Abstract
Amberlyst-15 supported cyclic alkyl amino carbene and bicyclic alkyl amino carbene ruthenium olefin metathesis catalysts for sustainable catalytic applications have been synthesized by the well-known wet impregnation method utilizing ionic complex/support interaction. Surface coverages are as high as 4 and 7 wt% were achieved in the case of the significantly higher pore volume Amberlyst-15, compared to Amberlyst-36. These phase separable catalysts show high activity in cross metathesis, ring closing metathesis and ethenolysis reactions compared to the reported heterogenized olefin metathesis catalysts. Leeching tests revealed no more than 1.5 ppm ruthenium content for the investigated metathesis reactions, which is well below the accepted 10 ppm limit in case of consumer products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The industrial application of sustainable catalysis is crucial from the point of contemporary chemical technologies view. It is estimated, that 90% of the commercial chemicals are manufactured via some catalytic process [1]. Recent trends in the widespread use of catalysis in sustainable and green chemical applications have created further challenges requiring robust solutions [2]. Olefin metathesis is one of the novel organometallics-catalyzed reactions discovered in the last fifty years, that initiated new industrial technology avenues [3]. Its application on the field of green chemistry—especially in oleo chemistry—is growing [4, 5]. Although in general, the homogeneous olefin metathesis catalysts render higher activity and functional group tolerance than heterogeneous systems [6], their separation from the reaction mixture is often cumbersome limiting their industrial application [7,8,9]. This is especially important in the pharmaceutical industry, where the acceptable remaining ruthenium content cannot be exceed 10 ppm [10,11,12], causing a sudden interest in the purification of metathesis products [13, 14]. One method to overcome this recurring problem is to combine the advantages of homogeneous and heterogeneous catalysis, by the heterogenization of homogeneous catalysts [15, 16]. Since the first solid-supported well-defined metathesis catalyst [17], numerous reviews summarized this growing area of metathesis chemistry [18,19,20,21,22,23], while their commercial availability is readily growing [24]. As of today, both primary and secondary interactions are used to deposit catalyst to different supports. Examples for the covalent bond based immobilizations include silica [25,26,27] and SBA-15 supported species [28], substituted polystyrenes [29] and polynorbornenes [30, 31] among others [32]. An interesting primary ionic bond-based solution is the application of tertiary amine-tagged ruthenium species together with acidic ion-exchange resin precipitated on glass Raschig rings [33]. Although many olefin metathesis reactions using solid-supported ruthenium based catalyst have been demonstrated, their application in ethenolysis reactions are rare: besides heterogeneous systems are proven to be effective catalysts for ethenolysis [34, 35], solid- (MOF) [36] or phase-supported (biphasic/SLIP) [37] complexes have been reported as effective catalysts. Turnover number of supported systems in olefin metathesis exceeds the order of one hundred thousand using Schrock systems [38], but only a few thousand at most when applying Grubbs-systems [37, 39, 40].
An emerging, new class of olefin metathesis catalyst bearing cyclic and bicyclic alkyl amino carbene (CAAC and BICAAC) family ligands appeared recently showing superior catalyst activity and stability. In our laboratories new CAAC (1 and 2) and latent BICAAC (3 and 4) ruthenium alkylidene complexes bearing dimethyl amino group have been synthesized recently [41, 42], showing exceptional stability and catalytic activity even in protic solvents or at elevated temperature (Scheme 1).
In this paper, the preparation and application of first Amberlyst-15 supported CAAC-Ru and BICAAC-Ru complexes in olefin metathesis including ethenolysis reactions will be demonstrated.
Experimental methods and materials
Materials
Ruthenium CAAC [33] and BICAAC [34] complexes (1 and 3) were synthesized according to literature procedure. Amberlyst-15 (hydrogen form, dry, total pore volume: 0.2 mL/g, average pore size: 24 nm, ACROS) and Amberlyst-36 (hydrogen form, 55% water content, total pore volume: 0.06 mL/g, average pore size: 27 nm, Sigma Aldrich) were dried in vacuum at 75 °C overnight before use.
