Abstract
Co-based catalysts were synthesized and studied as novel oxidation catalysts, exploring and optimizing the effect of synthesis method on the redox behavior, the oxygen storage ability and thus the catalytic performance of the derived Co3O4 materials in the complete CH4 and/or CO oxidation reactions. Thus, a series of Co-based catalysts were synthesized applying either the precipitation and/or the hydrothermal method, using different precipitating agents (Na2CO3, NH3, urea or NaOH), Co precursor salt (nitrate or acetate) and finally varying the Co/Na ratio. In addition, the reaction time (6 or 24 h aging) was also investigated for the hydrothermally prepared Co3O4. The best catalysts for the CH4 oxidation are the precipitated Co3O4, using cobalt acetate as precursor salt and NaOH as precipitating agent, presenting the highest surface areas and the lowest Co3O4 particle sizes. On the other side, hydrothermally prepared cobalt oxides reveal higher performance for CO oxidation, with Co3O4 prepared with cobalt acetate, NaOH and low aging time shown as the optimum materials. The best catalysts were further promoted with incorporation of Pd (0.5wt.%) and explored for both reactions. The addition of Pd enhanced the activity of Co3O4 for CH4 oxidation, while Pd did not improve any further the catalyst performance for CO oxidation, presenting thus the same activity with pure cobalt oxides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Compressed natural gas (CNG), composed of 85–95% of methane (CH4) has recently received increasing attention as an attractive alternative fuel for motor vehicles, as it has many advantages over traditional fossil fuels, such as gasoline and diesel; it is cheaper, has lower sulphur and nitrogen content (less SOx and NOx emissions), produces smaller amount of carbon dioxide (CO2) due to the high H/C ratio and much lower number of particulates as compared to diesel engines [1]. Today’s heavy-duty (HD) natural gas-fueled vehicles is estimated to represent less than 2% of the total fleet. However, over the next couple of decades, predictions are that the percentage could grow to as much as 50% [2]. CNGVs under lean burn conditions present increased fuel efficiency as compared to stoichiometric conditions. Unfortunately, even with efficient lean conditions, CO and unburned HC are always released in the exhaust; about 90% of the unburned fuel is CH4, typically equivalent to 500–1,000 ppmv. Both CH4 and CO gases are harmful to the living beings and environment [1]. CO is a colorless, odorless, tasteless, non-irritating, poisonous gas, while CH4 is a substantially more potent greenhouse gas (GHG) than CO2, due to its 25 times higher global warming potential. Hence, leakage of only a small percentage of CH4 from the supply chain could alter the net climate benefits of NG as a HD vehicle fuel [2]. Therefore, it is imperative to eliminate CO and CH4 emissions and thus catalytic oxidation converters are needed for their abatement [3]. In contrast to thermal oxidation, catalytic oxidation is considered as green and cost-effective way to reduce the GHG emissions, as it is performed at much lower temperatures, thus being economically favorable and also preventing the formation of nitrous oxides [4].
Up to date, the catalytic combustion of CH4 has been extensively investigated over noble metals, transition metal oxides, and mixed metal oxides. Among the noble metals, Pd and Pt catalysts belong to the most active for CH4 combustion at low temperatures. However, despite of being expensive, they also usually deteriorate due to sintering. Alternative oxide materials such as spinels, hexaalumimnates, and perovskites have been considered as promising catalysts for total oxidation of CH4 due to their high activity, abundant resources and distinctly lower costs when compared to the noble metals [5]. Among the spinel-type oxide catalysts, cobalt oxide (Co3O4) usually stands out as an earth-abundant material and possible alternative to expensive noble metals as it exhibits high activity for the catalytic combustion of CH4. This is possibly attributed to the structure and electronic properties of spinel-type cobalt oxides with variable valence states of transition metal cations in, either tetrahedron (Co2+), or octahedron (Co3+) interstices, and multifold surface lattice oxygen anions, as well as the lower bonding energy of Co–O bond and the high turnover frequency [4]. In addition, Co3O4 holds several other potential advantages such as a lower tendency to sinter at high temperature compared to other transition metal oxides (e.g., Cu or Mn), a larger thermodynamic driving force for reaction with hydrocarbon fuels, and greater rates of reduction and (re)oxidation. Reduction of Co3O4 to metal Co provides the highest amount of available oxygen among common metal oxides used for CH4 combustion, as Co3O4 offers the highest ratio of available oxygen moles/mole of metal. Activity and selectivity of Co3O4 catalysts depend greatly on their morphology, possibly related to the electro transfer properties of different Co3O4 materials, thus enormous efforts have been devoted to develop Co3O4 based materials of different dimensions and morphologies (cubes, sheets, belts, wires, tubes, plates, flowers, rods, boxes and nanoparticles) to enhance their catalytic activity [6].
