Abstract
Aims
Inoculation with climate-adapted rhizobia is able to increase legume productivity in drought-prone regions of Sub-Saharan Africa. Enhanced nodulation might additionally affect plant-soil interactions and control rhizosphere carbon (C) and nitrogen (N) pools.
Methods
We investigated inoculation effects on nodulation and biological N2 fixation (BNF) of Vigna unguiculata and consequent effects on C and N pools in two Namibian soils. Three treatments (Bradyrhizobium sp.1–7 inoculant, non-inoculated, N-fertilised with 50 kg N ha−1) were applied in rhizoboxes at 45% and 20% maximum water holding capacity. Nodule development was photo-documented, and rhizobia-DNA sequences were identified. BNF was assessed by δ15N enrichment, and organic C and N pools were analysed in bulk and root adherent soil.
Results
Plant growth initially enhanced mineral N losses from the rhizosphere at flowering stage (6 weeks growth), but led to a re-increase of N, and organic C contents after ripening (10 weeks). Inoculation had no effect on nodulation and soil C and N pools, indicating that both soils contained sufficient indigenous rhizobia to allow effective nodulation. However, the inoculant strain was more competitive in establishing itself in the root nodules, depending on the local conditions, showing a need for regional adjustment of inoculation strategies.
Conclusion
Water stress was the main limitation for nodulation and, in combination with soil type, substantially affected rhizosphere and bulk soil C and N contents. The temporally enhanced rhizodeposition after ripening could be able to maintain soil C and N pools after legume cultivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Smallholder farming systems in Sub Saharan Africa is characterized by low productivity, which is mainly caused due to declining soil fertility, insufficient fertiliser availability and unreliable water supply (Dakora and Keya 1997; Wall et al. 2014). Sandy soils in Sub-Saharan Africa contain low amounts of N and soil organic carbon (SOC), and hold a generally low nutrient level (de Blécourt et al. 2019; Gröngröft 2013). However, a potential enhancement of soil fertility through mineral fertilisers is limited because these are not affordable by the majority of subsistence farmers in the region (Grönemeyer et al. 2013). Considering projected climate-change impacts and growing populations (IPCC 2018; United Nations 2019), pressure on crop production areas will increase substantially, and therefore a sustainable and efficient way towards an intensification of agricultural crop production is required to ensure food security in the future.
A promising approach to achieve this is the improvement and adoption of legume crops for sustainable agriculture (Rehman et al. 2019). Many legumes are relatively drought resistant and have the capacity to grow in low-fertile sandy soil (Vanlauwe et al. 2019). Since they produce high protein grain and leaves (Hamid et al. 2016; Kerr et al. 2007), legumes are already one of the main food as well as fodder crops growing in Southern Africa (Vanlauwe et al. 2019). A characteristic feature of legume plants is their ability to establish a mutualistic symbiosis with Rhizobia (i.e. formation of nodules) for the utilisation of atmospheric dinitrogen (N2) as an N source (Sugiyama and Yazaki 2012). This legume-rhizobia symbiosis might improve soil fertility via crop residues acting as N-rich green manure which can improve soil physicochemical characteristics and increase SOC content and N availability for succeeding crops (O’Dea et al. 2015). Further, through exudation of organic compounds and nutrients in the rhizosphere, which can trigger plant-microbial interactions (Lynch and Leij 2012). The N2 fixation by rhizobia could for example stimulate microbial activity and increase nutrient concentrations in the rhizosphere (Dakora and Phillips 2002; Sammauria and Kumawat 2018), enhancing plant responses against pathogens especially under unfavourable environmental conditions (Reinhold-Hurek et al. 2015). While the incorporation of legume residues is known to increase SOC sequestration and improves crop nutrient uptake (Amusan et al. 2011; Lal 2015a), direct effects of plant-soil and rhizosphere interactions are less clear (Fustec et al. 2010) or were not given emphasis in agronomy (Gogoi et al. 2018). However, recent research has pointed out the importance of legume derived rhizodeposition for soil C and N status (Virk et al. 2022). These direct effects of plant-soil interaction are particularly important for smallholder farmers who rely on removing legume residues for livestock feeding (Paul et al. 2020).
Cowpea (Vigna unguiculata) is the major food crop and a source of cheap protein for most resource poor households in Namibia (Horn and Shimelis 2020). The north-central regions (such as Oshana and Omusati) and the north-eastern Kavango region are the most significant production areas in the country, where smallholder farmers cultivate cowpeas for personal consumption or use them in intercrop** and crop rotation practices to enhance soil fertility. While cowpeas are generally able to fix adequate amounts of N (Salgado et al. 2021), the efficiency of nodulation can be hindered by local conditions and previous studies have reported such low root nodulation in cowpeas in northern regions of Namibia. (Grönemeyer et al. 2013). Previous analyses have shown that local rhizobia phylotypes are well adapted to the local environment (Delamuta et al. 2013; Grönemeyer et al. 2016, 2014; Grönemeyer and Reinhold-Hurek 2018), and particularly the genus Bradyrhizobium spp. provides the majority of rhizobia for a potential symbiosis in these soils (Grönemeyer et al. 2014). Low water holding capacity, and water scarcity in general might also negatively influence biological N2 fixation (BNF) by reducing nodule nitrogenase activity (Pararajasingham and Knievel 1990), as well as reducing nodulation by limiting rhizobia mobility or leading to desiccation and subsequent cell death (Hamdi 1971; Vlassak et al. 2010). Particularly, the long dry period that occurs yearly in Namibia is putatively adverse to rhizobial survival and abundance. Hence, a possible solution to improve nodulation potential and consequent nutrient supply and growth under drought conditions, is the treatment of legumes with climate adapted native rhizobial bio-inoculants. It is particularly promising for increasing crop productivity of smallholder farming systems in drought-prone regions of Sub-Saharan Africa (Smaling et al. 2008). In this approach, a selected rhizobial inoculant strain, enriched as biofertilizer is directly applied to the legume seeds, at the time of sowing thereby enhancing the chance of root nodulation of the legume mostly by the bio-inoculant strain which ideally should also outcompete the infection from possibly small or inactive populations present in the soil.