1-Octene (9), diethyl diallylmalonate (5) and cyclopentene (7) were purchased from Sigma Aldrich and used as received.
Methyl oleate (11) was received from Sigma Aldrich and passed through a plug of dried alumina in a glove box prior to use.
7-Tetradecene (10) was prepared according to literature procedure [43].
Amberlyst supported catalyst preparation
Amberlyst supported CAAC and BiCAAC catalysts were prepared via wet impregnation method. First, the ion-exchange resin (Amberlyst-15 or Amberlyst-36) was dried in vacuo at 75 °C and transferred to a glove-box. In the glove-box, the ruthenium alkylidene complex (1 or 3) was dissolved in dichloromethane (DCM, 5 mM) and added in excess to the resin. After 15 h of continuous stirring at room temperature, the resin was filtered and washed with DCM, followed by drying in vacuo. The combined DCM phase was evaporated and the mass of the residual ruthenium complex in the liquid phase was measured, indicating the amount of impregnated ruthenium alkylidene. This gravimetric ruthenium content was in good agreement with the following ICP-OES measurement, namely: 1-AL-15: 3.4 m%, 1-AL-36: 0.03 m%, 3-AL-15: 6.1 m% and 3-AL-36: 0.05 m% ruthenium complex content.
Characterization methods
Inductively coupled plasma—optical emission spectrometry (ICP-OES)
For the ruthenium content analysis, sample solutions were measured with a simultaneous ICP-OES spectrometer with axial plasma viewing (Spectro Genesis, Germany). For the calibration of the instrument, the following standard solutions were applied: CPAChem Ltd. multi-element standard solution (33 elements, M8A96.K1.5 N.L5) and Inorganic Ventures multi-element standard solution (8 elements including Ru, 31-ICPMS-71C).
Scanning electron microscopy (SEM) with energy dispersive X‑ray spectrometer (EDS)
SEM measurements of the gold- (carbon- in case of EDS analysis) coated support resins and the impregnated catalyst beads were carried out with a ZEISS EVO 40 scanning electron microscope operated at 5 keV, coupled with an Oxford Instruments, INCAx-sight x-ray detector.
Electrospray time-of-flight mass spectrometry (ESI-TOF MS)
ESI-TOF MS measurements were carried out using a Bruker MicroTOF Q instrument (Bruker Daltonik, Bremen, Germany) equipped with an ESI source operated in the positive ion mode. The voltage on the spray was 3.5 kV, nitrogen was used as nebulizer and drying gas (180 °C, 4 L/min). The mass spectra were recorded using a digitalizer at a sampling rate of 2 GHz. The calibration was performed using the exact masses of clusters formed from the electrosprayed solution of sodium trifluoroacetate (NaTFA). The mass spectra were assessed with the DataAnalysis 3.4 software from Bruker.
Sample preparation for ESI-TOF MS
2.1 mg (CAAC) and 2.3 mg (BICAAC) were added to 200 μL methanol and placed in an ultrasound bath, where they were stirred for 20 min. After filtration, the samples were injected directly into the ion-source of ESI-TOF MS instrument and the MS spectra were recorded (Figs. S1–S4).
Model reactions
Representative example of solid-supported ring-closing metathesis (RCM) reaction (Table 1 Entry 6)
In a glove box, diethyl diallylmalonate (5, 1000 mg, 4.2 mmol) and solid-supported catalyst (1-AL-15, 0.01% Ru, 6.8 mg) was measured in a small vial. The reaction mixture was stirred at RT for 24 h in the box. Portion of the crude material was dissolved in CDCl3 and measured via 1H-NMR spectroscopy. Yields were calculated from the internal and terminal alkene proton signals. To determine the ruthenium content of the reaction mixture, part of the crude product was evaporated and stirred with aqua regia at 65 °C overnigth. After diluting with water, this sample was analyzed by ICP-OES.
