Abstract
The temporal dynamics of the Bray–Liebhafsky reaction (iodate-based catalytic decomposition of hydrogen peroxide in an acidic aqueous solution) was experimentally characterized in a continuous stirred tank reactor by independently varying the temperature and the mixed inflow hydrogen peroxide concentration. When the temperature was the bifurcation parameter, the emergence/disappearance of oscillatory behavior via a supercritical Andronov–Hopf bifurcation was observed for different mixed inflow hydrogen peroxide concentrations. An increase in the mixed inflow hydrogen-peroxide concentration resulted in a shift of the bifurcation point towards higher values of temperature, but did not alter the bifurcation type.



Similar content being viewed by others
Notes
A batch reactor is a closed chemical reaction system where reactants are placed into the reaction vessel at the beginning and chemical reactions proceed thereafter without exchanging matter with the surroundings. The reaction mixture in a batch reactor is usually thermostated using a circulating water bath to maintain constant temperature [3].
The mixed inflow concentration of reactant Xi ([Xi]) is the concentrations of the reactant Xi established in the reaction vessel after mixing the separate inflows, before any chemical reaction started. It is calculated by multiplying the concentration in the reservoir ([Xi]0) by the relative contribution of the flow of this species to the overall volume flow rate.
These concentrations refer to stock concentrations of reactants Xi in the reservoirs ([Xi]0).
The BL oscillatory reaction dynamics was examined when the concentration of hydrogen peroxide as control parameter varied from 0.007 to 0.2 mol L−1; other experimental conditions were: [KIO3] = 0.03 mol L−1, [H2SO4] = 0.125 mol L−1, j0 = 0.00625 min−1 and o = 700 rpm.
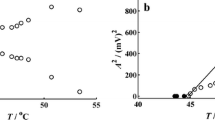
The BL oscillatory reaction dynamics was examined when temperature as control parameter varied from 45.0 to 57.5.0 °C; other the experimental conditions were: [KIO3] = 0.059 mol L−1, [H2SO4] = 0.055 mol L−1, j0 = 0.0296 min−1 and o = 900 rpm. In those experiments, the rate at which gaseous phase removed was 5.8 mL min−1.
References
Bray WC (1921) Periodic reaction in homogenous solution and its relation to catalysis. J Am Chem Soc 43:1262–1267
Bray WC, Liebhafsky HA (1931) Reaction involving hydrogen peroxide, iodine and iodate ion I. Introduction. J Am Chem Soc 53:38–44
Epstein IR, Pojman JA (1998) An introduction to nonlinear chemical dynamics. Oscilations, waves, patterns, and chaos. Oxford University Press, Oxford
Lj Kolar-Anić, Schmitz G (1992) Mechanism of the Bray–Liebhafsky reaction: effect of the oxidation of iodous acid by hydrogen peroxide. J Chem Soc Farady Trans 88:2343–2349
Noyes MR, Kalachev LV, Field RJ (1995) Mathematical model of the Bray–Liebhafsky oscillations. J Phys Chem 99:3514–3520
Lj Kolar-Anić, Mišljenović Đ, Anić S, Nicolis G (1995) Influence of the reduction of iodate ion by hydrogen peroxide on the model of the Bray–Liebhafsky reaction. React Kinet Catal Lett 54:35–41
Anić S, Lj Kolar-Anić, Körös E (1997) Methods to determine activation energies for the two kinetic states of the oscillatory Bray–Liebhafsky reaction. React Kinet Catal Lett 61:111–116
Ševčík P, Lj Adamčíková (1997) Effect of a pressure decrease and stirring on the oscillating Bray–Liebhafsky reaction. Chem Phys Lett 267:307–312
Stanisavljev D, Begović N, Vukojević V (1998) J Phys Chem A 102:6887–6891
Genigová J, Melicherčik Olexová A, Triendl L (1999) Influence of the MnO4 − ions on the Bray–Liebhafsky oscillatory reaction. J Phys Chem A 103:4690–4692
Ćirić J, Anić S, Čupić Ž, Lj Kolar-Anić (2000) The Bray–Liebhafsky oscillatory reaction. Kinetic investigations in reduction and oxidation pathways based on hydrogen peroxide concentration monitoring. Sci Sinter 32:187–196