To investigate inoculation effects on crop productivity and soil properties, a preceding field study was conducted in Northern Namibia, comparing different cowpea cultivars, and identify suitable inoculants for local pulses that are not only adapted to current dry climatic conditions during the vegetation period but also to future exacerbating conditions due to climate change. In a previous experiment, Bradyrhizobium sp. strain 1–7 led to increased cowpea yields (Chaluma Luchen et al. 2018). Strain 1–7 belongs to the B. japonica lineage of Bradyrhizobium, was isolated from root nodules of peanut grown in the Mashare area of the Namibian Kavango, and was used in the current study because it is particularly heat tolerant and active at up to 38 °C, while also being more competitive than other strains (Grönemeyer et al. 2014; Grönemeyer and Reinhold-Hurek 2018). Among the different cultivars in these studies, particularly’Lutembwe’ variety reacted positively to inoculation. However, it is yet unclear how inoculation with specific Bradyrhizobium sp. influences nodulation of cowpea cultivar’Lutembwe’ and subsequent N supply via BNF. Previous studies mainly investigated legume effects on soil fertility, but it has yet to be explored how inoculation affects legume-soil interaction and thereby control rhizosphere N dynamics over time and under varying environmental conditions. Faster and more efficient nodulation could e.g. enhance root productivity and exudation (Concha and Doerner 2020), eventually affecting soil C and N status.The inoculation with rhizobia can also modify the physical and chemical properties of the soil, creating a more favorable environment for the growth of other soil microbes (Alami et al. 2000), which can lead to an increase in soil C pools. To our knowledge, there is no present study comparing inoculation effects on rhizosphere and bulk soil, before and after nodule senescence, and studies from our study area are generally scarce. Our objectives were (1) to asses whether inoculation of cowpea with specific strains of rhizobia improve plant and nodule growth as well as BNF, (2) to identify consequent effects on C and N pools in the rhizosphere, and (3) to assess how these effects are influenced by soil water availability and soil origin.
To answer these questions, we conducted an experiment under laboratory conditions using rhizoboxes to monitor temporal changes in root and nodule development after inoculation with Bradyrhizobium sp. strain 1–7. Effects on rhizosphere soil were assessed by comparing values of N pools, SOC content and pH in root adherent soil to initial values. These effects were compared between two Northern Namibian soils, a loamy sand and a sand, under optimum water and water stress conditions.
Material and Methods
Studied soils
The study was conducted using mixed topsoil (0–10 cm) samples from two study sites, representing major agricultural regions in Northern Namibia. The Mashare study site is located in the north-eastern Kavango region at an altitude of 1068 m a.s.l. (S: 17° 53′ 27’’; E: 20° 10′ 17’’), embedded in an old floodplain of the Okavango river. The soil was classified as Haplic Luvisol (Arenic) according to IUSS Working Group WRB (2015), and developed on translocated sands and dunes of the Kalahari Basin with interspersed clay and silt layers from fluvial deposits. The climate is semi-arid with a mean annual air temperature of 22.3 °C and an annual precipitation of 571 mm, which mainly occurs during the rainy season between November and March.
The Ogongo study site is located in the Omusati region, central-northern Namibia, at an altitude of 1108 m a.s.l. (S: 17° 40′ 56’’; E: 15° 17′ 59’’). The area is covered by Kalahari sands on slightly elevated terraces that are partly flooded by water courses during the austral summer arising in the Angolan highlands (Jürgens et al. 2012). The soil was classified as Eutric Sideralic Arenosol (Aridic) (IUSS Working Group WRB 2015). The climate is semi-arid with a pronounced seasonal rainfall pattern. Mean annual air temperature is 22.7 °C, with a mean annual precipitation of 469 mm. The majority of rainfall occurs in summer season peaking in February with over 100 mm (Jürgens et al. 2012).
Experimental setup
The experiment was conducted in summer 2020 under controlled conditions at the Institute of Soil Science, Universität Hamburg. The study setup followed a three factorial approach, comparing three fertilization levels, two water availability levels on two soil types. Rhizoboxes (28 × 18 × 1 cm), were filled with air-dried, 2 mm-sieved and well homogenized bulk soil (Table 1). Three fertilization treatments of cowpea (variety: Lutembwe) were applied as follows: (1) inoculated with strain 1–7 of Bradyrhizobium sp. (inoc), (2) non-inoculated (non-inoc), (3) non-inoculated and fertilised with urea equivalent to 50 kg N ha−1 (N). One box of each soil served as blank and unplanted comparison. Four replicates of each treatment and soil type were subjected to a near constant water availability of 45% maximum water holding capacity (WHC) and 20% WHC, respectively. This was ensured by weighting and watering each box every 48 h, and reflects optimum water conditions for cowpea with moisture and oxygen availability for roots to grow (Leenaars et al. 2015), as well as water stress conditions. Plants were grown for 40 days until reaching flowering stage. Additionally, three rhizoboxes per soil type were planted with inoculated seeds and maintained for 75 days until reaching fruit maturity stage (hereinafter: ripening stage). The ripening stage was only reached by plants grown under optimum water conditions, and one replicate plant in Mashare soil died. Seedlings and inoculant were prepared by the Department of Microbe-Plant interactions of the University of Bremen. The cowpea seeds (variety Lutembwe) were surface sterilized with freshly prepared 2.5% sodium hypochlorite for six minutes, washed repeatedly with sterile distilled water and allowed to germinate on 1% water agar plates for twenty four hours at 30 °C in dark before inoculation. Fresh culture of inoculant strain Bradyrhizobium sp. 1–7 was adjusted to optical density of 0.2 at 600 nm with Modified Arabinose Gluconate (MAG) medium. For the inoculated treatment, germinated seedlings were inoculated with 1 ml of bacterial suspension immediately before sowing, whereas seedlings treated with 1 ml of sterile liquid MAG medium served as non-inoculated control. Dissolved urea, equivalent to 50 kg N ha−1, was injected into the fertilised treatment soil at sowing. To ensure plant survival and avoid phosphorus deficiency leading to reduced nodule mass and decreased N production (Dhakal et al. 2016), 100 kg ha−1 granulated superphosphate with 18% phosphorus pentoxide was added to each rhizobox soil. The rhizoboxes were put in a fixture construction at a 60° angle to ensure root growth towards the transparent screen. The screen was covered with aluminium foil to exclude light from the rooting zone. Plants were grown under artificial illumination provided through four 1200-Watt LED grow lights (Wakyme, Monterey Park, CA, USA). Time of illumination was 12 h per day, from 6am to 6 pm. Temperature was maintained at 25 °C and relative humidity was kept at 50%.