Representative example of solid-supported cross-metathesis (CM) reaction (Table 1 Entry 11)
In a glove-box, 1-octene (9, 2000 mg, 17.8 mmol) and solid-supported catalyst (1-AL-15, 0.01% Ru, 26 mg) were measured in a small vial and stirred at RT for 24 h. Portion of the crude material was dissolved in CDCl3 and measured via 1H-NMR spectroscopy. Yields were calculated from the internal and terminal alkene proton signals. To determine the ruthenium content of the reaction mixture, part of the crude product was evaporated and stirred with aqua regia at 65 °C overnight. After diluting with water, this sample was analyzed by ICP-OES.
Representative example of solid-supported ethenolysis reaction (Table 1 Entry 15)
In a glove-box, 7-tetradecene (10, 2000 mg, 10.2 mmol) and solid-supported catalyst (1-AL-15, 0.01% Ru, 15 mg) was measured in a 150 mL Fischer-Porter bottle. The vessel was sealed and connected to a high pressure system outside the box. The bottle was flushed with ethylene gas (purity: 4.5) five times and filled to 10 bar. The reaction mixture was stirred at RT for 24 h. Portion of the crude material was dissolved in CDCl3 and measured by 1H-NMR spectroscopy. Yields were calculated from the internal and terminal alkene proton signals. To determine the ruthenium content of the reaction mixture, part of the crude product was evaporated and stirred with aqua regia at 65 °C overnight. After diluting with water, this sample was analyzed by ICP-OES.
Results and discussion
As catalyst 1 and 3 have shown exceptional activity comparing to their bis-carbene anologues (2 and 4) their impregnation to Amberlyst ionexchange resin have been investigated. Catalyst 1 and 3 have been synthesized according to the literature procedure [41, 42] and impregnated onto pre-treated Amberlyst resins (Amberlyst 15 yielding 1-AL-15 and 3-AL-15 and Amberlyst-36 resulting in 1-AL-36 and 3-AL-36) via the well-known wet-impregnation method (Scheme 2). Upon impregnation of the complexes to the Amberlyst solid support followed by multiple washing greenish pearls could be obtained (Fig. 1). Solid-supported catalyst characterization has been carried out using Scanning Electron Microscopy coupled with energy dispersive X-ray (SEM/EDX) spectroscopy (Fig. 2). The EDX spectra revealed that there is no ruthenium (Kα 19.233, Lα 2.558) on the outer surface of the solid support, meanwhile the ruthenium complexes were clearly detectable by ESI-TOF MS (Figs. S1–S4) upon treatment of the catalyst with MeOH in ultrasonic bath for 20 min. ICP-MS also clearly indicated the presence of ruthenium. Based on these investigations, it can be assumed that the catalyst species are located primarily in the pores of the solid support. It should be noted that the main differences between the applied supports is their acidity (or the number of the acidic species on the surface) and overall pore volume. It was found that the catalyst coverage—determined by ICP—strongly depends on the Amberlyst type. The highest coverage could be achieved using Amberlyst-15 support and catalyst 3 (6.1% for 3-AL-15). Impregnation of catalyst 1 gave a solid-supported catalyst having lower catalyst coverage, 3.4% for 1-AL-15. Using Amberlyst-36, significantly lower coverage was observed for both complexes, 0.03% for 1-AL-36 and 0.05% for 3-AL-36. This can be explained by the almost one order of magnitude higher pore volume of Amberlyst-15 (0.2 mL/g) compared to Amberlyst-36 (0.06 mL/g) and also supports that the catalytically active ruthenium-alkylidene species are located mainly in the resin pores.
The catalytic activity of 1-AL and 3-AL has been investigated on representative olefin metathesis model reactions including cross-metathesis, ethenolysis, ring-closing metathesis and ring-opening metathesis polymerization (ROMP) depicted in Scheme 3. It was found that all the reactions can be carried out at as low as 0.01 mol% catalyst loading. High product yields could be achieved using catalyst 1-AL-15 at ambient condition, meanwhile latent catalyst 3-AL-15 showed limited activity even at elevated temperature (Table 1). While preliminary reactions indicated some catalyst leaching in methanol or DCM solvents, changing the solvent to hexane or carrying out the reactions in neat resulted in less than 1.5 ppm ruthenium content in all cases based on ICP-OES.