In general, the synthesis method (e.g., type and parameters during the preparation process and/or type of catalysts precursor, etc.) has an important effect on the structural properties of the catalysts, such as surface area, the surface cobalt valence states, etc. Hydrothermal synthesis is reported with significant advantages over others methods, while hydrothermal conditions, such as precursor nature and concentration, pH value, temperature, and time, have critical impacts on the crystal structure and morphology of the product material [7, 8]. However, only few pioneering studies have been reported to explore the influence of different cobalt precursors on the catalytic combustion of CH4 over Co3O4 catalysts [9]. Recently [10], we have investigated the preparation of novel Co-Ce catalytic materials targeting in the complete combustion of CH4. Co incorporation on an optimum selected CeO2 support aimed to promote the catalytic activity for the oxidation reactions, via an attempted improvement of the redox property and high oxygen storage capacity induced by the synergistic effect between CeO2 and CoOx. We purposely explored the effect of both the synthesis method of the CeO2 support (following either the hydrothermal or precipitation technique) and the metal loading (0, 2, 5 and 15 wt. % Co) on the catalytic performance and durability for complete CH4 oxidation. Moreover, the addition of water vapor was studied in the catalytic activity of the optimum selected 15Co/CeO2 prepared with hydrothermal method. The corresponding material was very promising for methane oxidation purposes, as it presented good water resistance properties in wet CH4 combustion [10]. Effect of water presence (either as gas humidity or as a combustion product) is suggested to cause deactivation mainly for Pd-based catalysts [11], due to the formation of OH groups, which block the interaction between methane and active sites [12], while a promotional effect is even reported for metal oxide materials (e.g., cobalt-manganese mixed oxides studied for methane combustion [13]. On the other hand, when testing water tolerance of CeO2–MOx catalysts (M = Cu, Mn, Fe, Co, and Ni) tested for CH4 or CO oxidation, it seems that catalytic performance in the absence or presence of water depends on the mixed oxide [14]. In another study [12], water effect during methane oxidation seems to also depends not only on active site (Cu or Co catalyst) but also on oxygen deficiency (O2/CH4 ratios). More recently, investigation of reaction kinetics of wet lean methane combustion over Co3O4- and Pd/Co3O4 catalysts outlined the beneficial contribution of Co3O4 support towards tolerance to water poisoning [15].
In the present work, we focus on pure cobalt oxide catalysts systematically investigating the effect of synthesis method on the physicochemical properties and catalytic efficiency of the derived cobalt oxide materials. We varied step by step several synthesis parameters such as the method (precipitation or hydrothermal procedure), the type of cobalt precursor (nitrate or acetate), the type of precipitating agent (Na2CO3, NH3, urea or NaOH) and the Co precursor/precipitant molar ratio (0.2 or 0.4), trying to optimize their effect on the catalytic properties and thus oxidation performance of the derived materials. In addition, selected Co3O4 samples were also promoted with low amount of Pd (0.5 wt.%), in order to enhance further their catalytic performance in CH4 and CO oxidation reactions. Last but not least catalytic performance of all materials is explored under the same reaction conditions, focusing further on the effect of differently synthesized catalysts.
2 Experimental
2.1 Materials
A series of Co-based catalysts were synthesized with two different methods; the precipitation method (Co3O4–P samples) and the hydrothermal method (Co3O4–H samples). The Co precursor salt and the precipitating agents during catalyst synthesis were also varied, using either cobalt acetate tetrahydrate (Co(CH3CO2)2.4H2O, Fluka, 99%) or cobalt nitrate hexahydrate (Co(NO3)2.6H2O, Merck, 99%), with Na2CO3, NH3, urea and NaOH. For the preparation of Co3O4–P samples an aqueous solution of cobalt precursor, acetate (ace) or nitrate (nit) was initially heated to 70 °C and then the precipitating agent (1 M solution of Na2CO3, NH3 or NaOH) was rapidly added, kee** the resulting mixture at 70 °C for 1 h under continuous stirring. The resulting precipitate was filtered, washed several times with double distilled water and ethanol, dried overnight at 110 °C and then calcined at 600 °C for 3 h under air flow. The samples prepared with precipitation method are labeled herein as P-ace or nit-Na2CO3–, NH3– or NaOH–x, where x is the investigated molar ratios of Co/Na (0.2 and 0.4).
For the preparation of Co3O4–H samples, cobalt precursor (acetate or nitrate), was dissolved in double distilled water. Then, urea or NaOH solution (1 M) was rapidly added, under vigorous stirring at room temperature. The mixed solution was placed in a 1L Teflon bottle, which was sealed and heated at 110 °C for 6 or 24 h (aging time). The as-obtained material was washed several times with double distilled water and ethanol, and then dried and calcined at 600 °C for 3 h under air flow. The hydrothermally prepared samples are labeled herein as H-ace or nit-urea or NaOH–x–taging, where x is the investigated molar ratios of Co/Na (0.2 and 0.4) and taging the aging time (6 and 24 h).
The addition of 0.5 wt.% Pd over selected Co3O4 samples was performed via incipient wetness impregnation method, using Pd nitrate (Pd(NO3)2.2H2O, Sigma-Aldrich) as the precursor salt. The derived samples were dried at 110 °C overnight and finally calcined under air flow at 500 °C for 5 h. All the code names of the as synthesized Co3O4 samples and the synthesis parameters (precursor salt, Co/Na ratio, aging time, precipitating agent) are presented in Table 1.