Photo documentation
To document root and nodule growth over time, photos of rhizobox surfaces were taken three times per week (2–3 day intervals) until harvest, at the same angle and distance with a digital compact camera. The photos were analysed using ImageJ software (v. 1.8.0). Visible nodules were counted, and their cumulative area was measured.
Sampling of soil and plant
Before the rhizoboxes were opened at one side, shoots were cut off. Roots were then carefully taken out to preserve them in one piece. Root adherent soil (in this study referred to as’rhizosphere soil’) was sampled by gently brushing off adhering soil from roots after being slightly shaken three times by hand. The remaining soil in the rhizobox was sieved (2 mm) to separate it from roots and was declared as bulk soil. Residual fine roots were removed with a tweezer under a magnifying glass. Photos of soil-free roots including nodules were taken. Next, particular nodules were cut out, sampled onto dried silica gel in closed tubes and cooled at 4 °C until use. Fresh soil was immediately stored at 4 °C until further analyses to minimise the mineralisation of labile rhizodeposits. After rhizosphere soil was sampled, roots were washed and, as well as shoot samples, dried at 60 °C for 72 h. Shoot and root biomass were measured as dry mass. Bulk soil of unplanted boxes was sampled in triplicates.
Laboratory analyses
In order to analyse which rhizobial symbionts occupied nodules, collected nodules were surface sterilized using 5% sodium hypochlorite for 2 min, followed by repeated washes with sterile distilled water. The nodules were finally crushed in water, and a small aliquot streaked on MAG agar plates to obtain the pure culture of the nodule symbiont. The rest of the nodule lysate was diluted, and a part used directly to isolate the genomic DNA of respective nodule symbionts with the help of the NucleoSpin Tissue Kit (Macherey–Nagel, Düren, Germany) using the method described by the manufacturer with some modifications. Thereafter, the partial 16S-23S rDNA internally transcribed spacer (ITS) region from individual isolated template DNA was PCR amplified as described by Laguerre et al. (1996). Amplicons were purified by Monarch® Nucleic Acid Purification Kit (NEB, Ipswich, Massachusetts, United States). Sanger sequencing was carried out by LGC Genomics (Berlin, Germany). The obtained nucleotide sequences of respective ITS-PCR products were compared with the National Center for Biotechnology Information (NCBI) sequence database using the Basic Local Alignment Search Tool (BLASTN). Phylogenetic analyses were conducted using MEGA7 free software (Kumar et al. 2016). Alignments were generated by MUSCLE (Edgar 2004; Larkin et al. 2007). DNA sequences of selected type species and reference strains were retrieved from LPSN database (Parte 2014). A Neighbor-Joining Phylogram was constructed with the aligned sequences based on the number of nucleotide differences without gap penalty as suggested by Willems et al. (2001); (2003).
For ammonium and nitrate analysis (Nmin) an aliquot of 25 g both fresh rhizosphere and bulk soil was extracted by shaking for 1 h in 100 ml 0.0125 M CaCl2 solution. Ammonium was analysed in a 2:1-water-extract dilution at 655 nm at photometer (Thermo Fisher Scientific, Waltham, MA, USA). To determine nitrate content, the same extract (0.0125 M CaCl2) was analysed using high performance liquid chromatography (Agilent Technologies, Santa Clara, CA, USA). Samples were measured in a 2:1-diluted water-extract solution. In case of very low concentrations (NO3− content < 0.1 mg kg−1), extract solutions were spiked with a 100 µM KNO3−-standard in 1:2 sample ratio to ensure clear peak separation from baseline noise.
Rhizosphere and bulk soil samples were dried at 105 °C, shoot and root samples at 60 °C until constant weight. Samples were milled and analysed for total C and total N content using a vario MAX cube (Elementar, Langenselbold, Hesse, Germany). In our previous analyses of Mashare and Ogongo soil samples, no inorganic carbon was detected, hence total carbon was declared as SOC. The δ15N-ratio was quantified at an Isotope ratio mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The bulk soil pH was measured with a pH-electrode in a suspension with 0.01 M CaCl2 (1:2.5).
Data analyses
The amount of biologically fixed N (%Ndfa) was estimated by calculating the difference between δ15N signatures of shoot and root biomass (Δδ15Nshoot-root) and inserting this value into an empirical linear equation (Khadka and Tatsumi 2015; Wanek and Arndt 2002). This method is based on a strong linear relationship with the common 15N depletion method and produces well representative data for relative group comparisons – independent of the mineral N signature in the soil (Wanek and Arndt 2002). However, absolute values can strongly vary depending on crop species. We therefore calculated a specific transfer function for cowpea derived from data by Wanek and Arndt (2002) (Eq. 1), and interpreted absolute %Ndfa values very carefully.
