Abstract
Agastache rugosa (Fisch. & C. A. Mey.) Kuntze known as Korean Mint is a medicinal and aromatic plant containing rosmarinic acid as a major bioactive polyphenol but its content in this herbal drug is variable. Plant in vitro culture is one of the approaches used to overcome the complexity of environmental factors influencing phytochemical profiles in medicinal plants. In this study, hairy root cultures of A. rugosa were established through the infection of Rhizobium rhizogenes. Four hairy roots lines were selected on the basis of biomass production in liquid media. Transformation was confirmed by PCR using rol C specific primers. The hairy roots were successfully cultured in 300 mL conical flasks and scaled-up using three bioreactor types (nutrient sprinkle bioreactor—NSB and two modular temporary immersion systems—RITA® and Plantform®). The UPLC analysis of A. rugosa transformed roots methanol extracts showed the presence of 24 polyphenolic compounds with the predominance of rosmarinic acid (RA), which level ranged between 3.82 and 9.16 mg/g of dry weight, depending on the culture system. Nineteen compounds were identified in hairy roots growing in NSB system, 9 of them were identified in roots cultured in RITA® or Plantform® and 7 compounds were identified in roots from Erlenmeyer flasks. The R. rhizogenes infection (strain A4) was found to be an effective method of hairy root culture establishment of A. rugosa.
Key message
Nutrient sprinkle bioreactor was the most efficient system for A. rugosa hairy roots growth and the production of specialized metabolites with rosmarinic acid a the major constituent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant natural compounds sourcing become an issue since the need of using them has grown. Paclitaxel, shikonin, carthinin, betacyanins and many others are the most recognizable examples of specialized plant metabolites, the acquisition of which in larger quantities required many tedious biotechnological treatments.
Plant in vitro culture based approaches have been used since almost a century to study plant specialized metabolism and to obtain selected phytochemicals via manipulating their composition and content for pharmaceutical, cosmetic and food industry purposes. Among the commercially relevant compounds known to be produced industrially via plant biotechnology are coptisine, paclitaxel, shikonin, and several other with new examples being added on a regular basis (reviewed by Espinosa‑Leal et al. 2018).
The difficulty in sustained and predictable recovery of these substances from naturally grown plant sources results for example from the complexity of their biosynthesis pathways and dynamic modifications which take place in planta in a response to environmental factors that often remain undefined. In this respect, the non-recombinant biotechnology offers an advantage of highly controlled experimental conditions, accelerated growth cycle, and independence on natural resources, being, however, more expensive and labor intensive than conventional cultivation. Therefore, the successful implementation of biotechnology for production of phytochemicals requires tedious optimization and upscaling experiments to fine tune the culture conditions to simultaneously achieve a possibly high biomass growth rate and superior metabolic profile. Hairy roots induced by infection of plant tissues with Rhizobium rhizogenes (previously used scientific name Agrobacterium rhizogenes) are a well-established system for such purposes in hundreds of medicinal plant species (Flores-Félix et al. 2020; Malarz et al. 2023). The hairy root cultures are also exploited as industrial source of extract for pharmaceutical and cosmetic industries, for example ginseng, Echinacea, Coleus (Roy 2021). Among the polyphenols which are considered superior antioxidants and antiinflammatory agents, RA is one of the common and intensively studied compounds which has been also obtained in spectacular amounts in plant cell, tissue and organ cultures, reaching over 30% of the cell dry mass in Coleus blumei (Gertlowski and Petersen 1993; Qian et al. Confirmation of Ri-plasmid integration To confirm the plant transformation, DNA isolation from the collected material was performed, followed by the DNA polymerase chain reaction (PCR). To carry out this reaction, specific primers were used, the sequence of which was designed based on the DNA sequence of the rol C gene located in the T-DNA region of the Ri plasmid of R. rhizogenes and vir G primers, a gene located outside the T-DNA region (Furner et al. 1986). Three clones (K17, K19, K21) from MS medium and four (K12, K14, K16, K21) from ½ MS medium, as well as one sample of untransformed A. rugosa roots derived from an in vitro cultures (negative control) were used in the transformation confirmation procedure. The material was triturated in liquid nitrogen in a mortar and accurately weighed approx. 100 mg into 1.5 mL test tubes. The extraction buffer of 396 µL and β-mercaptoethanol of 4 µL were added. It was vortexed and incubated at 60 °C for 30 min. Subsequently, 60 μL of a 24:1 mixture of chloroform and isoamyl alcohol was added to the test tube and vortexed for approximately 10 s. It was centrifuged at 10,000 rpm for 3 min, then the supernatant was transferred to a fresh tube. The mixture of chloroform and isoamyl alcohol was added again, mixed, centrifuged and the supernatant added to the previous portion. The cold isopropanol of 280 µL was added to the supernatant, the contents were mixed gently, centrifuged at 10,000 rpm for 3 min. The liquid phase was removed from the tube with a pipette. 70% ethanol (500 µL) was added to the DNA pellet of, and mixed, centrifuged at 10,000 rpm for 3 min, and then the ethanol was removed. The action was repeated and the DNA was allowed to dry. The dried pellets were dissolved in 50 μL of a 10 μg/mL RNAse water solution. The solution was incubated at 37 °C for one h and then stored at − 20 °C. The concentration of the isolated DNA was determined using a spectrophotometer. Then, two Master Mix mixtures were prepared consisting of: 111.6 μL of nuclease-free water, 36 μL of Q5 reaction buffer (5 × concentrated), 3.6 μL of free dNTPs nucleotides (10 mM), 1.8 μL of Q5 DNA polymerase (2000 U/mL). To one of them, 9 μL of rol CF and rol CR primers were added, and to the other—analogously vir GF and vir GR. 1 µL of template DNA of each sample was collected into two test tubes. Then 19 µL of Master Mix containing the rol C and vir G primers, respectively, were added to each. The contents of the tubes were mixed, centrifuged and inserted into a thermocycler T100™ Thermal Cycler (BIO-RAD Laboratories InC) for PCR. Nucleic acid electrophoresis was performed on an agarose gel to analyze the PCR product. A 50 mL gel containing 1.5% agarose was prepared