2.2 Catalysts Characterization
2.2.1 Textural and Structural Characterization (BET Method, XRD, ICP)
The physicochemical characteristics of the as prepared catalysts were determined by the adsorption–desorption isotherms at −196 °C, using the Nova 2200e (Quantachrome) flow apparatus. BET surface areas were obtained according to the Brunauer–Emmett–Teller (BET) method, at the relative pressure in the range of 0.05–0.30. The total pore volume was calculated based on nitrogen volume at the highest relative pressure, whereas the average pore size diameter was determined by the Barrett-Joyner-Halenda (BJH) model, using the desorption branch of the isotherm. Prior to measurements the samples were degassed at 250 °C under vacuum.
The crystalline structure of the catalysts was determined by X-ray powder diffraction (XRD) on a Siemens D 500 diffractometer at 40 kV and 30 mA, with Cu Kα radiation (λ = 0.154 nm). Diffractograms were recorded in the 2–80° 2θ range at a scanning rate of 0.02° over 2 s. The Scherrer equation was employed to determine the particle size of Co3O4, based on their most intense diffraction peaks (~ 36.9° and ~ 59.4°).
Inductive coupled plasma-atomic emission spectroscopy (ICP-AES) was performed for the determination of Pd content on Co3O4 samples, using a 4300 DV PerkinElmer Optima spectrometer.
2.2.2 Oxygen Storage Ability and Mobility (O2–TPD and CO–TPR)
Oxygen temperature-programmed desorption (O2–TPD) experiments were performed in a bench-scale unit, with a fixed bed reactor (0.1 g of sample loading). The temperature was increased to 400 °C (heating rate 5 °C/min) under 20 vol.% O2/N2 (50 cm3/min), for 30 min and was cooled to room temperature to adsorb oxygen for 30 min. After that, the samples were purged under He flow for 1 h, in order to remove physically adsorbed oxygen. The desorption step was conducted under He flow (50 cm3/min) up to 600 °C at a rate of 10 °C/min. The reactor exit was coupled with a mass spectrometer (MS), detecting the signal of O2 (m/z = 32).
Temperature-programmed reduction with CO (CO–TPR) was performed in the same unit. The reactor was loaded with the sample (0.1 g) and a pre-treatment was initially performed at 400 °C for 1 h under He flow, in order to remove the humidity and any other physiosorbed species from the sample surface. The reduction step was performed with 5 vol.% CO/He (50 cm3/min), from room temperature up to 600 °C (heating rate: 5 °C/min). The effluent gases were analyzed by mass spectrometry (MS). The main m/z fragments registered were CO: 28 and CO2: 44.
2.3 Catalytic Activity Measurements
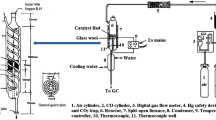
Catalytic performance experiments were carried out in a quartz fixed-bed reactor, loaded with 0.6 g of catalyst. The total gas flow feed rate was 900 cm3/min corresponding to a gas hourly space velocity (GHSV) of 40,000 h−1. For the CH4 combustion experiments, the composition of the feed was 0.5 vol.% CH4 and 10 vol.% O2, balanced with He. The CH4 conversion was monitored in the range 300 – 600 °C (in a decreasing temperature mode). For the CO oxidation, the feed composition was 1 vol.% CO, 1 or 10 vol.% O2, balanced with He and the CO conversion was monitored in the 80–300 °C range (in a decreasing temperature mode). The composition of the effluent gas for both reactions, was analyzed using a FT-IR gas analyzer from MKS instruments (MKS-MG2030Germany).
The conversion of CH4 and CO was calculated using the Eqs. (1) and (2) respectively, where the CCH4, in and CCO, in represent the CH4 and CO concentration of inlet and CCH4, out and CCO, out the concentration of the outlet stream.
3 Results and Discussion
3.1 Catalytic Activity of Co3O4 Catalytic Materials for CH4 Oxidation
3.1.1 Effect of Synthesis Method and Precipitating Agent
The catalytic performances of Co3O4 materials, prepared by different synthesis methods and parameters, in complete oxidation of CH4 were investigated using a fixed-bed reactor [10]. Figure 1 presents the CH4 oxidation performance of Co3O4 catalysts prepared with precipitation and hydrothermal methods, using different precipitating agents, like Na2CO3, NH3, urea and NaOH.
Co3O4 prepared with the precipitation method from Co acetate and using Na2CO3 as the precipitating agent (P-ace-Na2CO3) presented the worse performance among the different solvents used with T50% ~ 597 °C, followed by P-ace-NH3, with T50% ~ 492 °C. When NaOH was used for the synthesis, cobalt oxide catalytic performance was improved and the light-off curve shifted to lower temperatures, achieving 50% CH4 conversion at 434 °C for P-ace-NaOH-0.4. The same was observed for the hydrothermally prepared cobalt oxides (NaOH vs urea), where H-ace-NaOH-0.4-24 h present higher activity than the urea catalyst (T50% ~ 458 °C and T50% ~ 478 °C, respectively).