Group comparisons were conducted using a three-way-analysis of variance (ANOVA), considering two sites (Mashare, Ogongo), two soil water levels (water stress: 20% WHC, water optimum: 45% WHC) and three fertilization treatments (inoculated, non-inoculated, mineral N), with factor interaction. Residual diagnostics were conducted using Shapiro–Wilk test for normality and Levene’s test for variance homogeneity (p < 0.05). In case ANOVA assumptions were not met, results were verified using robust ANOVA with trimmed means. If at least one factor or one factor interaction was significant, a Tukey’s HSD post-hoc test was performed to test the significant deviation between means of each group at p-level = 0.05. Treatment and water effects on nodule development over time were assessed using a generalized additive mixed model (GAMM) with rhizobox-ID as random factor to account for repeated measurements. Relationships between variables were assessed by linear regression analysis. Simple linear, log-linear and quadratic fits were selected according to reduction in Akaike Information Criterion values. Spearman rank correlation was used to additionally account for clustering and non-linear relationships. Statistical analyses were conducted in R 4.0.3 (R-Core-Team 2021).
Results
Inoculation and water availability effects on plant biomass, nodulation and N fixation
Under optimum water, the plants reached flowering stage after 40 days. Under water stress, plants did not flower until sampling at day 40. Shoot and root biomass under optimum water were 3.4 and 2.7 times higher than under water stress, respectively (Table 2). For shoot biomass, this effect was stronger in Mashare compared to Ogongo soil. Roots in all treatments established faster under optimum water than under water stress (Fig. 1), and reached generally higher biomass in Ogongo compared to Mashare soil (Table 2). All effects were independent from fertilization treatment.
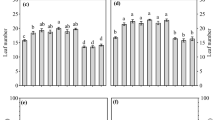
Development of visible nodules over time (A) and maximum number of visible nodules (B). Solid lines and dashed lines represent 20% and 45% water holding capacity (WHC), respectively. Lines and shaded areas indicate GAMM fit at 95% confidence. Small letters indicate significant differences according to ANOVA at p-level = 0.05
The nodulation increased over time and was independent from site and fertilization treatment but affected by water content (ANOVA, p < 0.001) (Fig. 1). Under optimum water, the first nodules appeared about 10 days earlier than under water stress, where the first nodules appeared around day 26 (Fig. 1). However, nodule morphology and distribution along the root varied between sites. While in Mashare soil, nodules distributed evenly along the root system, in Ogongo soil nodules formed mainly around the root base under both water contents.
The fixation of atmospheric N2 was reflected by calculating the difference between δ15N in shoot and root biomass (Δδ15Nshoot-root) and using an empirical transfer function to calculate %Ndfa (Eq. 1). Calculated values of %Ndfa ranged between 32 and 58% between groups (Table 2). The inoculation treatment did not enhance N2 fixation, but had tendency for decreased %Ndfa compared to non-inoculated treatment. In Mashare soil, N2 fixation was reduced under water stress compared to optimum water (ANOVA, p < 0.001). In Ogongo soil, %Ndfa was in the same range as in Mashare soil at 45% WHC and no differences between water contents were observed (ANOVA, p > 0.05).
Treatment and water availability effects on rhizobia infection
To evaluate whether the inoculant established in root nodules, nodule occupancy was analysed according to marker gene sequences. The sequenced ITS DNA-PCR products from the root nodule isolates collected from all rhizoboxes had closest hits to the members of the genus Bradyrhizobium. For a good taxonomic assignment with appropriate resolution of the branches of the group, phylogenetic analysis of ITS sequences was carried out as previously described. The ITS sequences from most of the nodule isolates were distributed together with two reference species in two major clusters supported by high bootstrap values, cluster I with Bradyrhizobium vignae 36 3–2, and cluster 2 with our inoculant strain Bradyrhizobium sp. 1–7 (Fig. 2). B. vignae 36 3–2 is related to the type strain of this species, 7-2 T [DSM 100297 T = LMG 28791 T = NTCCM0018T (Windhoek)]. As the gene bank submitted partial DNA sequence of ITS region from strain 7-2 T is 0.814 kb (KM378504), the relatively long ITS sequence (KM378523) from strain 36 3–2 was used for constructing the ITS-based phylogram although the ITS sequences from both the strains, share 99.9% sequence identity with each other over the complete length. B. vignae I in cluster I was a dominant cowpea nodule occupant also in previous Ogongo field trials (Sarkar and Reinhold-Hurek, unpublished observations). Apparently, the inoculant strain was partially outcompeted in the Ogongo soil, since also some inoculated cowpeas were nodulated by this strain (red circles of cluster I in Fig. 2). The majority of B. vignae induced nodules were from non-inoculated and N fertilizer treatments. Probably the dose of fertilizer treatment used in rhizobox experiments was not sufficient to completely eliminate root nodulation. Interestingly, under drought conditions (Rb2), after inoculation only the inoculant strain in cluster II was found in the inspected nodules and not B. vignae. Cluster II represented by our inoculant strain contained mainly nodules from Mashare, irrespective of the treatment (Fig. 2).
Root nodule occupancy by strains of Bradyrhizobium spp. in rhizobox experiments of cowpea cv. Lutembwe under different treatments. Neighbor-Joining phylogenetic tree from 16S-23S intergenic spacer (ITS)- sequences) of Bradyrhizobium strains amplified from DNA extracted from individual cowpea nodules. (variety: Lutembwe) root nodules collected from two independent Rhizobox experiments under different treatments. The percentage of bootstrap test (1000 replicates) are shown next to the branches, and values under 50% were not considered. ITS sequence of Rhodopseudomonas palustris AB498825 served as outgroup. The evolutionary distances were computed using the Maximum Composite Likelihood method. The analysis involved 48 nucleotide sequences. Evolutionary analyses were conducted in MEGA7. Pink or Red represent Mashare or Ogongo soil, respectively. Treatment with inoculant Bradyrhizobium sp. 1–7 (+ B) and harvested either after 40 days (filled circle) or after 75 days (empty circle); triangle: no inoculant treatment (-B); square: N fertilizer treatment without inoculation. Rb1 or Rb2: rhizobox experiments under 45% or 20% soil water holding capacity, respectively. The major inoculant strain, Bradyrhizobium sp. 1–7 is indicated by a star (*) in the phylogram
Under optimal water conditions with bio-inoculant treatment, B. vignae already present in Ogongo soil can compete with the inoculant for cowpea nodule occupancy. Therefore, strain 1–7 could be less effective to be applied as bio-inoculant under optimal water conditions in Ogongo. However, under drought conditions, inoculant treatment was successful in yielding cluster II nodule occupants, the bio-inoculant strain 1–7 could successfully outcompete the indigenous Ogongo strains.