with TBE buffer and heated in the flask to reflux temperature. Then, ethidium bromide—a fluorescent dye of 5 μL, visible under UV light, was added to it, which allowed for the subsequent interpretation of the results. The gel was poured into a previously prepared and leveled mold and allowed to cool for 30 min. Prepared samples: 10 μL of PCR product were collected from each of 8 samples into new tubes. Then 2 μL of loading buffer was added, mixed and centrifuged. The gel comb was removed from the set gel, then the gel and the tray were placed in the electrophoresis apparatus Sub-Cell GT (BIO-RAD Laboratories InC). TBE buffer was poured into the apparatus so as to cover the gel. The PCR product mixture of 10 µL and loading buffer were taken and then applied to the wells. The electrodes were connected and the separation was started on 110 V for 30 min. Measurements of the biomass growth were made for the four clones selected on the basis on preliminary observations: K17, K19 from MS medium and K12, K14 from ½ MS medium without growth regulators. For better characteristics of hairy roots growth, apart from biomass, growth rate (µ) as well as time required for double the biomass (doubling time) were calculated for three intensively growing hairy root lines (K12, K17 and K19). The root growth rate was calculated according to the formula: μ—culture growth rate [day per day]. x—mean fresh weight [g/flask] in time t. x0—mean fresh weight [g/flask] in time t0. t0—day of the beginning of the exponential growth phase of the culture. t—the day when the exponential growth phase of the culture ends. ∆t = t − t0 Doubling time (dt) was calculated using the formula: μ—culture growth rate [day per day]. On the basis of the growth, morphology as well as RA content, studied in our preliminary experiments (unpublished), for further experiments K12 clone was chosen. To increase the scale of culture, hairy roots of A. rugosa were cultured in three types of bioreactors: nutrient sprinkle bioreactor (NSB), and two commercially available temporary immersion systems: Plantform® (PlantForm AB, Sweden) and RITA® (Récipient à Immersion Temporiaire Automatiqe, VITROPIC, France). For comparison, the hairy roots were also cultured in conical flasks (300 mL of volume, 80 mL of medium) in the same medium as bioreactor cultures for the same period of time (17 days). The NSB system (volume 5.0 L, working volume 3.0 L) has been described in details by Piątczak et al. (2014). The bioreactor contained 1000 mL of liquid medium. The medium was supplied to the spraying nozzle by a CH-8604 peristaltic pump (Chemap AG, Switzerland). The operating time of the pump was 40 s with 3.0 min breaks (60 mL of medium/1 cycle of pump working). The Plantform® bioreactor (size 180 × 150 × 150 mm) containing 500 mL of liquid medium was serially connected with an Optima pump (Hagen, Canada) with an inlet capacity of 0.6 m3/h. The 800 mL round RITA® bioreactor contained 250 mL of the liquid medium, which delivery was controlled by a DT4.4 pressure pump (Becker, Germany) with a capacity of 4.2 m3/h and pressure of 1000 mbar. The operating time of the pumps in the both temporary bioreactors was 10 min every 80 min. The hairy root cultures in each bioreactor were cultured in liquid MS medium with reduced to half the content of macro- and micronutrients (1/2 MS) supplemented with 3% of sucrose without growth regulators. In the study, hairy roots from 4 subsequent subcultures were used. The inoculum for all the cultures derived from 14th day of culture. The mean fresh weight (FW) of inoculum in NSB system was 12.67 ± 3.08 g/L (1.18 ± 0.1 g/L DW), in RITA® was 12.46 ± 0.5 g/L FW (1.13 ± 0.11 g/L DW), in Plantform® was 7.53 ± 0.28 g/L FW (0.74 ± 0.04 g/L DW) and in 300 mL culture flasks was 15.85 ± 0.47 g/L FW (1.50 ± 0.05 g/L DW). All the cultures were cultured at 26 ± 2 °C in the darkness for 17 days. At the end of the culture period, a biomass of the culture, means of fresh weight (FW) and dry weight (DW) of culture (g/L) and a growth index (GI), calculated as final fresh or dry weights—initial fresh or dry weight/initial fresh or dry weight, were recorded. 100 mg lyophilized and grounded plant material was extracted twice using 2.5 mL solution of 0.1% formic acid and methanol (v/v) in a 4:1 ratio every time. The extraction procedure was performed according to previously published methodology (Zielińska et al. 2019). The separation of polyphenolic compounds was carried out using a UPLC BEH C18 column (1.7 μm, 2.1 × 100 mm, Waters Corporation, Milford, MA). The mobile phase consisted of solvent A (0.1% formic acid in LC–MS grade water, v/v, Merck, Germany) and solvent B (0.1% formic acid in LC–MS grade acetonitrile, Merck Germany). The chromatographic procedure as well as quality and quantity analyses were performed according to previously published methodology (Zielińska et al. 2019). Compounds were optimized to their estimated molecular masses in the negative mode, before and after fragmentation. The authentic standards were used for the comparison of retentions times and spectra of analyzed compounds. The calibration curve for acacetin was used to quantify tilianin. Caffeic, chlorogenic, rosmarinic, p-coumaric and feruloylquinic acids as well as acacetin, apigenin, and phloridzin were quantified with theirs own standards. The quantities were expressed in mg of a compound per g of plant material dry weight (mg/g DW). Calculations of arithmetic means and standard errors (SE) were made using the EXCEL 2010. Each chromatographic analysis was performed six times. Results were reported as mean values ± SE. Experiments in bioreactor systems were repeated four times. Statistical significance was calculated with STATISTICA 13.3 version (TIBCO) using Tukey HSD or Kruskal–Wallis tests at p ≤ 0.05. Scanning electron microscopy. For SEM, the hairy roots fragments were prepared according to previously published methodology (Piątczak et al. 2020) using a Hitachi S-4700 scanning electron microscope (Hitachi, Tokyo, Japan), housed in the Institute of Geological Sciences, Jagiellonian University in Kraków).Hairy root biomass production in culture flasks
Hairy root biomass production in different bioreactor systems
Phytochemical analysis
Extracts preparation
Chromatographic analysis
Statistical analysis
Results
Induction of hairy roots
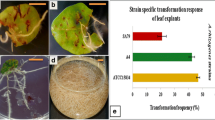
Hairy root cultures of A. rugosa were established through the infection with R. rhizogenes strain (A4). The ability to form roots at the site of infection depended on the type of explant. Leaf blades, for which the transformation frequency was 55%, turned out to be the best explants. In case of nodes, none of the explants responded. Roots were formed through direct rhizogenesis. They were thin, delicate, light-beige-colored (Fig. 1).