Tables 2 and 3 present the textural properties of the as prepared catalysts and it is worth to mention that their physicochemical properties seem to follow their catalytic performance. Thus, the limited activity of the samples prepared with Na2CO3, NH3 and urea is attributed to the low surface area and mainly to the high mean particle size, which is 57.5 nm, 66.7 nm and 65.3 nm, respectively. On the other side, when NaOH was used the size of the formed crystallites was smaller, ranging between 47 and 49 nm. The mean particle size of all the samples were calculated applying Scherrer equation at 2θ: 36.9° and 59.4° from the XRD diffractograms.
XRD patterns (not shown here) of all Co3O4 samples prepared with either the precipitation or the hydrothermal method exhibits characteristic diffraction peaks at 2θ: 19.0°, 31.3°, 36.9°, 38.5°, 44.8°, 55.9°, 59.4° and 65.2°, which are attributed to the Co3O4 and suggest that the cobalt precursor was completely turned into a face centered cubic unit cell of Co3O4 (space group Fd3m) with a spinel type structure after calcination [10].
Comparing the two synthesis methods with NaOH as precipitating agent, it seems that the precipitation method led to higher surface areas and smaller crystallites, enhancing the CH4 oxidation performance of P-ace-NaOH-0.4. Thus, NaOH was selected as the precipitating agent to further investigate the effect of the synthesis parameters on the CH4 oxidation performance and the physicochemical properties of Co3O4 for both synthesis methods.
3.1.2 Effect of Synthesis Parameters: Cobalt Precursor, Co/Na Molar Ratio and Aging Time
The effect of cobalt precursor, Co/Na ratio and the aging time (for hydrothermal Co3O4 catalysts) for the CH4 oxidation is presented in Figs. 2 and 3, respectively. The superiority of the Co acetate precursor during precipitation synthesis is evidenced from the whole CH4 conversion vs temperature curves (Fig. 2a). The curve is shifted to lower temperatures, as compared to the catalyst prepared with cobalt nitrate and the difference in T50% is > 20 °C (T50% ~ 434 °C for P-ace-0.4 and 458 °C for P-nit-0.4). More significantly, Co/Na molar ratio (0.2 vs 0.4) does not seem to affect the CH4 oxidation performance of Co3O4 prepared by precipitation method (Fig. 3a). It should be also mentioned that when attempting to use Co/Na molar ratios higher 0.5, complete precipitation was not achieved, as evidenced by significant Co leaching in the filtrate. On the other hand, lower surface areas are observed when applying the hydrothermal method (Co3O4–H samples), thus leading to bigger crystallites. In this case, the aging time (6 or 24 h) seems to be more crucial than the type of Co precursor salt (Fig. 2b) and Co/Na molar ratio (Fig. 3b). As expected, longer aging (24 h) and higher Co/Na ratio (0.4), leads to larger crystallites, directly affecting the surface area of the derived materials. Use of rather low Co/Na ratio (0.2 instead of 0.4) interestingly results in similar surface area and crystallite size.
Among all the Co3O4 samples, the best catalysts for the CH4 oxidation are P-ace with Co/Na ratio 0.2 and 0.4 and H-ace-0.4-6 h presenting the highest surface areas (~ 16—18 m2/g) and the lowest Co3O4 particle sizes (~ 33—43 nm). High surface areas and small crystallite sizes enhance the catalytic activity, providing more active sites (Co3+) [16]. All three catalysts present more or less the same CH4 oxidation performance with T50% ~ 430 °C. Different morphology (crystallite shape and size) is generally suggested, when varying the synthesis parameters; a property expected to affect the catalytic activity [6] for both CH4 and CO oxidation reactions and currently explored via HR-TEM.
3.2 Oxygen Storage Mobility (O2-TPD) and Reducibility (CO-TPR) of Co3O4
Catalyst efficiency is directly associated with the oxygen availability/mobility of the catalysts and possibly related with their morphology. In addition, besides the interaction of Co3O4 catalytic materials with O2, further investigation was also performed with CO (CO-TPR) to study the reducibility of the catalysts.
The oxygen mobility of Co3O4 catalysts was investigated with O2-TPD technique and the results are presented in Fig. 4. Selected samples, with low (P-ace-Na2CO3, H-ace-urea-24 h) and high (P-ace-0.2, P-ace-0.4 and H-ace-NaOH-0.2-6 h) CH4 oxidation performance were studied and the corresponding desorption curves revealed two desorption areas; the low (< 250 °C) and the high (250 – 600 °C) temperature area. The peaks at low temperatures can be assigned to the desorption of surface adsorbed oxygen, while at high temperatures the desorption of surface lattice oxygen takes place [17,18,19].
It is obvious that the desorption ability of the adsorbed surface oxygen species is better when NaOH is used as the precipitating agent, instead of Na2CO3 and/or urea. P-ace-Na2CO3 presented two peaks at temperatures higher than 250 °C, with maximum at 320 °C and 450 °C, while H-ace-urea-24 h presented very low intensity peaks at 155 °C and 346 °C (with a shoulder at 430 °C).