Additionally, few of the nodule isolates (Rb2 26 and Rb2 27) occupied distinct positions in well-separated branches within the ITS phylogram supported by high bootstrap values. They are closely related to B. kavangaense, a second bio-inoculant strain with potential for enhancement of cowpea growth and yield in the field trials in Namibia (Grönemeyer et al. 2015). Members of such novel clusters may be interesting candidates for future novel species descriptions.
Inoculation and water availability effects on C and N in the rhizosphere
The initial SOC and total N contents in the homogenized soil were about 30% higher in Mashare compared to Ogongo, with CN ratios of 10 at both sites (Table 1). Mineral N content was 31.2 mg kg−1 and 9.4 mg kg−1 respectively. Changes in the rhizosphere are hereafter presented as relative changes compared to these initial soil contents. For absolute values, please refer to Appendix Table A1.
The total N as well as SOC content in the rhizosphere increased by 88% to 112% compared to initial soil (one sample t-test: df = 47, p-value ≤ 0.001). This rhizosphere effect occurred independent of site and water availability (Fig. 3). Non-inoculated and N-fertilized treatments showed a stronger increase than inoculated treatments. The average rhizosphere CN ratio increased from 10.0 to 11.8 compared to initial soil (t-test: df = 47, p-value ≤ 0.001) However, this effect did not occur in Mashare under water stress and was generally stronger in Ogongo, particularly in the inoculated treatment (Fig. 3). The Nmin concentration in the rhizosphere decreased under optimum water conditions. In contrast, an enrichment of rhizosphere Nmin occurred under water stress, reaching 51.33 mg kg−1 in Mashare, and 6.63 mg kg−1 in Ogongo soil. This effect of water availability on rhizosphere Nmin was stronger in Mashare compared to Ogongo soil. Fertilization treatments did not influence rhizosphere Nmin.
Change of SOC (A-B), total N (C-D), CN ratio (E–F) and mineral N (G-H) contents in the rhizosphere at flowering stage in relation to initial soil conditions (zero line). Treatments are indicated as inoculated (inoc), non-inoculated (non-inoc) and with organic N addition (N) for soils from Mashare and Ogongo at 20% and 45% water holding capacity (WHC). Small letters (a-c) indicate significant differences according to ANOVA at p-level = 0.05
Inoculation and water availability effects on C, N and pH in bulk soil
Water stress had a major effect on bulk soil condition (C, total N, Nmin and pH) during flowering stage. The SOC content in bulk soil under optimum water decreased by 10–20% compared to pre-planting content in Mashare and Ogongo soil respectively (Table 3). Under water stress, bulk soil SOC content remained at pre-planting content in both soils. Total N content in bulk soil under optimum water decreased by 40% in Mashare and Ogongo soil compared to pre-planting content. Under water stress, this effect was reduced to 25% loss in Mashare and 37% loss in Ogongo soil, respectively. Mineral N content in bulk soil decreased under both water contents on both sites (Table 3). The decrease was higher in Mashare than in Ogongo soil, with the largest depletion in Mashare soil under optimum water with -28.74 mg kg−1 compared to the initial content. The bulk soil pHCaCl2 in both soils decreased compared to initial values. This effect was stronger under optimum water than under water stress and more pronounced in Ogongo compared to Mashare soil. Fertilization treatments showed no direct effect on bulk soil.
Growth-period effects on soil C and N pools
Temporal effects on rhizosphere characteristics were compared between maturity (40 days) and ripening (75 days) stage in inoculated water-optimum treatments (Fig. 4). In the long-term observation, nodule senescence appeared after 68 days for both sites, indicated by visible cavities (Appendix Figure A1). Rhizosphere SOC content increased by 77% after ripening compared to maturity stage (Fig. 4A). This increase was lower in Ogongo compared to Mashare soil, with 0.30% to 0.46% in Ogongo, and 0.32% to 0.67% in Mashare soil, respectively. The total N content in the rhizosphere increased after the ripening stage, reaching a maximum of 0.07%. This effect was stronger in Mashare compared to Ogongo soil. After reaching ripening stage, CN ratios and mineral N content in the rhizosphere developed in the direction of pre-planting levels (Fig. 4C-D). In both soils, rhizosphere Nmin increased after ripening compared to flowering stage. Particularly in Ogongo soil, rhizosphere Nmin content rose from 4.67 mg kg−1 at flowering to 5.89 mg kg−1 at ripening stage, exceeding the pre-planting level.
Change of SOC (A), total N (B), CN ratio (C) and mineral N (D) contents in the rhizosphere compared to initial soil conditions (zero line) at two growth stages (flowering, ripening) for inoculated and optimum water treatments. The ripening stage in Mashare soil was limited to n = 2 and statistical significances are accepted under reserve. Small letters indicate significant differences according to ANOVA at p-level = 0.05
In bulk soil, the effect was less pronounced than in rhizosphere soil, and only SOC contents reached pre-planting levels (Appendix Table A2). However, SOC, total N, Nitrate as well as pH values significantly increased after ripening compared to flowering stage.
Interactions between soil and plant parameters
During the flowering stage, the number of nodules as well as average nodule size were directly related, and nodulation in general (number of nodules as well as average nodule size) was positively correlated to plant biomass, plant C and N content (Fig. 5, Appendix Figure A3). Particularly shoot biomass was positively related to nodulation and %Ndfa, resulting in increased shoot to root ratios. Nodulation and %Ndfa were negatively correlated to Nmin in the rhizosphere and bulk soil (total values as well as changes compared to initial condition), but showed no relationship with total N contents.