The RCM of diethyl diallylmalonate (5) is a well-known, simple model reaction used for the comparison of catalyst activities. The reactivity of both homogenous 1 and the supported 1-AL-15 catalysts in the RCM reaction of 5 was examined under similar conditions to investigate the effect of heterogenization of homogeneous catalysts. (Table 1 Entry 1–4). While homogeneous catalyst 1 completed the reaction within a few minutes (95%, Table 1 Entry 1), it took 24 h for the solid-supported 1-AL-15 catalyst to achieve similar yield (94%, Table 1 Entry 4) under the same conditions. Heterogenized ruthenium metathesis catalysts often render a slower reaction and a reduced reactivity compared to their homogeneous analogues. Based on this, all model reactions have been carried out for 24 h. Lowering the ruthenium catalyst loading to 0.01% revealed yields up to 28% for the CAAC based 1-AL-15 catalyst; however, the BiCAAC containing 3-AL-15 showed only traces of the expected product, even at elevated temperatures (Table 1 Entry 5 and 8). Amberlyst-36 supported CAAC species (1-AL-36) showed significantly lower yields of RCM of 5 and generally less reactivity than 1-AL-15 even at 0.1% load.
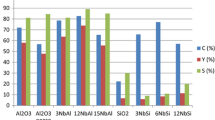
Self-metathesis of 1-octene (9) was carried out in hexane solution and in neat, as well (Table 1 Entry 10 and 11), showing as high yields as 44% at low, 0.01% 1-AL-15 catalyst loading. Since homogenous catalyst 3 catalyzes olefin metathesis reaction only over 75 °C [41], the CM reaction of 9 using catalyst 3-AL-15 was carried out at the same temperature. When hexane was used as solvent (Table 1 Entry 12), only the formation of the expected 7-tetradecene (10) was observed as the sole product. Interestingly, neat reaction conditions (Table 1 Entry 13) lead to a product mixture, containing not only 7-tetradecene (10), but homologues of terminal and internal alkenes, ranging from C7 to C14 (Fig. 3) indicating that not only metathesis, but also double bond isomerization were taking place at the same time. This finding can be explained by the partial intrinsic decomposition of the ruthenium complexes giving Ru–H species showing double bond isomerization activity [44], i.e., isomerization-metathesis (ISOMET) reaction was observed. For these reactions (Table 1 Entry 13) yields were calculated for tetradecene isomers based on the components’ GC–MS peak area.
Ethenolysis, which is usually more challenging than CM reaction because of the increased concentration of the less stable Ru = CH2 methylidene species also showed similar results when 7-tetradecene (10) was used as starting material (Table 1 Entry 15), comparable to the CM of 1-octene at RT with 1-AL-15. Performing the reaction using 3-AL-15 at 75 °C yielded only minor amount of terminal alkenes and only trace amounts of ISOMET products (Table 1 Entry 16 and 17). Unexpectedly, the ethenolysis of methyl oleate (11) yielded only 3% of the expected terminal alkenes when carried out under similar condition as the ethenolysis of 10. This reduced reactivity to the esters is also reflected in the CM reaction of 10 and cis-1,4-diacetoxy-2-butene, which was carried out under the same conditions as above and gives only traces (< 3%) of the expected product. even at a catalyst load of 0.1%.
The equilibrium metathesis polymerization of cyclopentene (7) showed 17% polymer formation, corresponding to 30% yield since the equilibrium polymer/monomer rate is 1.2 on RT [45].