On the other side, the use of NaOH as a precipitating agent during Co3O4 synthesis improves the mobility of the oxygen species, shifting the peaks at lower temperatures and increasing the intensity of the desorption profiles, indicating the desorption of higher amount of oxygen. Between the precipitation and hydrothermal method, the cobalt oxides prepared via precipitation present higher oxygen mobility, with the desorption peak of P-ace-NaOH-0.2 starting at 70 °C, with a maximum at 150 °C and 430 °C.
The oxygen mobility of P-ace-NaOH-0.2 enhances the formation of oxygen vacancies on the surface of the catalyst, which reflects the amount of Oads that could be formed. This oxygen species reacts with CO adsorbed on Co3+ ions and produces CO2. Thus, Co3+ species are the active sites for CH4 and CO oxidation, while Co2+ is almost inactive. In combination with the oxidation state of cobalt, the morphology of Co3O4 is strongly related to its catalytic performance [20,21,22].
The formation of oxygen vacancies enhances the redox properties of the catalysts, improving the reducibility of Co3O4 [23]. To further investigate the reducibility of the Co3O4 catalysts, CO–TPR experiments were performed. Figure 5(a) presents the intensity of CO signal (28), while Fig. 5(b) the CO2 (44) during CO–TPR. In all cases, it is obvious that the consumed CO was converted to CO2 and both signal profiles are perfectly matched. All the CO profiles present two main peaks, centered at 315 °C the first one and a broad peak, with shoulders in the temperature range of 335 – 550 °C. The reduction of Co3O4 is a two-step reduction process; Co3O4 is reduced to CoO and then, to metallic Co [19, 24]. The first peak at 315 °C may be assigned to the reduction of Co3+ to Co2+. In addition, all the CO profiles of Co3O4 present one low intensity peak at 120 °C, probably related to the reduction of bulk Co3O4 and the reaction of CO with the catalysts surface lattice oxygen species [25]. The broad peak starts at 335 °C and the reduction of CoO to metallic cobalt under CO was completed at 500 – 540 °C.
It is obvious that in the case of the samples prepared with urea and Na2CO3 as precipitating agents (H-ace-urea-24 h and P-ace-Na2CO3), the reduction profiles are shifted to higher temperatures, from 315 °C to 330 °C for H-ace-urea-24 h and 322 °C for P-ace-Na2CO3, for the first peak, while the second broad peaks started at temperatures higher than 350 °C, compared with the samples prepared with NaOH, indicating that the precipitating agent affect the reducibility of the cobalt catalysts. It is probably related with the size of cobalt crystallites; as the size increases, i.e., from 43 nm for P-ace-NaOH-0.2 to 57 nm for P-ace-Na2CO3, the peaks are shifted to higher temperatures. Li et al. [26] studied the size effect of Co3O4 nanoparticles and tested them for CO oxidation. The results revealed that the catalyst activity is depending on the size of the Co3O4 nanoparticles, with the smallest being highly active. In addition, the areas of the reduction peaks are smaller, indicating that these catalysts have less active oxygen species [19].
The Co3O4 prepared with NaOH present peaks at the same temperature area, with different reduction areas. P-ace-NaOH-0.2 present the highest CO consumption (and CO2 formation), followed by P-ace-NaOH-0.4 and H-ace-NaOH-0.2-6 h. Especially the first peak at 315 °C due to the reduction of Co3+, revealed that P-ace-NaOH-0.2 present higher amount of Co3+ active sites, which is in line with the O2-TPD profiles. The combination of oxygen adsorption capacity, mobility and cobalt’s oxidation state of P-ace-NaOH-0.2 enhances the efficiency of the catalyst for the CH4 oxidation reaction.
3.3 Catalytic Activity of Optimum Co3O4 Catalysts for CO Oxidation
Selected Co3O4 catalysts were also tested for the oxidation of CO using two different reaction feeds: 1 vol.% CO, 1 vol.% O2 and 1 vol.% CO, 10 vol.% O2, balanced with He and the results are presented in Fig. 6. The profiles are shifted to lower temperatures, when 10% vol. O2 was used in the feed, with a simultaneous important decrease of T50%. The order of the catalyst’s performance is the same under both feeds, with H-ace-NaOH-0.2-6 h (T50% ~ 131 °C under 1 vol.% O2 and 114 °C under 10 vol.% O2 in the feed) shown as the optimum catalysts, followed by P-ace-NaOH-0.2 and P-ace-NaOH-0.4. In addition, P-ace-Na2CO3 was tested for CO oxidation reaction. The lower catalyst performance is also evident for the oxidation of CO, with T50% ~ 190 °C. The low oxidation performance (as presented above) is related to the large particle size (57 nm), while the same trend was observed when urea was used as precipitating agent (resulting in particle sizes of 65 nm).