Relationship between nodule amount and %Ndfa (A), shoot biomass (B), change in rhizosphere Nmin (C) and change in rhizosphere pH (D) with Spearman rank correlation coefficients (ρ). Red lines indicate linear or logarithmic regression lines with 95% confidence bands and respective coefficients of determination (R2). Relationships with p > 0.05 are denoted as not significant (n.s.)
The statistical power of correlation analyses at ripening stage was limited (n = 5). However, it indicated a tendency for decoupling of nodule amount and nodule size (Appendix Figure A4). While nodule size was positively related to Nmin and %Ndfa, the nodule amount was negatively related.
Discussion
Inoculation and water availability effects on nodulation and biological N2 fixation
The application of bio-inoculants to improve N supply of legume crops is seen as a promising tool to improve small-holder agriculture in Sub-Saharan Africa (Nyaga and Njeru 2020; Rehman et al. 2019). The actual potential of inoculation to improve legume nodulation can depend on local site characteristics and might affect soil–plant interaction in the rhizosphere (Henneron et al. 2020; Maltais-Landry 2015), independent of plant residue inputs. We investigated the effects of inoculation on crop and nodule development, as well as rhizosphere-soil conditions in a rhizobox setup. Inoculation was compared to conventional N fertilization and untreated soil, under two different water availabilities and in two Namibian soils.
The inoculation treatment did not improve nodulation or N2 fixation in our experiment, neither were soil conditions in the rhizosphere largely affected by fertilization treatments. The calculated %Ndfa values were comparatively low but within previously reported ranges for cowpea under field conditions in Ghana (Belane and Dakora 2009). Previous studies explained the absence of an inoculation effect on N2 fixation by cowpeas and other legumes with the presence of an adequate number of native rhizobia existing in their respective soils (Awonaike et al. 1990; Makgato et al. 2020). Accordingly, Grönemeyer et al. (2013) and Grönemeyer et al. (2014) reported occurrence of strains of Bradyrhizobium spp.—which relatively heat resistant and common in the region—in nodules in Mashare field soil. They related the concurrently low number of rhizobial infections to environmental stress, such as high temperatures and drought. The latter was also supported by the dominant effect of water availability on nodule development in our study. Water scarcity not only reduced the maximum number and size of pronounced nodules, but also led to a retarded nodule development. Under water stress, nodulation occurred about ten days later than under optimum water, reducing BNF fixation and consequently plant growth. Inhibited nodulation under water stress is well described in previous studies (Kurdali et al. 2006), and commonly related to reduced motility and chemotaxis of rhizobia in discontinuously water-filled pores (Hamdi 1971). Compared to our study, we would expect even stronger drought effects under field condition in the Kavango region, where even lower soil moisture levels (e.g. 4%vol) are prevalent. Even more so, since soil heat effects—which were not covered by our approach—might further reduce nodulation under field conditions (Andrés et al. 2012). That the bradyrhizobium inoculant did not positively affect the plant or grain yield in our lab setup was quite surprising, as in a previous field trail in Mashare, grain yield had increased by inoculation with our strains even over the N-fertilized controls (Chaluma Luchen et al. 2018). It is conceivable that under more stressful environmental field conditions in comparison with laboratory rhizoboxes under controlled climate and constant water supply, inoculants are more important for successful nodulation and BNF. Moreover, soil samples were well mixed and thus did not contain natural gradients; in the field, uppermost layers of soil are prone to high temperatures and drying, which are adverse conditions for survival of rhizobia during the dry season.
Contrary to our expectation, N fertilization did not inhibit nodulation or BNF completely. It is already known that little to moderate levels of N-fertilization can facilitate root nodulation by rhizobia (Getachew Gebrehana and Abeble Dagnaw 2020). We calculated the added amount of N to reflect 50 kg N ha−1 under field conditions, a common value for small-holder farmers in the study region. Apparently, this amount was not sufficient to suppress symbiosis with rhizobia under strong N limitation. The rate of BNF is strongly influenced by the initial soil N content and commonly decrease with higher availability of mineral N (Reinprecht et al. 2020). Given the low N status in Mashare and Ogongo soils, the N2 fixation rates in this study are comparably high (Kermah et al. 2018), and further highlight the importance of legume crops for the study region.
Biological nitrogen fixation by rhizobium-legume symbioses constitutes an ecological friendly and inexpensive alternative to the use of chemical nitrogen fertilizers for legume crops (Herridge et al. 2008). Rhizobial inoculants, often applied as bio-fertilizers, play an important role in sustainable agriculture (Kebede 2021; Soumare et al. 2020). However, inoculants often fail to compete for nodule occupancy against native rhizobia resulting in low yields (Thies et al. 1991). Strains with excellent performance under controlled conditions are typically selected as inoculants, but the rates of nodule occupancy compared to native strains are also important factors that need to be investigated (Batista et al. 2015; Irisarri et al. 2019; Mendoza-Suarez et al. 2021). Therefore, knowledge from laboratory studies assessing competition and even from field trials, may allow us to select climate adapted elite strains specific for a certain cultivar and successful in establishing in majority of the root nodules. In our study, regional separation (Mashare or Ogongo), with different soil properties, played a major role for cowpea nodule occupancy by our Namibian inoculant strain. Apparently, the inoculant strain originating from sandy soil in north-east of Namibia (Mashare in the Kavango region (Grönemeyer et al. 2014)) was not fully competitive in the north-west of Namibia (Ogongo with loamy soil), except for drought conditions. Existence of such site-specific rhizobial genotypes have already been reported from different organic fields in Minnesota (Wongphatcharachai et al. 2015). Identification of such strains might prove useful as novel inoculants for organic bean production systems. Even a study involving mycorrhizal fungi inoculant has shown that establishment of the fungus inoculant at the two distant locations was not related to crop** or inoculation practices but site specific (Kokkoris et al. 2019).
