology, and the application of such lithotripsy tools in the treatment of ureteral incarcerated stones may also cause irreversible damage to the ureteral mucosa. Ureteral stenosis that occurs as a complication of lithotripsy of lower ureteral stones is becoming more common in clinical practice, and this type of ureteral stenosis is a challenge for physicians [1A).
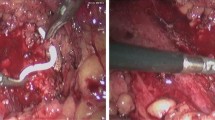
After fully dissociating the ureter, the ureteroscope was used again to support the position of the stricture. Using a pair of laparoscopic scissors close to the front of the ureteroscope, the distal end of the stenotic segment is cut first; then, the ureter toward the proximal end of the stenotic segment where ureteral dilatation is evident is slowly cut until urine is ejected. Then, after the normal mucosa of the ureteral lumen or normal mucosa of the renal pelvis could be seen, the proximal ureter was cut obliquely (If the lumen is small, cut 0.5–1 cm lengthwise at 8 o 'clock), and the distal normal ureter was cut 0.5–1.0 cm longitudinally at 3 o 'clock. With the assistance of ureteroscopy, the first three needles of the posterior ureteral wall were sutured with 4–0 absorbable suture (Fig. 1B), and then the zebra guidewire crossed the suture position into the renal pelvis. Under the monitoring of ureteroscopy and laparoscopy, the 7F double J tube was pushed retrograde. After withdrawing from the ureteroscope, the anterior ureteral wall was sutured with 4–0 absorbable sutures using 4–5 stitches intermittently. Appropriate reinforcement of the needle was performed to prevent urine leakage according to the suture situation (Fig. 1C). Complete hemostasis of the surgical area was performed after suturing was completed, and the drainage tube and urinary catheter were routinely placed beside the anastomosis.
The control group also used endotracheal intubation general anesthesia, and after catheterization, they were put in a supine position (head lower than feet) to perform simple laparoscopic ureteroplasty, 36 cases successfully carried out the removal of the narrowed section of the ureter and end-to-end anastomosis operation, Ten patients underwent ureter-bladder reimplantation, of which, three cases involved rolling the bladder muscle flaps, Normally, a drainage tube is placed next to the anastomosis.
Postoperative management, follow-up, and efficacy evaluation
Patients should rest in bed for 1–3 d after surgery (depending on the anastomotic tension). Antibiotics should be used to prevent infection routinely, proper fluids should be administered to keep urine flowing well, prevent small blood clots, and prevent urine sediments from blocking the urinary catheter. Remove the abdominal drain when it is confirmed to be clean, remove the urinary catheter 2 weeks after it was placed, and return to the hospital three months later to remove the ureteral stent. Patients need to keep a urinary catheter in place for 2 weeks because early removal after complex ureteral stricture repair can lead to urine reflux and anastomotic leakage. Patients with bladder irritation symptoms may take M receptor blockers, such as tolterodine tartrate. Follow-up is conducted for 3 to 36 months after the tube is removed, with an average of (12.6 ± 5.3) months, patients return to the hospital one week after the double J stent is removed for a urinary system CT scan and kidney function test, patients return to the hospital 1 week after the double J stent is removed for a urinary system CT scan and kidney function test, if the treatment is effective, then a urinary system CT scan and kidney function test are performed every 3 months, with at least two consecutive checks.
Observational indicators
-
1.
Success rate and treatment effects of end-to-end ureteral anastomosis surgery performed on two groups of patients.
-
2.
Perioperative indicators for both patient groups, including operation time, intraoperative blood loss, postoperative hospital stay, time of catheter removal, and occurrence of surgical complications.
Evaluation of the therapeutic efficiency
All patients showed reduced renal hydronephrosis on CT of the urinary system 2 weeks after catheter removal. The following conditions are deemed effective: follow-up kidney function shows a decrease in creatinine levels compared to before; the patient's lumbar pain and other clinical symptoms have eased; renal hydronephrosis remains stable over two consecutive follow-ups.
Statistical methods
Data were analyzed using SPSS 26.0 statistical software. Measurement data were expressed as mean ± standard deviation (x ± s) and subjected to a t test (when variances of two samples are equal) or an approximate t test (when variances of two samples are not equal). Count data were represented by the number of cases (%), and a χ2 test was performed. Differences with P < 0.05 were considered statistically significant.
Results
Population characteristics
There were no significant statistical differences in general clinical data such as gender, age, cause of stenosis, length of stenosis, and hydronephrosis between the observation group and control group, making them comparable (Table 1).
Comparison of completion rate, effectiveness, and incidence of complications of plastic surgery between patients in the observation group and the control group
In the observation group, 46 patients successfully underwent ureteroscopy combined with laparoscopic ureteroplasty, and 3 patients underwent ureteral-bladder reimplantation; in the control group, 36 patients successfully underwent laparoscopic ureteroplasty, and 10 patients underwent ureteral-bladder reimplantation, of which 3 patients underwent bladder muscle flap rolling, the success rate of ureteroplasty operation in the observation group, which was laparoscopic excision of narrowed ureter and end-to-end anastomosis, was higher than that in the control group (P < 0.05). In the observation group, only one patient developed significant gross hematuria after surgery, while in the control group, two patients developed urinoma, two patients developed postoperative fever, one patient developed vesicovaginal fistula, and one patient developed obvious gross hematuria; no patients in the observation group developed re-narrowing after catheter removal, with an effectiveness rate of 100%. The control group had three cases of re-narrowing and two cases of reflux symptoms, with an effectiveness rate of 89.13%. The total incidence of complications in the observation group was significantly lower than that in the control group, and the difference was statistically significant (P < 0.05) (Table 2).