3.4 Pd-Promoted Co3O4 Catalysts
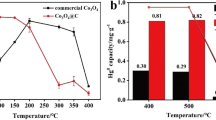
As already mentioned, selected Co3O4 catalysts were further promoted with the addition of 0.5 wt.% Pd. Among the precipitated catalysts, the impregnation of Pd was performed over the samples originally synthesized with cobalt acetate and Co/Na ratio 0.2 and 0.4, while H-ace-NaOH-0.2-6 h was selected among the hydrothermally prepared cobalt oxides. The catalysts were tested for both CH4 and CO oxidation (1% vol. and 10% vol. O2 in feed) reactions and the results are presented in Fig. 7a and Fig. 7b, c, respectively.
The incorporation of Pd over the optimum pure cobalt oxides improved further their CH4 oxidation performance (Fig. 7a). The curves were shifted to lower temperatures and the T50% was decreased up to 60 °C for Pd/P-ace-NaOH-0.2 (T50% ~ 373 °C), 67 °C for 0.5Pd/H-ace-NaOH-0.2-6 h (T50% ~ 387 °C) and 54 °C for Pd/P-ace-NaOH-0.2 (T50% ~ 388 °C). The 0.5Pd/P-ace-NaOH-0.2 sample is shown as the best catalyst for CH4 oxidation, while H-ace-NaOH-0.2-6 h exhibited the highest improvement with the addition of Pd.
On the other side, CO oxidation efficiency of Pd-doped Co3O4 catalysts (Fig. 7b) did not follow the same activity sequence as CH4 oxidation. A similar trend is observed in the literature [27], where the effect of Pd was different for CH4 and CO oxidation; Pd enhances the activity of Co3O4 for CH4 oxidation, while a significant decline was observed for the CO oxidation. In our case, Pd enhanced the activity of P-ace-NaOH-0.4 (under both feeds), shifting light-off curves to lower temperatures and decreasing T50% ~ 17 °C (from 153 °C for P-ace-NaOH-0.4 under 1% vol. O2 to 137 °C with Pd impregnation and from 137 °C to 118 °C, under 10% vol. O2). The 0.5Pd/P-ace-NaOH-0.2 cobalt oxide presents more or less the same catalytic performance for CO oxidation with pure cobalt oxide (P-ace-NaOH-0.2), especially under 1% vol. O2 in the feed, while under 10% vol. O2 the curve is slightly shifted to lower temperatures, with up to 5 °C lower T50%.
The best catalyst for CO oxidation, with or without Pd, is Co3O4 prepared by hydrothermal method and with Co/Na ratio 0.2 and aging time 6 h (H-ace-NaOH-0.2-6 h sample). The addition of Pd did not improve further its catalytic performance, presenting the same activity with pure cobalt oxide, with T50% ~ 134 °C and 114 °C under 1 vol.% and 10 vol.% O2 in the feed, respectively.
The addition of Pd on Co3O4 catalysts prepared with precipitation method (P-ace-NaOH-0.2 and -0.4) slightly decreased the surface area and increased the pore diameter, as shown in Table 4. The opposite was observed for 0.5Pd/H-ace-NaHO-0.2-6 h, where the incorporation of Pd did not affect the surface area or slightly increased it (from 11.4 nm to 11.7 nm). The XRD patterns (not presented here) revealed peaks of Co3O4, without the existence of PdO phase, probably due to the low Pd loading (0.5% wt.) and the good dispersion of the metal over cobalt oxide. The calculated crystal size of Co3O4 (applying the Scherrer equation) was slightly increased for the Pd on Co3O4-P, while for the hydrothermally prepared cobalt oxide, the impregnation of Pd decreased the crystallites size from 53.7 nm to 48.5 nm.
No important changes were observed on the reduction profiles of Co3O4 (CO-TPR) after the incorporation of Pd (Fig. 8). The same double peak, at the same temperature area was observed for Pd-based Co3O4 catalysts. The main differences observed relates with the intensity of the peaks (CO consumption), where 0.5Pd/P-ace-NaOH-0.4 and 0.5Pd/H-ace-NaOH-0.2-6 h present more or less the same intensity and higher than 0.5Pd/P-ace-NaOH-0.2. These two catalysts present the same catalytic performance for both CH4 and CO oxidation, with higher performance in CO oxidation, while 0.5Pd/P-ace-NaOH-0.2 in CH4 oxidation. This is probably related to the existence of more Co3+ active sites according the intensity of the first peak in CO-TPR profiles of Pd-based cobalt oxides P-ace-NaOH-0.4 and H-ace-NaOH-0.2-6 h.
In addition, O2-TPD profiles of Pd-based cobalt oxides are presented in Fig. 9. For all Pd/Co3O4 catalysts the first peak is the same with the pure cobalt oxides, with the peak of 0.5Pd/P-ace-NaOH-0.2 shifted to 167 °C, compared to P-ace-NaOH-0.2, which was centered at 150 °C. On the other side, the addition of Pd over H-ace-NaOH-0.2-6 h shifted the first peak to 168 °C (from 176 °C for pure hydrothermally prepared Co3O4). The high temperature area (250 – 500 °C) of the desorption of surface lattice oxygen presented higher oxygen adsorption with the incorporation of palladium.