An intensive root growth and elongation, as well as numerous axillary branched roots development were observed on both liquid media. The most intensively growing four clones K17, K19 (on MS medium), and K12, K14 (on ½ MS medium) were selected for further investigation on RA production. For comparison, untransformed roots were also cultured on liquid MS and ½ MS media, in which no further growth was observed. The MS medium with half the content of macro-, microelements and iron turned out to be more effective for the growth of root cultures.
Confirmation of transformation
Transformation status of the roots was confirmed by PCR using rol C and vir G specific primers.
PCR reaction was performed to show the presence of a bacterial R. rhizogenes plasmid fragment (rol C gene) in the genome of A. rugosa hairy roots. DNA was isolated from several clones: K17, K19, K21 (from MS medium) and K12, K14, K16, K21 (from ½ MS medium) and from untransformed A. rugosa roots derived from an in vitro cultures (negative control). Primers sequence was designed based on the DNA sequence of the rol C gene, located in the T-DNA region of the Ri R. rhizogenes plasmid. Primers for vir G, a gene located in the plasmid outside the T-DNA region not involved in the genetic transformation, were also used (positive control). An electroforetogram showing the results of the PCR reaction is shown in Fig. 2.
An electrophoretogram showing the DNA separation of transformed and untransformed root samples, enabling the identification of rol C genes; 1—DNA isolated from hairy roots K17 clone, MS medium + rol C (626 bp), 1a—DNA isolated from hairy roots (K17 clone, MS medium) + vir G (273 bp), 2—K19 clone, MS + rol C, 2a—K19 clone, MS + vir G, 3—K21 clone, MS + rol C, 3a—K21 clone, MS + vir G, 4—K12 clone, ½ MS + rol C, 4a—K12 clone, ½ MS + vir G, 5—K14 clone½ MS + rol C, 5a—K14 clone, ½ MS + vir G, 6—K16 clone, ½ MS + rol C, 6a—K16 clone, ½ MS + vir G, 7—K21 clone, ½ MS + rol C, 7a—K21 clone, ½ MS + vir G, 8—DNA isolated from untransformed A. rugosa roots + rol C, 8a—DNA isolated from untransformed A. rugosa roots + vir G. M—GeneRulerTM 100 bp DNA ladder (Fermentas)
On the basis of the electrophoretograms, the genetic transformation of K17, K19, K12 and K14 clones was confirmed. In samples containing isolated DNA from K21 clones (both MS and ½ MS medium) and K16, the vir G gene was additionally identified, the presence of which was most likely due to contamination of the plant material with R. rhizogenes bacteria, therefore further research was carried out only on the aforementioned four clones. In the control sample containing DNA isolated from untransformed A. rugosa roots, none of the identified genes were present.
Hairy root biomass production in Erlenmeyer flasks
For the three intensively growing clones with transformation confirmation (K19 and K17 on MS medium and K12 on ½ MS medium), an average growth rate (µ) and doubling time (dt) were calculated. Regardless of the type of clone and culture medium, the growth cycle lasted 43–50 days. The most intensive growth of cultures were observed between 30 and 40th day of culture. The K19 clone cultured on MS medium achieved a slightly higher biomass than K12 clone, but without the significant difference (at p ≤ 0.05) (Table 1) but both the average growth rate and the time required to double the biomass (dt) was shorter for K12 clone (μ = 0.156; dt = 4.463, respectively) than for K19 (μ = 0.141; dt = 4.906, respectively).
With an aim to test the possibility to scale up, the hairy roots (K12) were cultured for 17 days in three types of bioreactors. Two of them (RITA® and Plantform®) are commercially available temporary immersion systems, where the explants are immersed in the liquid medium only by the short, pre-programmed period of time (in our study 10 min of immersion with 80 min breaks). In the NSB system, the explants are situated at the stainless net in the middle of bioreactor and they are sprinkled with the liquid medium with 3 min intervals. Liquid ½ MS medium without growth regulators was used in the all types of bioreactors. For comparison, the hairy roots were also cultured in Erlenmeyer flasks in the same medium. It was observed that hairy roots cultured in RITA® and NSB bioreactors after 17 days of culture have occupied all available space in each bioreactor. They were thin, brown in the middle and creamy-white at their distal edges. Additionally, roots growing in NSB and Plantform® bioreactors created thin, white, fluffy hair in upper part of culture (Fig. 3). The highest biomass (FW and DW) was recorded for hairy roots growing in RITA® system (160 g/L of FW and 16 g/L of DW). It was almost 1.5 times higher than the biomass of roots growing in 300 mL glass flasks and NSB system, respectively. The lowest biomass and the slowest growth of the hairy roots were observed in Plantform® system (26 g/L of FW and 3 g/L of DW). The biomass increases were 6-times lower in terms of FW and 5.5-times lower in terms of DW than the biomass of hairy roots growing in the RITA and 3–4 times lower than the biomass of roots growing in the flasks and NSB system (Fig. 4A).
Biomass (A) and growth index—GI (B) for FW and DW in g/L of A. rugosa hairy roots (K12 line) after 17 days of growth in ½ MS liquid medium without growth regulators in different bioreactor systems. Bars indicated with the same letter within each series were not significantly different in Tukey test at p ≤ 0.05
After a consideration of the inoculum size, the highest growth index—GI was observed in the RITA® system (GI = 11.8 for FW and 13.5 for DW) followed by the NSB system (GI = 7.5 for FW and 7.3 for DW) and 300 mL flasks (GI = 5.5 for FW and 5.2 for DW). GI in Plantform® bioreactor was 1.7–2.2-times, 2.4–2.5-times and 4.5–4.7 times lower than GI values for DW and FW for hairy roots cultured in flasks, NSB and RITA®, respectively (Fig. 4B).