Summary
Styrene–divinylbenzene ion exchange resins with strongly acidic sulfonic groups were wet-impregnated by CAAC and BICAAC ruthenium alkylidene complexes having dimethylamino funcionalities to obtain solid-supported metathesis catalysts. It was found that the active complex is mostly adsorbed in the pores of the solid support. The pore volume was shown to have significant impact on the adsorption capacity of the resin. Resin Amberlyst-36, having low pore volume, bound almost an order of magnitude less complex than the higher porosity resin Amberlyst-15. Model metathesis reactions, such as RCM, CM, ethenolysis and ROMP, showed high yields at low catalyst loadings for the investigated systems. It was found that solvent can play crucial role in the cross metathesis (CM) reactivity of 1-octene. In solvent hexane at 75 °C, the expected 7-tetradecene was formed. However, in neat and at elevated temperature isomerization-metathesis reaction was observed, producing a mixture of internal and terminal alkenes. Tests revealed no more than 1.5 ppm ruthenium leaching in the investigated metathesis reactions.
References
Catalysis Definition in Chemistry.https://www.thoughtco.com/definition-of-catalyst-604402%0D%0A. Accessed date 2021
Rafael Luque Frank Leung-Yuk Lam (2018) Sustainable catalysis: energy-efficient reactions and applications. Wiley-VCH Verlag GmbH & Co KGaA, Weinheim
Mol JC (2004) Industrial applications of olefin metathesis. J Mol Catal A Chem 213:39–45. https://doi.org/10.1016/j.molcata.2003.10.049
Mol JC (2002) Application of olefin metathesis in oleochemistry: an example of green chemistry. Green Chem 4:5–13. https://doi.org/10.1039/b109896a
Marx VM, Sullivan AH, Melaimi M et al (2015) Cyclic alkyl amino carbene (CAAC) ruthenium complexes as remarkably active catalysts for ethenolysis. Angew Chemie Int Ed 54:1919–1923. https://doi.org/10.1002/anie.201410797
Buchmeiser MR (2004) Recent advances in the synthesis of supported metathesis catalysts. New J Chem 28:549–557. https://doi.org/10.1039/b315236g
Szczepaniak G, Urbaniak K, Wierzbicka C et al (2015) High-performance isocyanide scavengers for use in low-waste purification of olefin metathesis products. Chemsuschem 8:4139–4148. https://doi.org/10.1002/cssc.201500784
Wang H, Goodman SN, Dai Q et al (2008) Development of a robust ring-closing metathesis reaction in the synthesis of SB-462795, a cathepsin K inhibitor. Org Process Res Dev 12:226–234. https://doi.org/10.1021/op700288p
Wang H, Matsuhashi H, Doan BD et al (2009) Large-scale synthesis of SB-462795, a cathepsin K inhibitor: the RCM-based approaches. Tetrahedron 65:6291–6303. https://doi.org/10.1016/j.tet.2009.06.022
European Medicines Agency (2008) Specification limits for residues of metal catalysts CHMP/SWP/4446/2000
Arumugasamy J, Arunachalam K, Bauer D et al (2013) Development of related HCV protease inhibitors: macrocyclization of two highly functionalized dienyl-ureas via ring-closing metathesis. Org Process Res Dev 17:811–828. https://doi.org/10.1021/op300296t
Farina V, Shu C, Zeng X et al (2009) Second-generation process for the hcv protease inhibitor biln 2061: a greener approach to ru-catalyzed ring-closing metathesis. Org Process Res Dev 13:250–254. https://doi.org/10.1021/op800225f
Vougioukalakis GC (2012) Removing ruthenium residues from olefin metathesis reaction products. Chem A Eur J 18:8868–8880. https://doi.org/10.1002/chem.201200600
Skowerski K, Gułajski Ł (2014) Purification strategies in olefin metathesis. In: Grela K (ed) Olefin metathesis. Wiley, Hoboken, pp 559–571