Table 5 presents several Co3O4 catalysts reported in the literature, prepared either with hydrothermal or precipitation method, showing different morphologies and similarly tested for CH4 and/or CO oxidation. As obvious from Table 5, the metal oxide morphology, actually configuring the surface topology is suggested in the literature as the most crucial property, mainly defining the catalytic efficiency. On the other hand, catalytic performance of Co-based materials synthesized during the current study may not reach the low T50% efficiency of the catalysts reported in the literature, but this trend is possibly related not only with the morphology (currently explored for catalysts of the present study) but also with the Co3O4 crystallite size. In conclusion, both from literature data and our current investigation not only morphology but crystallite size (depending on synthesis parameters) is suggested to be very important for both CH4 and CO oxidation reactions. Interestingly, when incorporating Pd on cobalt oxide, the differences in catalytic performance between our catalysts and those reported in the literature are not so big for both reactions. This is an important observation, if we consider that materials of the present study are loaded with much lower Pd content than other catalysts in Table 5. In this case, that is for Pd/Co3O4 catalysts, optimization of cobalt oxide support should focus more on catalyst stability (e.g. durability, water tolerance, etc.) and not only on the efficiency of the materials studied. Nevertheless optimally synthesized cobalt oxide support may minimize if not replace the expensive noble metal.
4 Conclusions
The present study systemically investigates the effect of synthesis method (type and synthesis parameters) on the physicochemical properties and catalytic efficiency of the derived Co3O4 for oxidation of CH4 and CO. Differentiating synthesis parameters directly affects the porosity and morphological characteristics (surface area and crystallite size) and other crucial properties (oxygen storage/redox performance) of the derived catalysts, thus influencing their catalytic performance in both oxidation reactions. Among the different precipitating agents, when NaOH was used, the size of the formed cobalt oxide crystallites was smaller, presenting higher catalytic activity for CH4 oxidation. Between the precipitation and the hydrothermal method, the precipitation led to higher surface areas and smaller crystallites than hydrothermal, enhancing CH4 oxidation performance of Co3O4.
The investigation of other synthesis parameters revealed the superiority of cobalt acetate as a precursor salt, while for the hydrothermally prepared cobalt oxides the aging time (6 or 24 h) seem to be more crucial than the type of Co precursor salt and Co/Na molar ratio. Longer aging (24 h) led to larger crystallites, directly affecting the surface area of the derived materials. The best Co3O4 for CH4 oxidation was prepared with precipitation method, using NaOH and cobalt acetate (P-ace-NaOH-0.2). The combination of oxygen adsorption capacity, mobility and cobalt’s oxidation state of P-ace-NaOH-0.2 enhanced the efficiency of the catalyst for the CH4 oxidation reaction.
Concerning CO oxidation on the other side, the hydrothermally prepared cobalt oxide H-ace-NaOH-0.2-6 h presented higher catalytic performance than the precipitated samples. The effect of Pd (0.5wt.%) was different for CH4 and CO oxidation reactions. Pd enhance the activity of Co3O4 for CH4 oxidation, while the same catalytic performance was observed for the CO oxidation.
Concerning Co3O4 materials it seems that besides morphology (currently explored) crystal size is a very crucial parameter affecting surface topology and thus catalytic performance. On the other hand, for Pd/Co3O4 catalysts, optimization of cobalt oxide support should focus more on catalyst stability (e.g. durability, water tolerance, etc.) and not only efficiency of the materials studied, simultaneously aiming to minimize noble metal loading.
References
Trivedi S, Prasad R (2016) Reactive calcination route for synthesis of active Mn–Co3O4 spinel catalysts for abatement of CO-CH4 emissions from CNG vehicles. J Environ Chem Eng 4:1017–1028
Clark NN, Johnson DR, McKain DL, Wayne WS, Li H, Rudek J, Mongold RA, Sandoval C, Covington AN, Hailer JT (2017) Future methane emissions from the heavy-duty natural gas transportation sector for stasis, high, medium, and low scenarios in 2035. J Air Waste Manag Assoc 67(12):1328–1341
Dey S, Dhal GC, Mohan D, Prasad R (2019) Synthesis of highly active cobalt catalysts for low temperature CO oxidation. Chemical Data Collections 24:100283