Phytochemical analysis
The selected hairy root lines were used for the extraction and UPLC analysis of phenolic compounds. Out of the four examined hairy root lines, only one (K12) was highly productive. The line was growing in liquid ½ MS medium and when maintained in the 300 mL flasks, the RA content was 20 to even 40 times higher than in the other lines growing in MS media (data not shown). Therefore, the K12 line was chosen for further experiments with bioreactors. The selected line was continuously cultured in the 300 mL flasks as well as in NSB and two temporary immersed systems (RITA® and Plantform®). The chromatographic analysis shown the presence of polyphenolic compounds and their derivatives (Table 2, Fig. 5).
The poorest phytochemical profile shown the hairy roots cultured in the flasks, consisting of only seven detected phenolic compounds. The major constituent was RA, but its amount was rather low (4.6 mg/g DW). The second in abundance phenolic compound found in hairy roots from culture flasks was a flavonoid—apigenin derivative (peak no 21). Its amount (0.6 mg/g DW) was the highest among all tested culture systems. The other phenolic compounds produced by the roots in the flasks were: tilianin, phloridzin, RA methyl ester, two caffeic acid derivatives, which were present in lower amounts (0.001–0.07 mg/g DW). Interestingly, one of the caffeic acid derivatives (peak no 11) was detected only in roots cultured in the flasks, but its amount was also low (0.029 mg/g DW).
Phytochemical profiles of phenolic compounds in RITA® and Plantform® were similar and consisting of nine compounds in low amounts. Only two compounds: RA and the apigenin derivative were present in higher levels (3.82–4.46 mg/g DW and 0.24–0.46 mg/g DW, respectively). Two compounds: RA hexoside (peak no 15) and RA isomer I (peak no 18) were present only in roots from RITA® and Plantform®, but not in flasks or NSB system.
The most effective for phenolic compounds accumulation in hairy roots of A. rugosa was NSB. After 17 days of culture in this type of bioreactor, 19 phenolic compounds were identified: 13 phenolic acids derivatives and 5 flavonoid derivatives, including acacetin and apigenin derivatives and one dihydrochalcone—phloridzin. Among these compounds, 14 were detected only in the NSB. There were mainly caffeic and caffeoylquinic acids derivatives, acacetin and apigenin derivatives. Similarly to other described culture systems, roots in the NSB produced RA as the major metabolite, but the amount (9.1 mg/g DW) was 2 times higher than in other culture systems (3.8—4.6 mg/g DW). Moreover, the total amounts of phenolic acid and flavonoid derivatives (10.193 and 1.941 mg/g DW, respectively) were also higher than in other tested culture systems (Table 2, Fig. 5).
Discussion
In several previous studies large differences in the RA content were observed in A. rugosa plants from different locations and different experimental treatments (Lee et al. 2008; Tuan et al. 2012; Desta et al. 2015; Zielińska et al. 2016, 2019; Lam et al. 2020a, 2020b, 2020c). In the plants cultivated in Korea, the RA content did not exceed 50 µg/g DW in all plant organs examined individually (Tuan et al. 2012; Desta et al. 2015). In turn, plants of the species cultivated in Poland produced much larger amounts of RA in leaves and inflorescences (0.03–10.76, 0.06–5.27 mg/g DW, respectively) (Zielińska et al. 2016). Moreover, samples derived from in vitro shoot cultures were found to be a rich source of RA (nearly 25 mg/g DW dependent on the treatment) (Zielińska et al. 2019). The establishment of A. rugosa hairy root cultures was reported for the first time by Korean group of Lee et al. in (2008), who also detected large amounts of RA—116.3 mg/g of dry weight in this kind of plant material. Unfortunately, no transformation confirmation have been delivered. This amount of RA was much higher than that detected in our current studies. The observed differences may be due to several factors such as R. rhizogenes strains used to obtain hairy root cultures or growth medium. We used A4 strain of R. rhizogenes for the hairy root obtainment, while in Lee et al. (2008) study R 1000 strain was used. Other studies also showed the effect of different strains of R. rhizogenes on secondary metabolite production. For example Sathasivam et al. (2022) noticed that the best strain was R1601 for phenolic acid content in Ocimum basilicum hairy roots. Moreover it was stated that among the different strains tested for both, hairy roots induction and secondary metabolites accumulation the most effective strains were R. rhizogenes ATCC 13333 and LBA9402 in the plants such as A. rugosa and Morus alba respectively (Park et al. 2017a, 2017b). The composition of media can also influence the biomass yield and the accumulation of metabolites in hairy root cultures. This correlation was noticed not only for A. rugosa hairy roots as in current study and previously described by Lee et al. (2008), but also for other transformed root cultures for example Coleus forskohlii (Li et al. 2005) or Salvia viridis (Grzegordzyk-Karolak 2020).
Due to the huge differences in the content of RA in the plants grown both in vivo and in vitro, we made an attempt to control and upscale biomass production of A. rugosa hairy roots using three different bioreactor systems. This methodological approach let to verify the possible RA content rage production as well as accompanying phenolic compounds in A. rugosa hairy root cultures derived from A4 R. rhizogenes strain infected tissue. RA was efficiently produced in various in vitro cultures of many plant species, such as Coleus blumei Benth. (Petersen 1991; Bauer et al. 2004), Anchusa officinalis L. (Su et al. 1993), Salvia officinalis (Santos-Gomes et al. 2002), Lavandula vera DC. (Georgiev et al. 2006; Georgiev et al. 2009), S. miltiorrhiza (Dong et al. 2010), including A. rugosa (Xu et al. 2008). A. officinalis and C. blumei suspension cell cultures were used in the experiments to discover the enzymes involved in RA biosynthesis. At the early stage of the pathway, which is the conversion of phenylalanine to cinnamic acid, activity is undertaken by enzymes fundamental to the synthesis of all phenylpropanoids (e.g. flavonoids or lignins) (Petersen and Simmonds 2003). The correlation between PAL activity and RA accumulation has been known for a long time (Razzaque and Ellis 1977). In isolated C. blumei protoplasts grown in suspension, a higher content of this metabolite was found in the vacuoles than in the cytoplasm (Häusler et al. 1993).