Kovács K, Hussami LI, Rábai G (2005) Temperature compensation in the oscillatory Bray reaction. J Phys Chem A 109:10302–10306
Schmitz G (2010) Iodine oxidation by hydrogen peroxide in acidic solutions, Bray–Liebhafsky reaction and other related reactions. Phys Chem Chem Phys 12:6605–6615
Olexová A, Mrákavová M, Melicherčik M, Treindl L (2010) Oscillatory system I−, H2O2, HClO4: the modified form of the Bray–Liebhafsky reaction. J Phys Chem A 114:7026–7029
Maksimović JP, Čupić ŽD, Lončarević D, Pejić N, Vasiljević-Radović D, Anić S (2011) Kinetics of the Bray–Liebhafsky oscillatory reaction perturbed by polimer supported cobalt catalyst. Sci Sinter 43:55–62
Szabo E, Ševčik P (2013) Reexamination of gas production in the Bray–Liebhafsky reaction: what happened to O2 pulses. J Phys Chem A 117:10604–10614
Stanković B, Anić S (2013) In: Katica Stevanovic Hedrih (ed) Scientific review series: scientific and engineering—special issue nonlinear dynamics, S2:89–112, Serbian Scientific Society, Belgrade
Buchholtz FG, Broecker S (1998) Oscillations of the Bray–Liebhafsky reaction at low flow rates in a continuous flow stirred tank reactor. J Phys Chem 102:1556–1559
Vukojević V, Anić S, Kolar − Anić LJ (2000) Investigation of dynamic behavior of the Bray–Liebhafsky reaction in the CSTR. Determination of bifurcation point. J Phys Chem A 104:10302–10306
Kolar-Anić, Lj, Vukojević V, Pejić N, Grozdić T, Anić S (2004) In: Boccaletti S, Gluckman BJ, Kurths J, Pecora L, Meucci R, Yordanov Q (eds) Experimental chaos, American Institute of Physics, AIP Conference Proceedings, Vol. 742: Melville, New York
Pejić N, Maksimović J, Ribič D, Lj Kolar-Anić (2009) Dynamic states of the Bray–Liebhafsky reaction when sulfuric acid is the control parameter. Russ J Phys Chem A 83:1666–1671
Pejić N, Vujković M, Maksimović J, Ivanović A, Anić S, Čupić Ž (2011) Kolar-Anić Lj. Russ J Phys Chem 85:2310–2316
Vukojević V, Anić S, Lj Kolar-Anić (2002) Investigation of dynamic behavior of the Bray–Liebhafsky reaction in the CSTR. Properties of the system examined by pulse perturbations with I−. Phys Chem Chem Phys 4:1276–1283
Ivanović-Šašić AZ, Marković VM, Anić SR, LjZ Kolar-Anić, Čupić ŽD (2011) Structures of chaos in open reaction systems. Phys Chem Chem Phys 13:20162–20171
Vukojević V, Pejić N, Stanisavljev D, Anić S, Lj Kolar-Anić (1999) Determination of Cl−, Br−, I−, Mn2+, malonic acid and quercetin by perturbation of a non-equilibrium stationary state in the Bray–Liebhafsky reaction. Analyst 124:147–152
Pejić N, Blagojević S, Vukelić J, Lj Kolar-Anić, Anić S (2007) Analyte pulse perturbation technique for the determination of 6-monoacetylmorphine in seized street drug sample. Bull Chem Soc Jpn 80:1942–1948
Jimenez-Prieto R, Silva M, Perez-Bendito D (1998) Application of oscillating reaction-based determinations to the analysis of real samples. Analyst 23:1R–8R
Gao JZ (2005) Application of oscillating chemical reaction to analytical chemistry: recent developments. J Biol Sci 8:512–519
Ren J, Zhang X, Gao J, Zang W (2013) The application of oscillating chemical reaction to analytical determination. Cent Eur J Chem 11:1023–1031
Jimenez-Prieto R, Silva M, Pérez-Bendito D (1995) Analyte pulse perturbation technique: a tool for analytical determinations in far-from-equilibrium dynamic systems. Anal Chem 67:729–734
Hassard BD, Kazarinoff NC, Wan Y-H (1981) Theory and applications of Hopf bifurcation. University Press, Cambridge
Maselko J (1982) Determination of bifurcation in chemical systems. Exp method Chem Phys 67:17–26
Gáspár V, Galambosi P (1986) Bifurcation diagram of the oscillatory Belousov–Zhabotinskii system of oxalic acid in a continuous flow stirred tank reactor. Further possible evidence of saddle node infinite period bifurcation behavior of the system. J Phys Chem 90:2222–2226