Topka P, Dvořáková M, Kšírová P, Perekrestov R, Čada M, Balabánová J, Koštejn M, Jirátová K, Kovanda F (2020) Structured cobalt oxide catalysts for VOC abatement: the effect of preparation method. Environ Sci Pollut Res 27:7608–7617
Zasada F, Janas J, Piskorz W, Gorczyńska M, Sojka Z (2017) Total oxidation of lean methane over cobalt spinel nanocubes controlled by the self-adjusted redox state of the catalyst - experimental and theoretical account for interplay between the langmuir-hinshelwood and mars van krevelen mechanisms. ACS Catal 7(4):2853–2867
Liu J, Zhou C, Yue W, Yan B, Lin Y, Huang A (2020) Facile and green template-free synthesis of morphology-controllable Co3O4 catalysts for CO oxidation. Chem Phys Lett 756:137817
Choya A, De Rivas B, González-Velasco JR, Gutiérrez-Ortiz JI, López-Fonseca R (2018) Oxidation of residual methane from VNG vehicles over Co3O4-based catalysts: comparison among bulk, Al2O3-supported and Ce-doped catalysts. Appl Catal B 237:844–854
Lykaki M, Pachatouridou E, Iliopoulou E, Carabineiro SAC, Konsolakis M (2017) Impact of the synthesis parameters on the solid-state properties and the CO oxidation performance of ceria nanoparticles. RSC Adv 7:6160–6169
Zheng Y, Yu Y, Zhou H, Huang W, Pu Z (2020) Combustion of lean methane over Co3O4 catalysts prepared with different cobalt precursors. Royal Society of Chemistry 10:4490–4498
Darda S, Pachatouridou E, Lappas A, Iliopoulou E (2019) Effect of preparation method of Co–Ce catalysts on CH4 combustion. Catalysts 9(3):219–234
Ciuparu D, Katsikis N, Pfefferle L (2001) Temperature and time dependence of the water inhibition effect on supported palladium catalyst for methane combustion. Applied Catalysis A 216:209
Geng H, Yang Y, Zhang L, Ran J, Yan Y (2017) Methane oxidation with low O2/CH4 ratios in the present of water: combustion or reforming. Energy Convers Manage 32:339–346
Li W, Lin Y, Zhang Y (2003) Promoting effect of water vapor on catalytic oxidation of methane over cobalt/manganese mixed oxides. Catal Today 83:239
Qiao D, Lu G, Guo Y, Wang Y, Guo Y (2010) Effect of water vapor on the CO and CH4 catalytic oxidation over CeO2 -MOx (M = Cu, Mn, Fe Co, and Ni) mixed oxide. J Rare Earths 28:742
Nasr S, Semagina N, Hayes RE (2020) Kinetic modelling of Co3O4- and Pd/Co3O4-catalyzed wet lean methane combustion. Emission Control Sci Technol 6(2):269–278
Wang Y, Wei X, Hu X, Zhou Y (2019) Effect of formic acid treatment on the structure and catalytic activity of Co3O4 for N2O decomposition. Catal Lett 149:1026–1036
Xue L, Zhang C, He H, Teraoka Y (2007) Catalytic decomposition of N2O over CeO2 promoted Co3O4 spinel catalyst. Appl Catal B 75:167–174
Yu Y, Takei T, Ohashi H, He H, Zhang X, Haruta M (2009) Pretreatments of Co3O4 at moderate temperature for CO oxidation at− 80° C. J Catal 267:121–128
Tang W, Weng J, Lu X, Wen L, Suburamanian A, Nam CY, Gao PX (2019) Alkali-metal poisoning effect of total CO and propane oxidation over Co3O4 Nanocatalysts. Appl Catal B 256:117859
Chen Z, Wang S, Liu W, Gao X, Gao D, Wang M, Wang S (2016) Morphology-dependent performance of Co3O4 via facile and controllable synthesis for methane combustion. Appl Catal A 525:94–102
Hu L, Peng Q, Li Y (2008) Selective synthesis of Co3O4 nanocrystal with different shape and crystal plane effect on catalytic property for methane combustion. J Am Chem Soc 130:16136–16137
Jia Y, Wang S, Lu J, Luo M (2016) Effect of structural properties of mesoporous Co3O4 catalysts on methane combustion. Chem Res Chin Univ 32(5):808–811
Wang Z, Wang W, Zhang L, Jiang D (2016) Surface oxygen vacancies on Co3O4 mediated catalytic formaldehyde oxidation at room temperature. Catal Sci Technol 6:3845
Zeng L, Li K, Huang F, Zhu X, Li H (2016) Effects of Co3O4 nanocatalyst morphology on CO oxidation: synthesis process map and catalytic activity. Chin J Catal 37:908–922
Lukashuk L, Föttinger K, Kolar E, Rameshan C, Teschner D, Hävecker M, Knop-Gericke A, Yigit N, Li H, McDermott E, Stöger-Pollach M, Rupprechter G (2016) Operando XAS and NAP–XPS studies of preferential CO oxidation on Co3O4 and CeO2–Co3O4 catalysts. J Catal 344:1–15
Li L, Yao Y, Tang Z, JiDaiShen WYX (2016) Size Effect of Co3O4 nanoparticles as catalysts for CO oxidation. J Nanosci Nanotechnol 16:7573–7578
Chen Z, Wang S, Ding Y, Zhang L, Lv L, Wang M, Wang S (2017) Pd catalysts supported on Co3O4 with the specified morphologies in CO and CH4 oxidation. Appl Catal A 532:95–104
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iliopoulou, E.F., Darda, S., Pachatouridou, E.P. et al. Exploring Synthesis Approaches of Co-based Catalysts for the Efficient Oxidation of CH4 and CO. Top Catal 66, 999–1012 (2023). https://doi.org/10.1007/s11244-022-01724-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-022-01724-0