In our study, for A. rugosa hairy roots induction, the R. rhizogenes strain A4 was chosen based on earlier experiments using several other plant species, such as R. elata (Piątczak et al. 2019), S. bulleyana (Wojciechowska et al. 2020) or S. viridis (Grzegorczyk-Karolak et al. 2017). Similarly to the results of Chaudhuri et al. (2005), Verma et al. (2007), Dehdashti et al. (2017) and Wojciechowska et al. (2020) we observed that the type of explant infected by the bacteria was crucial for hairy roots formation frequency. In all the cited above reports leaves were more susceptible to infection than stems or nodes. However, in many other plant species, the nodes/stems were more effective in hairy root induction than leaves (Grzegorczyk-Karolak et al. 2017; Piątczak et al. 2019). The differences may correspond to the physiological state of plant tissues, especially with the fact that leaves as younger tissues than shoots, may be more susceptible for the Rhizobium infection. Larger number of meristematic cells in leaves compared to stems, with higher proliferative potential may also be one of the explanation. Hairy root induction can be also influenced by the individual susceptibility of plant species (De Cleene and De Ley 1981).
The hairy root lines obtained in this study, with confirmed transformation status, differed in the level of fresh biomass production. The fastest growing lines were K12 and K19, which biomass accumulation was 6.67 times higher than in the slowest growing line (K14). The differences in the growth within different hairy root lines of the same plant species have been described earlier in R. glutinosa (Piątczak et al. 2012) and S. viridis (Grzegorczyk-Karolak 2020). It could be related to differences in the presence or expression level of the rol genes inserted from the Rhizobium Ri plasmid into the plant genome. It is known that rol genes could affect the growth of roots by activating transcription genes (Ono and Tian 2011).
Consequently, we attempted to scale-up the A. rugosa hairy root culture, for the first time, in RITA®, Plantform® and NSB systems and to examine the influence of the type of bioreactor on the biomass (both fresh and dry weight) production and polyphenolic compounds accumulation. In our preliminary studies (data not published) we have observed the best growth and morphology of roots after the 17th day of culture, therefore in this study, all results were recorded after 17 days of hairy roots growth.
The obtained results indicated that bioreactor systems, especially RITA® and NSB, efficiently promote growth of A. rugosa hairy roots. Growth index was 1.5–5 times higher than for other culture systems (300 mL flasks and Plantform®). NSB or RITA® systems have been earlier reported to be effective for growth of other plant cultures, for example Schisandra chinensis microshoots (Szopa et al. 2017, 2021) and Dracocephalum forrestii transformed shoots (Weremczuk-Jeżyna et al. 2020). The high biomass accumulation could be caused by better absorption of nutrients and plant growth regulators from the liquid medium in such types of bioreactors (Preil 2005; Mehrotra et al. 2007). Pavlov and Bley (2006) have also observed that temporary immersion systems, like RITA® could reduce the risk of morphology abnormalities occurring as a result of shear forces (Szopa et al. 2017). Similarly to the results obtained in the our study, other authors also reported advantageous biomass growth in RITA® bioreactors compared to NSB, for example in shoots of Rhododendron tomentosum Harmaja (Jesionek et al. 2017) or shoots of S. chinensis (Szopa et al. 2017, 2021).
On the other hand, lower growth parameters were observed for A. rugosa hairy roots growing in Plantform® system. After 17 days of culture, growth indexes for roots growing in Plantform® were 3 and 5 times lower than these for roots cultured in NSB and RITA®, respectively. It may be a result of lower oxygen availability while being flooded in this type of bioreactor. Therefore, this parameter turned out to be a main limiting factor for large scale cultivation of A. rugosa hairy roots. It may be assumed that the culture conditions in Plantform® bioreactor are not as advantageous for hairy roots growth and metabolite production as in other bioreactors used. Plantform® bioreactor has been designed and used earlier only for shoot cultures, especially for crops like Olea europaea (Benelli and De Carlo 2018) or Capparis spinosa (Gianguzzi et al. 2019), but not for hairy root cultures.
Phytochemical analysis
NSB was the most effective system for polyphenols accumulation at 17th day of culture in liquid growth regulator-free ½ MS medium. Hairy roots growing in the NSB accumulated more polyphenolic metabolites than roots growing in two other bioreactor systems. It was observed that among 19 phenolic compounds detected in extracts from roots cultured in NSB, 13 compounds (10 phenolic acid derivatives and 3 flavonoids derivatives) were found only in this type, whereas in hairy roots growing in RITA®, only 9 compounds were detected. However, the amounts of these metabolites were rather low and did not exceed 0.4 mg/g DW. Regardless of the bioreactor system used, the predominant metabolite was RA, which level varied from 3.8 mg/g DW in roots growing in Plantform® to 9.16 mg/g DW in the NSB. The highest amount (9.16 mg/g DW) of RA was similar to the level of the metabolite present in leaves of two years old plants growing in the field (Zielińska et al. 2016). Nonetheless, this level of RA in hairy roots was achieved in significantly shorter time—17 days. Earlier, other biotechnological treatments, such as precursor feeding, plant growth regulators supplementation or different illumination regime were found to be also effective for promoting RA accumulation (over 20 mg/g DW) (Zielińska et al. 2019), but taking into account the dry biomass (8.71 g), which is possible to achieve during one culture cycle in NSB, it is possible to obtain almost 80 mg of RA during 17 days of culture. Additionally, it is also possible to obtain other metabolites in high amounts, for example acacetin acylglycosyl isomer II (9.2 mg) and cryptochlorogenic acid (6 mg) during single cycle (17 days). These two compounds were produced only in NSB. Moreover, the phytochemical profile of the hairy roots in NSB was richer than in other cultures. For example, in shoot cultures only 7 metabolites were identified (6 phenolic acids derivatives and one flavonoid) (Zielińska et al. 2019). Therefore, taking into consideration all these results, the NSB bioreactor turned out to be effective and feasible system for polyphenolic compounds production in hairy roots of A. rugosa. It is possible to make an effort to further increase the metabolite production in the hairy roots by elicitation or precursor feeding. Earlier, Lee et al. (2008) reported 116.3 mg/g DW of RA in hairy root cultures of this species, but the transformation of the cultures has not been confirmed by molecular methods. NSB system has been also suitable for the growth and polyphenolic compounds production in Dracocephalum forrestii transformed and untransformed shoots (Weremczuk-Jeżyna et al. 2019, 2020), RA production in shoots and hairy roots of Salvia officinalis (Grzegorczyk and Wysokińska 2010), polyphenolic compounds production in shoots of Scutellaria alpina (Grzegorczyk-Karolak et al. 2017), phenolic and flavonoid production in shoots of Rehmannia glutinosa (Piątczak et al. 2014), lignin production in Schisandra chinensis (Szopa et al. 2017), secoiridoid production in shoots of Centaurium erythraea (Piątczak et al. 2005). It is suggested that the high metabolite production in this type of bioreactor is a result of the optimal medium circulation as well as nutrient and oxygen supply and reduced shear forces which often destroy delicate structure of hairy roots (Ziv 2005).