Buchmeiser MR (2014) Immobilization of olefin metathesis catalysts. In: Grela K (ed) Olefin metathesis. Wiley, Hoboken, pp 495–515
Grubbs RH (2003) Handbook of metathesis. Wiley/VCH, Weinheim
Nguyen SBT, Grubbs RH (1995) The syntheses and activities of polystyrene-supported olefin metathesis catalysts based on Cl2(PR3)2Ru = CH-CH = CPh2. J Organomet Chem 497:195–200. https://doi.org/10.1016/0022-328X(95)00122-7
Buchmeiser MR (2009) Polymer-supported well-defined metathesis catalysts. Chem Rev 109:303–321
Suriboot J, Bazzi HS, Bergbreiter DE (2016) Supported catalysts useful in ring-closing metathesis, cross metathesis, and ring-opening metathesis polymerization. Polymers (Basel). https://doi.org/10.3390/polym8040140
Hamad F, Kai C, Cai Y et al (2013) Solid supported ruthenium complexes for olefin metathesis. Curr Org Chem 17:2592–2608. https://doi.org/10.2174/13852728113179990111
Clavier H, Grela K, Kirschning A et al (2007) Sustainable concepts in olefin metathesis. Angew Chemie Int Ed 46:6786–6801. https://doi.org/10.1002/anie.200605099
Copéret C, Basset JM (2007) Strategies to immobilize well-defined olefin metathesis catalysts: supported homogeneous catalysis vs. surface organometallic chemistry. Adv Synth Catal 349:78–92. https://doi.org/10.1002/adsc.200600443
Grela K, Michrowska A, Bieniek M (2006) Catalysts for new tasks: preparation and applications of tunable ruthenium catalysts for olefin metathesis. Chem Rec 6:144–156. https://doi.org/10.1002/tcr.20079
(2018) Apeiron’s metal scavenging solutions. https://apeiron-synthesis.com/. Accessed date 2022
Prühs S, Lehmann CW, Fürstner A (2004) Preparation, reactivity, and structural peculiarities of hydroxyalkyl-functionalized “second-generation” ruthenium carbene complexes. Organometallics 23:280–287. https://doi.org/10.1021/OM0342006
Allen DP, Wingerden MMV, Grubbs RH (2009) Well-defined silica-supported olefin metathesis catalysts. Org Lett 11:1261–1264. https://doi.org/10.1021/OL9000153
Lim J, Seong Lee S, Ying JY (2010) Mesoporous silica-supported catalysts for metathesis: application to a circulating flow reactor. Chem Commun 46:806–808. https://doi.org/10.1039/B917986K
Skowerski K, Pastva J, Czarnocki SJ, Janoscova J (2015) Exceptionally stable and efficient solid supported hoveyda-type catalyst. Org Process Res Dev 19:872–877. https://doi.org/10.1021/acs.oprd.5b00132
Grela K, Tryznowski M, Bieniek M (2002) A PS-DES immobilized ruthenium carbene: a robust and easily recyclable catalyst for olefin metathesis. Tetrahedron Lett 43:9055–9059. https://doi.org/10.1016/S0040-4039(02)02283-9
Mayr M, Mayr B, Buchmeiser MR (2001) Monolithic materials: new high-performance supports for permanently immobilized metathesis catalysts. Angew Chemie Int Ed 40:3839–3842. https://doi.org/10.1002/1521-3773(20011015)40:20%3c3839::AID-ANIE3839%3e3.0.CO;2-O
Mayr M, Wang D, Kröll R et al (2005) Monolithic disk-supported metathesis catalysts for use in combinatorial chemistry. Adv Synth Catal 347:484–492. https://doi.org/10.1002/adsc.200404197
Skowerski K, Biatecki J, Czarnocki SJ et al (2016) Effective immobilisation of a metathesis catalyst bearing an ammonium-tagged NHC ligand on various solid supports. Beilstein J Org Chem 12:5–15. https://doi.org/10.3762/bjoc.12.2
Michrowska A, Mennecke K, Kunz U et al (2006) A new concept for the noncovalent binding of a ruthenium-based olefin metathesis catalyst to polymeric phases: preparation of a catalyst on raschig rings. J Am Chem Soc 128:13261–13267. https://doi.org/10.1021/ja063561k
Lee M, Han YH, Hwang DW (2020) Cross-metathesis of methyl oleate with ethylene over methyltrioxorhenium supported on ZnAl2O4 as a heterogeneous catalyst. Catal Commun 144:106088. https://doi.org/10.1016/J.CATCOM.2020.106088