Thus, future development of rhizobial bio-inoculants need to combine effectiveness and competitiveness under field conditions, and perhaps develop additional regional inoculant strains, e.g. for North-West Namibia.
Inoculation and water availability effects on soil C and N pools
Rhizobox cultivation of cowpea affected SOC and N pools in Namibian soils compared to their initial status. Until flowering, these effects followed an opposite direction for bulk and rhizosphere soil, and were weakened under water stress. While in bulk soil, SOC as well as total and mineral N contents generally decreased until flowering stage, rhizosphere contents increased – or showed increasing tendencies in most treatments. After ripening, SOC and N pools in the rhizosphere increased even further and also started to re-increase in the bulk soil.
The decrease of bulk-soil total N content during plant growth can be explained by microbial N mineralization and plant N uptake (Parkin et al. 2002). This is supported by a reduction of this process under water stress in our study, which inhibits plant N uptake (He and Dijkstra 2014) and reduces microbial activity (Manzoni et al. 2012). Also, in our unplanted controls, total N contents decreased until harvest. In turn, Nmin contents increased by 350—550%, indicating an effective microbial N mineralization – particularly under optimum water. These effects were mitigated or even reversed in the rhizosphere soil. Effects of legume cultivation on soil C and N stocks are well reported (Drinkwater et al. 1998; Lal 2015b), and often associated with the incorporation of N rich plant residues and consequent increase of overall soil quality (Meena et al. 2018; Webb et al. 2003). However, the release of rhizodeposits has a direct positive effect on SOC contents (Pausch and Kuzyakov 2018), and – mainly in form of ammonium, amino acids and ureides – on N pools in the rhizosphere (Fustec et al. 2010). This effect can comprise up to 20% of organic C (Kumar et al. 2018), and 10% of total plant derived N inputs in legume cover crop systems (De Notaris et al. 2019), and also increase gross N mineralization (Liu et al. 2022), independent of fertilization (Meier et al. 2017). The latter might also explain why fertilization treatments showed no or weak effects in our study. Furthermore, water availability hat a stronger effect on rhizosphere than bulk soil pH and Nmin, indicating a direct effect of rhizodeposition. However, this was not apparent for total C and N contents, and previous studies have shown that drought can affect rhizodeposition amount and composition in various ways –depending on plant species or cultivar (Naylor and Coleman-Derr 2018). More research would be required to distinguish these effects.
At ripening stage, the rhizodeposition effect was further enhanced, as Nmin, total N and SOC increased compared to flowering stage in rhizosphere but also bulk soils of both sites. Rhizodeposition is directly related to plant growth, leading to C and N enrichment in the Rhizosphere over time (Kumar et al. 2018). However, this follows a non-linear relationship and aboveground productivity as well as the amount of fresh rhizodeposition decrease after the vegetative stage (Villarino et al. 2021). Therefore, it is unlikely that our observed increase of SOC and N contents in the rhizosphere from flowering to ripening stage is solely a result of continuous inputs. Instead, we expect that the visible nodule senescence and dissolution at ripening stage (Appendix Figure A2, day 75) led to a pulse of rhizodeposits, explaining strong increases of SOC and N contents in the rhizosphere. Generally, the N rhizodeposition is expected to increase with increased senescing of roots and nodules (Fustec et al. 2010). While we are not aware of any study that identified the composition of shed nodule tissues, legumes are assumed to provide highly N-rich deposits (De Notaris et al. 2019), which would lead to a pulse of easily available N-rich organic matter inputs into the rhizosphere. This effect might also occur well before maturity if nodule senescence is prematurely induced by water stress (Kasper et al. 2019). Consequently, legume effects on soil conditions are undergoing a strong temporal variation, which could alter C and nutrient availability in the rhizosphere and would be important to consider in multi-crop** and cover crop systems (Hassen et al. 2017).
Conclusion
In this study, we used a rhizobox setup to investigate the effects of rhizobial inoculation (Bradyrhizobium sp.) and water stress on nodule development and BNF of cowpea in two Namibian soils and analysed consequent effects on rhizosphere soil C and N content. We conclude that inoculation was unable to enhance nodulation, BNF and yields under these well-controlled laboratory conditions, in contrast to field studies in the Kavango. However, it was able to ensure successful root nodulation by rhizobia, even under drought. Identification of site specific strains, which are more competitive under extreme conditions, might prove useful to improve novel inoculants. Successful nodule development was mainly controlled by sufficient soil water availability and was independent of soil type.
However, despite the marginal difference, the soil type did control the response of SOC and total N pools to cowpea cultivation, indicating a strong effect of regional soil characteristics. These effects were further dependent on soil moisture and growth stage, and differed between rhizosphere and bulk soil. Microbial activity, mineralization and plant N uptake led to a decrease in bulk soil SOC and N pools at flowering stage. In the rhizosphere, this effect was buffered and partly reversed over time. Eventually, SOC and N pools in the rhizosphere increased to pre-planting levels after ripening. This indicates that legume cultivation can be able to – at least – maintain soil C and N pools without the additional input of harvest residues. However, the fate of these deposits until the following growing season remains unknown, and understanding consequences of farming practices on these mechanisms requires further research.
Data Availability
A data summary is available as an electronic supplementary.