Conclusions
The infection of R. rhizogenes strain A4 was found to be an effective method of A. rugosa hairy root culture establishment. The scale-up of A. rugosa hairy root culture allowed to obtain a significant amounts of plant material rich in bioactive metabolites. The most effective system for the growth of biomass was RITA, where biomass of roots increased almost 15 times after 17 days of culture, but for phenolic compounds production, the most effective was NSB system. Further research is necessary to elucidate exogenous factors influencing the phytochemical composition and optimize the biomass growth with possibly highest accumulation of desired constituents.
Data availability
The data that support the findings of this study are available from the corresponding author on a reasonable request.
References
Bauer N, Leljak-Levanic D, Jelaska S (2004) Rosmarinic acid synthesis in transformed callus culture of Coleus blumei Benth. Z Naturforsch 59:554–560. https://doi.org/10.1515/znc-2004-7-819
Benelli C, De Carlo A (2018) In vitro multiplication and growth improvement of Olea europaea L. cv Canino with temporary immersion system (Plantform™). 3 Biotech 8(7):317. https://doi.org/10.1007/s13205-018-1346-4
Chaudhuri KN, Ghosh B, Tepfer D, Jha S (2005) Genetic transformation of Tylophora indica with Agrobacterium rhizogenes A4: growth and tylophorine productivity in different transformed root clones. Plant Cell Rep 24(1):25–35. https://doi.org/10.1007/s00299-004-0904-x
De Cleene M, De Ley J (1981) The host range of infectious hairy root. Bot Rev 47:147–194
Dehdashti SM, Acharjee S, Kianamiri S, Deka M (2017) An efficient Agrobacterium rhizogenes-mediated transformation protocol of Withania somnifera. Plant Cell Tiss Organ Cult 128:55–65. https://doi.org/10.1007/s11240-016-1081-7
Desta KT, Kim GS, Kim YH, Lee WS, Lee SJ, ** JS, Abd El-Aty AM, Shin HC, Shim JH, Shin SC (2015) The polyphenolic profiles and antioxidant effects of Agastache rugosa Kuntze (Banga) flower, leaf, stem and root. Biomed Chromatogr 30(2):225–231. https://doi.org/10.1002/bmc.3539
Dong J, Wan G, Liang Z (2010) Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J Biotechnol 148(2–3):99–104. https://doi.org/10.1016/j.jbiotec.2010.05.009
Espinosa-Leal CA, Puente-Garza CA, García-Lara S (2018) In vitro plant tissue culture: means for production of biological active compounds. Planta 248:1–18. https://doi.org/10.1007/s00425-018-2910-1
Flores-Félix JD, Menéndez E, Peix A, García-Fraile P, Velázquez E (2020) History and current taxonomic status of genus Agrobacterium. Syst Appl Microbiol 43:126046. https://doi.org/10.1016/j.syapm.2019.126046
Furner IJ, Huffman GA, Amasino RM, Garfinkel DJ, Gordon MP, Nester EW (1986) An Agrobacterium transformation in the evolution of the genus Nicotiana. Nature 319:422–427. https://doi.org/10.1038/319422a0
Georgiev M, Kuzeva S, Pavlov A, Kovacheva E, Ilieva M (2006) Enhanced rosmarinic acid production by Lavandula vera MM cell suspension culture through elicitation with vanadyl sulfate. Z Naturforsch 61:241–244. https://doi.org/10.1515/znc-2006-3-414
Georgiev M, Abrashev R, Krumova E, Demirevska K, Ilieva M, Angelova M (2009) Rosmarinic acid and antioxidant enzyme activities in Lavandula vera MM cell suspension culture: a comparative study. Appl Biochem Biotechnol 159(2):415–425. https://doi.org/10.1007/s12010-008-8437-3
Gertlowski C, Petersen M (1993) Influence of the carbon source on growth and rosmarinic acid production in suspension cultures of Coleus blumei. Plant Cell Tissue Organ Cult 34:183–190. https://doi.org/10.1007/BF00036100
Gianguzzi V, Inglese P, Barone E, Sottile F (2019) In vitro regeneration of Capparis spinosa L. by using a temporary immersion system. Plants 8(6):177. https://doi.org/10.3390/plants8060177
Grzegorczyk I, Wysokińska H (2010) Antioxidant compounds in Salvia officinalis L. shoots and hairy roots cultures in the nutrient sprinkle bioreactor. Acta Soc Bot Pol 1:7–10. https://doi.org/10.5586/asbp.2010.001
Grzegorczyk-Karolak I (2020) Optimization of culture conditions and cultivation phase for the growth of Salvia viridis transformed roots and polyphenolic compound production. Plant Cell Tissue Organ Cult 142:571–581. https://doi.org/10.1007/s11240-020-01883-6
Grzegorczyk-Karolak I, Rytczak P, Bielecki S, Wysokińska H (2017) The influence of liquid systems for shoot multiplication, secondary metabolite production and plant regeneration of Scutellaria alpina. Plant Cell Tissue Organ Cult 128:479–486. https://doi.org/10.1007/s11240-016-1126-y
Häusler E, Petersen M, Alfermann AW (1993) Isolation of protoplasts and vacuoles from cell suspension cultures of Coleus blumei Benth. Plant Cell Rep 12:510–512
Hosseini B, Ayyobi N, Fattahi M (2018) Study of factors affecting hairy roots induction and rosmarinic acid production in Dracocephalum kotschyi Boiss. Iran J Horti Sci 49(1):255–269. https://doi.org/10.22059/ijhs.2017.215380.1078
Jesionek A, Kokotkiewicz A, Wlodarska P, Zabiegała B, Buciński A, Luczkiewicz M (2017) Bioreactor shoot cultures of Rhododendron tomentosum (Ledum palustre) for a large-scale production of bioactive volatile compounds. Plant Cell Tissue Organ Cult 131:51–64. https://doi.org/10.1007/s11240-017-1261-0