Kustov LM, Furman DB (2018) Catalytic synthesis of octadiene-1,7 from ethylene and cyclohexene. J Organomet Chem 867:261–265. https://doi.org/10.1016/J.JORGANCHEM.2018.01.059
Milewski M, Kajetanowicz A, Grela K (2020) Improved preparation of an olefin metathesis catalyst bearing quaternary ammonium tag (FixCat) and its use in ethenolysis and macrocyclization reactions after immobilization on metal-organic framework (MOF). Arkivoc 2021:73–84. https://doi.org/10.24820/ARK.5550190.P011.373
Aydos GLP, Leal BC, Perez-Lopez OW, Dupont J (2014) Ionic-tagged catalytic systems applied to the ethenolysis of methyl oleate. Catal Commun 53:57–61. https://doi.org/10.1016/J.CATCOM.2014.04.020
Sen S, Schowner R, Imbrich DA et al (2015) Neutral and cationic molybdenum imido alkylidene N-heterocyclic carbene complexes: reactivity in selected olefin metathesis reactions and immobilization on silica. Chem A Eur J 21:13778–13787. https://doi.org/10.1002/CHEM.201501615
Dewaele A, Van Berlo B, Dijkmans J et al (2016) Immobilized Grubbs catalysts on mesoporous silica materials: insight into support characteristics and their impact on catalytic activity and product selectivity. Catal Sci Technol 6:2580–2597. https://doi.org/10.1039/C5CY01897H
Dewaele A, Verpoort F, Sels B (2016) Opportunities of immobilized homogeneous metathesis complexes as prominent heterogeneous catalysts. ChemCatChem 8:3010–3030. https://doi.org/10.1002/cctc.201600591
Nagyházi M, Lukács Á, Turczel G et al (2022) Catalytic decomposition of long-chain olefins to propylene via isomerization-metathesis using latent bicyclic (alkyl)(amino)carbene-ruthenium olefin metathesis catalysts. Angew Chemie Int Ed. https://doi.org/10.1002/anie.202204413
Nagyházi M, Turczel G, Balla Á et al (2020) Towards sustainable catalysis – highly efficient olefin metathesis in protic media using phase labelled cyclic alkyl amino carbene (CAAC) ruthenium catalysts. ChemCatChem 12:1953–1957. https://doi.org/10.1002/cctc.201902258
Johns AM, Pederson RL, Kiser RC (2015) Preparation of surfactants via Cross-Metathesis
Nascimento DL, Foscato M, Occhipinti G et al (2021) Bimolecular coupling in olefin metathesis: correlating structure and decomposition for leading and emerging ruthenium-carbene catalysts. J Am Chem Soc 143:11072–11079. https://doi.org/10.1021/jacs.1c04424
Tuba R, Grubbs RH (2013) Ruthenium catalyzed equilibrium ring-opening metathesis polymerization of cyclopentene. Polym Chem 4:3959–3962. https://doi.org/10.1039/c3py00584d
Acknowledgements
Project no. TKP2021-NKTA-34 has been implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the TKP2021-NKTA funding scheme. The authors acknowledge the financial support of the project by the Economic Development and Innovation Operative Program of Hungary, GINOP-2.3.2-15-2016-00053: Development of liquid fuels having high hydrogen content in the molecule (contribution to sustainable mobility). The Project is supported by the European Union. R. Tuba thanks the Hungarian National Research, Development and Innovation Office – NKFIH under the Grant TKP-108-9/PALY-2021.
Funding
Open access funding provided by ELKH Research Centre for Natural Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deme, J., Nagyházi, M., May, Z. et al. Synthesis and catalytic olefin metathesis activity of amberlyst-15 supported cyclic and bicyclic alkyl amino carbene ruthenium complexes. Reac Kinet Mech Cat 135, 2519–2531 (2022). https://doi.org/10.1007/s11144-022-02261-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02261-3