Noszticzius Z, Wittman M, Stirling P (1987) Bifurcation from excitability to limit cycle oscillations at the end of the induction period in the classical Belousov–Zhabotinsky reaction. J Chem Phys 86:1922–1926
Richetti P, Roux JC, Argoul F, Arneodo A (1987) From quasiperiodicity to chaos in the Belousov–Zhabothinski reaction. II. Modeling and theory. J Chem Phys 86:3339–3356
Gray P, Scott SK (1990) Chemical oscillations and instabilities: nonlinear chemical kinetics. Clarendon Press, Oxford
Gaspard P (1990) Measurement of the instability rate of a far-from-equilibrium steady state at an infinite period bifurcation. J Phys Chem 94:1–3
Bar-Eli K, Brøns M (1990) Period lengthening near the end of oscillations in chemical system. J Phys Chem 94:7170–7177
Brøns M, Bar-Eli K (1991) Canard explosion and excitation in a model of the Belousov–Zhabotinsky reaction. J Phys Chem 95:8706–8713
Strogatz S (1994) Nonlinear dynamics and chaos. Addison-Wesley, Reading
Strizhak PE, Pojman JA (1996) Infinite period and Hopf bifurcations for the pH-regulated oscillations in a semibatch reactor (H2O2–Cu2+–S2O3–NaOH system). Chaos 6:461–465
Milošević M, Pejić N, Čupić Ž, Anić S, Lj Kolar-Anić (2005) Examinations of cross-linked polyvinylpyridine in open reactor. Mater Sci Forum 494:369–374
Gomila D, Jacobo A, Matías MA, Colet P (2007) Phase-space structure of two-dimensional excitable localized structures. Phys Rev E 75:026217-1–026217-10
Blagojević SM, Anić SR, Čupić ŽD, Pejić ND, LjZ Kolar-Anić (2008) Malonic acid concentration as a control parameter in the kinetic analysis of the Belousov–Zhabotinsky reaction under batch conditions. Phys Chem Chem Phys 10:6658–6664
Boissonade J, De Kepper P (1980) Transitions from bistability to limit cycle oscillations. theoretical analysis and experimental evidence in an open chemical system. J Phys Chem 84:501–506
Čupić Ž, Ivanović-Šašić A, Anić S, Stanković B, Maksimović J, Lj Kolar-Anić, Shmitz G (2013) Tourbillion in the phase space of the Bray–Liebhafsky nonlinear oscillatory reaction and related multiple-time-scale model. Match Commun Math Co 69:805–830
Schmitz G, Lj Kolar-Anić, Anić S, Čupić Ž (2008) Stoichiometric network analysis and associated dimensionless kinetic equations. Application to a model of the Bray–Liebhafsky reaction. J Phys Chem 112:13452–13457
Noszticzius Z, Stirling P, Wittmann M (1985) Measurement of bromine removal rate in the oscillatory BZ reaction of oxalic acid. Transition from limit cycle oscillations to excitability via saddle-node infinite period bifurcation. J Phys Chem 89:4914–4921
McCormick WD, Noszticzius Z, Swinney HL (1991) Interrupted separatrix excitability in a chemical system. J Chem Phys 94:2159–2167
Kolthoff M, Sandell EB (1952) Textbook of quantitative inorganic analysis. The MacMilan Co, New York
Stanisavljev D, Vukojević V (1995) Thermochemical effects accompanying oscillations in the Bray–Liebhafsky reaction. J Serb Chem Soc 60:1125–1134
Marsden J, McCracken H (1976) The Hopf bifurcation theorem and its application. Springer, New York
Argoul F, Arneodo A, Richetti P, Roux JC (1987) From quasiperiodicity to chaos in the Belousov–Zhabothinski reaction. I. Experiment. J Chem Phys 86:3325–3338
Acknowledgments
The present investigations were supported by The Ministry of Education, Science and Technological Development of the Republic of Serbia, under Project 172015 and 43009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pejić, N., Kolar-Anić, L., Maksimović, J. et al. Dynamic transitions in the Bray–Liebhafsky oscillating reaction. Effect of hydrogen peroxide and temperature on bifurcation. Reac Kinet Mech Cat 118, 15–26 (2016). https://doi.org/10.1007/s11144-016-0984-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-016-0984-y