Lam VP, Lee MH, Park JS (2020a) Optimization of indole-3-acetic acid concentration in a nutrient solution for increasing bioactive compound accumulation and production of Agastache rugosa in a plant factory. Agriculture 10(8):343. https://doi.org/10.3390/agriculture10080343
Lam VP, Kim SJ, Bok GJ, Lee JW, Park JS (2020b) The effects of root temperature on growth, physiology, and accumulation of bioactive compounds of Agastache rugosa. Agriculture 10(5):162. https://doi.org/10.3390/agriculture10050162
Lam VP, Kim SJ, Park JS (2020c) Optimizing the electrical conductivity of a nutrient solution for plant growth and bioactive compounds of Agastache rugosa in a plant factory. Agronomy 10(1):76. https://doi.org/10.3390/agronomy10010076
Lee SY, Xu H, Kim YK, Park SU (2008) Rosmarinic acid production in hairy root cultures of Agastache rugosa Kuntze. World J Microbiol Biotechnol 24:969–972. https://doi.org/10.1007/s11274-007-9560-y
Li W, Koike K, Asada Y et al (2005) Rosmarinic acid production by Coleus forskohlii hairy root cultures. Plant Cell Tiss Organ Cult 80:151–155. https://doi.org/10.1007/s11240-004-9541-x
Malarz J, Yudina YV, Stojakowska A (2023) Hairy root cultures as a source of phenolic antioxidants: simple phenolics, phenolic acids, phenylethanoids, and hydroxycinnamates. Int J Mol Sci 24:6920. https://doi.org/10.3390/ijms24086920
Mehrotra S, Goel KM, Kukreja AK, Mishra BN (2007) Efficiency of liquid culture systems over conventional micropropagation. A progress towards commercialization. Afr J Biotechnol 6:1484–1492
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nourozi E, Hosseini B, Hassani A (2016) Influences of various factors on hairy root induction in Agastache foeniculum (Pursh) Kuntze. Acta Agriculturae Slovenica 107(1):45–54. https://doi.org/10.14720/aas.2016.107.1.05
Ono NN, Tian L (2011) The multiplicity of hairy root cultures: prolific possibilities. Plant Sci 180(3):439–446. https://doi.org/10.1016/j.plantsci.2010.11.012
Park W, Baskar T, Yeo S, Park NII, Park J, Park SU (2017a) Response of different Agrobacterium rhizogenes strains for in vitro hairy root induction and accumulation of rosmarinic acid production in Agastache rugosa. J Biol Sci 17:136–142
Park CH, Zhao S, Yeo HJ et al (2017b) Comparison of different strains of Agrobacterium rhizogenes for hairy root induction and betulin and betulinic acid production in Morus alba. Nat Prod Commun. https://doi.org/10.1177/1934578X1701200403
Pavlov A, Bley T (2006) Betalains biosynthesis by Beta vulgaris L. hairy root culture in a temporary immersion cultivation system. Process Biochem 41(4):848–852. https://doi.org/10.1016/j.procbio.2005.10.026
Petersen MS (1991) Characterization of rosmarinic acid synthase from cell cultures of Coleus blumei. Phytochemistry 30(9):2877–2881. https://doi.org/10.1016/S0031-9422(00)98217-7
Petersen M, Simmonds M (2003) Rosmarinic acid. Phytochemistry 62:121–125. https://doi.org/10.1016/S0031-9422(02)00513-7
Piątczak E, Chmiel A, Wysokinska H (2005) Mist trickling bioreactor for Centaurium erythraea Rafn growth of shoots and production of secoiridoids. Biotechnol Lett 27:721–724. https://doi.org/10.1007/s10529-005-5189-9
Piątczak E, Królicka A, Wielanek M, Wysokińska H (2012) Hairy root cultures of Rehmannia glutinosa and production of iridoid and phenylethanoid glycosides. Acta Physiol Plant 34:2215–2224. https://doi.org/10.1007/s11738-012-1022-y
Piątczak E, Grzegorczyk-Karolak I, Wysokińska H (2014) Micropropagation of Rehmannia glutinosa Libosch.: production of phenolics and flavonoids and evaluation of antioxidant activity. Acta Physiol Plant 36:1693–1702. https://doi.org/10.1007/s11738-014-1544-6
Piątczak E, Jeleń A, Makowczyńska J, Zielińska S, Kuźma Ł, Balcerczak E (2019) Establishment of hairy root cultures of Rehmannia elata N.E. Brown ex Prain and production of iridoid and phenylethanoid glycosides. Ind Crops Prod 137:308–314. https://doi.org/10.1016/j.indcrop.2019.05.022
Piątczak E, Kuźma Ł, Kozłowska W, Lisiecki P, Szemraj M, Płachno BJ, Gonciarz W, Chmiela M, Matkowski A, Zielińska S (2020) Phenylethanoid and iridoid glycosides production in Rehmannia elata N.E. Ind Crops Prod 158:113050
Preil W (2005) General introduction: A personal reflection on the use of liquid media for in vitro culture. In: Hvoslef-Eide AK, Preil W (eds) Liquid culture systems for in vitro plant propagation. Springer, Dordrecht, pp 1–18
Qian J, Gui** L, **ujun L, **ncai H, Hongmei L (2009) Influence of growth regulators and sucrose concentrations on growth and rosmarinic acid production in calli and suspension cultures of Coleus blumei. Nat Prod Res 23:127–137. https://doi.org/10.1080/14786410801890338
Razzaque A, Ellis BE (1977) Rosmarinic acid production in Coleus cell cultures. Planta 137:287–291
Roy A (2021) Hairy root culture an alternative for bioactive compound production from medicinal plants. Curr Pharm Biotechnol 22:136–149. https://doi.org/10.2174/1389201021666201229110625
Santos-Gomes PC, Seabra RM, Andrade PB, Fernandes-Ferreira M (2002) Phenolic antioxidant compounds produced by in vitro shoots of sage (Salvia officinalis L.). Plant Sci 162:981–987. https://doi.org/10.1016/S0168-9452(02)00052-3