Comparison of perioperative clinical data between observation group and control group patients
The average operation time and intraoperative blood loss in the observation group were less than those in the control group (P < 0.05). There was no significant statistical difference in the postoperative hospital stay and drainage tube removal time between the two groups (P > 0.05) (Table 3).
Discussion
Currently, ureteral strictures are managed in a variety of ways [5]. Endovascular surgery is often the first choice for clinical physicians because of its minimally invasive nature and rapid associated postoperative recovery [6]. Many studies have shown that in patients with a history of pelvic surgery, endoluminal procedures (be they endotomy, balloon dilation, multiple stent retention, etc.) have poor long-term outcomes and may make patients undergo multiple operations [7,8,9]. Repeated ureteroscopic surgery will not only increase the difficulty of subsequent surgical procedures but also greatly increase the psychological and physiological burden of patients and also increase the economic burden on these patients [10]. Laparoscopic ureteral stricture resection and end-to-end anastomosis have been confirmed to be effective treatment options for such ureteral strictures that occur as a result of pelvic surgery [11]. However, after pelvic surgery, the ureteral stricture is often located and dissociated due to severe scar adhesion and normal anatomical stricture; thus, it can be difficult to free the ureter above and below the stricture. If the normal ureter is injured again during the process of dissociation, it may bring devastating consequences to the normal reconstruction of the ureter [12]. Therefore, accurately locating the ureteral stricture segment, safely dissociating the normal ureteral tissues above and below the stricture segment, and accurately cutting off the ureteral scar are critical to ensuring low-tension anastomosis of the ureter. They are also the difficult parts of the procedure [13].
In order to accurately determine the location of the ureteral stenosis, numerous scholars have tried different methods. Kim et al. [14] used indocyanine green near-infrared fluorescence imaging to accurately locate the narrow segment of the ureter; however, the indocyanine green dye may cause side damage to the ureter. Verbeek et al. [15] used low-dose methylene blue near-infrared fluorescence to guide the identification of ureteral stricture during surgery, however, due to the complexity of these methods, they have not been effectively promoted. There are also reports of scholars using flexible ureteroscopy to locate the ureteral stenosis during surgery, then using robot-assisted laparoscopy to treat lower ureteral stenosis, achieving good results [16,17,18].
In this study, we carried out laparoscopic ureteroplasty with the aid of a ureteroscope in the lithotomy position (head high and foot low), achieving good therapeutic results. We found during the surgery that freeing the dilated ureter above the stricture was relatively easier, but the ureter at and below the stricture often faced issues due to complications of previous surgeries, including intense inflammation, dense fibrotic scar tissue surrounding the ureter, and loss of normal anatomical structures, making the freeing process difficult [19, In conclusion, ureteroscopy-assisted laparoscopic ureteroplasty for ureteral stenosis after pelvic surgery is a safe and effective surgical method with certain clinical applications.Conclusion
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Satitniramai S, Manonai J (2017) Urologic injuries during gynecologic surgery, a 10-year review. J Obstet Gynaecol Res 43(3):557–563. https://doi.org/10.1111/jog.13238
**e B, Wang X, Zeng X, **e L, Zeng Z, Xu H (2024) Ureterocalicostomy for complex upper ureteral stricture: a narrative review of the current literature. Int Urol Nephrol 56(6):1899–1909. https://doi.org/10.1007/s11255-023-03911-8
Kim TN, Kim JH, Oh CK, Lee W, Nam JK, Lee KS (2021) Three different laparoscopic techniques for the management of iatrogenic ureteral injury: a multi-institutional study with medium-term outcomes. Asian J Surg 44(7):964–968. https://doi.org/10.1016/j.asjsur.2021.01.027
Yang K, Fan S, Wang J, Yin L, Li Z, **ong S et al (2022) Robotic-assisted lingual mucosal graft ureteroplasty for the repair of complex ureteral strictures: technique description and the medium-term outcome. Eur Urol 81(5):533–540. https://doi.org/10.1016/j.eururo.2022.01.007
Darwish AE, Gadelmoula MM, Abdelkawi IF, Abdellatif AM, Abdel-Moneim AM, Hammouda HM (2019) Ureteral stricture after ureteroscopy for stones: a prospective study for the incidence and risk factors. Urol Ann 11(3):276–281. https://doi.org/10.4103/ua.Ua_110_18