Sathasivam R, Choi M, Radhakrishnan R, Kwon H, Yoon J, Yang SH, Kim JK, Chung YS, Park SU (2022) Effects of various Agrobacterium rhizogenes strains on hairy root induction and analyses of primary and secondary metabolites in Ocimum basilicum. Front Plant Sci. https://doi.org/10.3389/fpls.2022.983776
Su WW, Lei F, Su LY (1993) Perfusion strategy for rosmarinic acid production by Anchusa officinalis. Biotechnol Bioeng 42:884–890. https://doi.org/10.1002/bit.260420713
Szopa A, Kokotkiewicz A, Luczkiewicz M, Ekiert H (2017) Schisandra lignans production regulated by different bioreactor type. J Biotechnol 247:11–17. https://doi.org/10.1016/j.jbiotec.2017.02.007
Szopa A, Kokotkiewicz A, Klimek-Szczykutowicz M, Luczkiewicz M, Ekiert H (2021) Different types of in vitro cultures of Schisandra chinensis and its cultivar (S. chinensis cv. Sadova): a rich potential source of specific lignans and phenolic compounds. In: Ramawat K, Ekiert H, Goyal S (eds) Plant cell and tissue differentiation and secondary metabolites. Reference series in phytochemistry. Springer, Cham. https://doi.org/10.1007/978-3-030-11253-0_10-1
Tuan PA, Park WT, Xu H, Park NI, Park SU (2012) Accumulation of tilianin and rosmarinic acid and expression of phenylpropanoid biosynthetic genes in Agastache rugosa. J Agric Food Chem 60:5945–5951. https://doi.org/10.1021/jf300833m
Verma PC, Rahman L, Negi AS, Jain DC, Khanuja SPS, Banerjee S (2007) Agrobacterium rhizogenes-mediated transformation of Picrorhiza kurroa Royle ex Benth.: establishment and selection of superior hairy root clone. Plant Biotechnol Rep 1:169–174. https://doi.org/10.1007/s11816-007-0029-0
Weremczuk-Jeżyna I, Kochan E, Szymczyk P, Lisiecki P, Kuźma Ł, Grzegorczyk-Karolak I (2019) The antioxidant and antimicrobial properties of phenol-rich extracts of Dracocephalum forrestii W. W. Smith shoot cultures grown in the nutrient sprinkle bioreactor. Phytochem Lett 30:254–260. https://doi.org/10.1016/j.phytol.2019.01.032
Weremczuk-Jeżyna I, Lisiecki P, Gonciarz W, Kuźma Ł, Szemraj M, Chmiela M, Grzegorczyk-Karolak I (2020) Transformed shoots of Dracocephalum forrestii W.W. Smith from different bioreactor systems as a rich source of natural phenolic compounds. Molecules 25:4533. https://doi.org/10.3390/molecules25194533
Wojciechowska M, Owczarek A, Kiss AK, Grąbkowska R, Olszewska MA, Grzegorczyk-Karolak I (2020) Establishment of hairy root cultures of Salvia bulleyana diels for production of polyphenolic compounds. J Biotechnol 20(318):10–19. https://doi.org/10.1016/j.jbiotec.2020.05.002
Xu H, Kim YK, ** X, Lee SY, Park SU (2008) Rosmarinic acid biosynthesis in callus and cell cultures of Agastache rugosa Kuntze. J Med Plants Res 2(9):237–241
Yeo HJ, Kwon MJ, Han SY, Jeong JC, Kim CY, Park SU, Park CH (2023) Effects of carbohydrates on rosmarinic acid production and in vitro antimicrobial activities in hairy root cultures of Agastache rugosa. Plants 12(4):797. https://doi.org/10.3390/plants12040797
Zielińska S, Matkowski A (2014) Phytochemistry and bioactivity of aromatic and medicinal plants from the genus Agastache (Lamiaceae). Phytochem Rev 13:391–416. https://doi.org/10.1007/s11101-014-9349-1
Zielińska S, Kolniak-Ostek J, Dziadas M, Oszmianski J, Matkowski A (2016) Characterization of polyphenols in Agastache rugosa leaves and inflorescences by UPLC–qTOF–MS following FCPC separation. J Liq Chromatogr Relat Technol 39:209–219. https://doi.org/10.1080/10826076.2016.1147461
Zielińska S, Dąbrowska M, Kozłowska W, Kalemba D, Abel R, Dryś A, Szumny A, Matkowski A (2017) Ontogenetic and trans-generational variation of essential oil composition in Agastache rugosa. Ind Crops Prod 97:612–619. https://doi.org/10.1016/j.indcrop.2017.01.009
Zielińska S, Dryś A, Piątczak E, Kolniak-Ostek J, Podgórska M, Oszmiański J, Matkowski A (2019) Effect of LED illumination and amino acid supplementation on phenolic compounds profile in Agastache rugosa in vitro cultures. Phytochem Lett 31:12–19. https://doi.org/10.1016/j.phytol.2019.02.029
Ziv M (2005) Simple bioreactors for mass propagation of plants. Plant Cell Tissue Organ CuLt 81:277–285. https://doi.org/10.1007/s11240-004-6649-y
Acknowledgements
The Authors would like to express sincere thanks to Mrs J. Bursy, M. Barwińska, K. Radecka and prof. B.J. Płachno for their help in methodology and photographic documentation.
Funding
This research was financially supported by Wroclaw Medical University Grant No. SUBK.D030.23.006 to Weronika Kozłowska.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by WK, SZ, EP, EK, JK-O, MS, BP, MS, MB and BJP. The first draft of the manuscript was written by SZ, EP, WK, and AM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare none conflict of interest.
Additional information
Communicated by Saikat Gantait.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kozłowska, W., Piątczak, E., Kolniak-Ostek, J. et al. Upscaling biomass production of rosmarinic acid-rich hairy root cultures of Agastache rugosa (Fisch. & C.A.Mey.) Kuntze. Plant Cell Tiss Organ Cult 156, 41 (2024). https://doi.org/10.1007/s11240-023-02626-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11240-023-02626-z