Lu C, Zhang W, Peng Y, Li L, Gao X, Liu M et al (2019) Endoscopic balloon dilatation in the treatment of benign ureteral strictures: a meta-analysis and systematic review. J Endourol 33(4):255–262. https://doi.org/10.1089/end.2018.0797
Klett DE, Mazzone A, Summers SJ (2019) Endoscopic management of iatrogenic ureteral injury: a case report and review of the literature. J Endourol Case Rep 5(4):142–144. https://doi.org/10.1089/cren.2019.0020
El-Abd AS, Suliman MG, Abo Farha MO, Ramadan AR, El-Tatawy HH, El-Gamal OM et al (2014) The development of ureteric strictures after ureteroscopic treatment for ureteric calculi: a long-term study at two academic centres. Arab J Urol 12(2):168–172. https://doi.org/10.1016/j.aju.2013.11.004
Gild P, Kluth LA, Vetterlein MW, Engel O, Chun FKH, Fisch M (2018) Adult iatrogenic ureteral injury and stricture-incidence and treatment strategies. Asian J Urol 5(2):101–106. https://doi.org/10.1016/j.ajur.2018.02.003
Stühler V, Bedke J, Stenzl A (2019) Surgical reconstruction of the ureter. Urologe A 58(6):651–657. https://doi.org/10.1007/s00120-019-0944-z
Pal DK, Wats V, Ghosh B (2016) Urologic complications following obstetrics and gynecologicai surgery: Our experience in a tertiary care hospital. Urol Ann 8(1):26–30. https://doi.org/10.4103/0974-7796.158502
Sforza S, Tellini R, Grosso AA, Maida FD, Mari A, Cocci A et al (2021) Robotic repair of iatrogenic ureteral stricture after pelvic surgery: a changing treatment paradigm. Minerva Urol Nephrol 73(1):133–135. https://doi.org/10.23736/s2724-6051.20.04138-7
Kapogiannis F, Spartalis E, Fasoulakis K, Tsourouflis G, Dimitroulis D, Nikiteas NI (2020) Laparoscopic and robotic management of ureteral stricture in adults. In Vivo 34(3):965–972. https://doi.org/10.21873/invivo.11864
Kim S, Fuller TW, Buckley JC (2020) Robotic Surgery for the Reconstruction of Transplant Ureteral Strictures. Urology 144:208–213. https://doi.org/10.1016/j.urology.2020.06.041
Verbeek FPR, van der Vorst JR, Schaafsma BE, Swijnenburg R-J, Gaarenstroom KN, Elzevier HW et al (2013) Intraoperative near infrared fluorescence guided identification of the ureters using low dose methylene blue: a first in human experience. J Urol 190(2):574–579. https://doi.org/10.1016/j.juro.2013.02.3187
Tsuru N, Mugiya S, Sato S (2016) Retrograde flexible ureteroscopy-assisted retroperitoneal laparoscopic ureteroureterostomy for refractory ureteral stricture: a case report. Int J Surg Case Rep 20:77–79. https://doi.org/10.1016/j.ijscr.2016.01.024
Yang KK, Asghar AM, Lee RA, Strauss D, Kuppa S, Lee Z et al (2022) Robot-assisted laparoscopic distal ureteroureterostomy for distal benign ureteral strictures with long-term follow-up. J Endourol 36(2):203–208. https://doi.org/10.1089/end.2021.0315
Slawin J, Patel NH, Lee Z, Dy GW, Kim D, Asghar A et al (2020) Ureteral reimplantation via robotic nontransecting side-to-side anastomosis for distal ureteral stricture. J Endourol 34(8):836–839. https://doi.org/10.1089/end.2019.0877
Wong JMK, Bortoletto P, Tolentino J, Jung MJ, Milad MP (2018) Urinary tract injury in gynecologic laparoscopy for benign indication: a systematic review. Obstet Gynecol 131(1):100–108. https://doi.org/10.1097/aog.0000000000002414
Li X, Qiao J, **ong S, Wang J, Wang Q, Li Z et al (2022) The surgical outcomes of reconstruction for the treatment of ureteral stricture after holmium laser lithotripsy: the comprehensive experiences. Asian J Surg 45(12):2713–2718. https://doi.org/10.1016/j.asjsur.2022.03.018
Funding
Funding information is not available.
Author information
Authors and Affiliations
Contributions
JX, TL and JG were primarily responsible for literature research, data collection, and drafting the initial manuscript. QH, LQ prepared figures 1, HC, as the corresponding author, provided guidance, supervision, and conceptual input. Additionally, TL was involved in revising the initial draft. All authors contributed to the article and approved the submitted version. All authors have read, provided substantial input during the manuscript development and revision, and have approved the final manuscript for submission. They agree to be accountable for the entirety of the work, ensuring its accuracy and integrity.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interests to declare.
Ethics approval
This study was approved by the Ethics Committee of Ganzhou People’s Hospital.
Informed consent
Informed consent was obtained from all patients, who signed informed consent forms.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
**ao, J., Liu, T., Zhu, Q. et al. Clinical efficacy of ureteroscopy-assisted laparoscopic ureteroplasty in the treatment of ureteral stricture after pelvic surgery. Int Urol Nephrol (2024). https://doi.org/10.1007/s11255-024-04115-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11255-024-04115-4