References
Alami Y, Achouak W, Marol C, Heulin T (2000) Rhizosphere Soil Aggregation and Plant Growth Promotion of Sunflowers by an Exopolysaccharide-Producing Rhizobiumsp. Strain Isolated from Sunflower Roots. Appl Environ Microbiol 66:3393–3398. https://doi.org/10.1128/AEM.66.8.3393-3398.2000
Amusan AO, Adetunji MT, Azeez JO, Bodunde JG (2011) Effect of the integrated use of legume residue, poultry manure and inorganic fertilizers on maize yield, nutrient uptake and soil properties. Nutr Cycl Agroecosyst 90:321–330. https://doi.org/10.1007/s10705-011-9432-6
Andrés JA, Rovera M, Guiñazú LB, Pastor NA, Rosas SB (2012) Interactions Between Legumes and Rhizobia Under Stress Conditions. Bacteria in Agrobiology: Stress Management
Awonaike KO, Kumarasinghe KS, Danso SKA (1990) Nitrogen-Fixation and Yield of Cowpea (Vigna-Unguiculata) as Influenced by Cultivar and Bradyrhizobium Strain. Field Crop Res 24:163–171. https://doi.org/10.1016/0378-4290(90)90035-A
Batista L, Irisarri P, Rebuffo M, Cuitiño MJ, Sanjuán J, Monza J (2015) Nodulation competitiveness as a requisite for improved rhizobial inoculants of Trifolium pratense. Biol Fertil Soils 51:11–20. https://doi.org/10.1007/s00374-014-0946-3
Belane AK, Dakora FD (2009) Measurement of N2 fixation in 30 cowpea (Vigna unguiculata L. Walp.) genotypes under field conditions in Ghana, using the15N natural abundance technique. Symbiosis 48:47–56. https://doi.org/10.1007/bf03179984
Chaluma Luchen C, Uzabikiriho J-D, Chimwamurombe PM, Reinhold-Hurek B (2018) Evaluating the Yield Response to Bio-Inoculants of Vigna unguiculata in the Kavango Region in Namibia. Journal of Plant Pathology & Microbiology 9. doi: https://doi.org/10.4172/2157-7471.1000456
Concha C, Doerner P (2020) The impact of the rhizobia-legume symbiosis on host root system architecture. J Exp Bot 71:3902–3921. https://doi.org/10.1093/jxb/eraa198
Dakora FD, Keya SO (1997) Contribution of legume nitrogen fixation to sustainable agriculture in Sub-Saharan Africa. Soil Biol Biochem 29:809–817. https://doi.org/10.1016/s0038-0717(96)00225-8
Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245:35–47. https://doi.org/10.1023/A:1020809400075
de Blécourt M, Gröngröft A, Baumann S, Eschenbach A (2019) Losses in soil organic carbon stocks and soil fertility due to deforestation for low-input agriculture in semi-arid southern Africa. J Arid Environ 165:88–96. https://doi.org/10.1016/j.jaridenv.2019.02.006
De Notaris C, Olesen JE, Sørensen P, Rasmussen J (2019) Input and mineralization of carbon and nitrogen in soil from legume-based cover crops. Nutr Cycl Agroecosyst 116:1–18. https://doi.org/10.1007/s10705-019-10026-z
Delamuta JRM, Ribeiro RA, Ormeno-Orrillo E, Melo IS, Martinez-Romero E, Hungria M (2013) Polyphasic evidence supporting the reclassification of Bradyrhizobium japonicum group Ia strains as Bradyrhizobium diazoefficiens sp. nov. Int J Syst Evol Microbiol 63:3342–3351. https://doi.org/10.1099/ijs.0.049130-0
Dhakal Y, Meena RS, Kumar S (2016) Effect of INM on nodulation, yield, quality and available nutrient status in soil after harvest of greengram. Legume Res Intl Jhttps://doi.org/10.18805/lr.v0iOF.9435
Drinkwater LE, Wagoner P, Sarrantonio M (1998) Legume-based crop** systems have reduced carbon and nitrogen losses. Nature 396:262–265. https://doi.org/10.1038/24376
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. https://doi.org/10.1093/nar/gkh340
Fustec J, Lesuffleur F, Mahieu S, Cliquet J-B (2010) Nitrogen rhizodeposition of legumes A review. Agron Sustain Dev 30:57–66. https://doi.org/10.1051/agro/2009003
Getachew Gebrehana Z, Abeble Dagnaw L (2020) Response of soybean to Rhizobial inoculation and starter N fertilizer on Nitisols of Assosa and Begi areas, Western Ethiopia. Environmental Systems Research 9. doi: https://doi.org/10.1186/s40068-020-00174-5.
Gogoi N, Baruah KK, Meena RS (2018) Grain Legumes: Impact on Soil Health and Agroecosystem. Legumes for Soil Health and Sustainable Management
Grönemeyer J, Berkelmann D, T. M-J, D. H, Chimwamurombe PM, B. K, Hurek T, Reinhold-Hurek B (2013) A survey for plant-growth-promoting rhizo-bacteria and symbionts associated with crop plants in the Okavango region of Southern Africa. Biodiversity and Ecology 5. doi: https://doi.org/10.7809/b-e.00282
Grönemeyer JL, Hurek T, Bunger W, Reinhold-Hurek B (2016) Bradyrhizobium vignae sp. nov., a nitrogen-fixing symbiont isolated from effective nodules of Vigna and Arachis. Int J Syst Evol Microbiol 66:62–69. https://doi.org/10.1099/ijsem.0.000674
Grönemeyer JL, Hurek T, Reinhold-Hurek B (2015) Bradyrhizobium kavangense sp. nov., a symbiotic nitrogen-fixing bacterium from root nodules of traditional Namibian pulses. Int J Syst Evol Microbiol 65:4886–4894. https://doi.org/10.1099/ijsem.0.000666
Grönemeyer JL, Kulkarni A, Berkelmann D, Hurek T, Reinhold-Hurek B (2014) Rhizobia Indigenous to the Okavango Region in Sub-Saharan Africa: Diversity, Adaptations, and Host Specificity. Appl Environ Microbiol 80:7244–7257. https://doi.org/10.1128/AEM.02417-14
Grönemeyer JL, Reinhold-Hurek B (2018) Diversity of Bradyrhizobia in Subsahara Africa: A Rich Resource. Front Microbiol 9:2194. https://doi.org/10.3389/fmicb.2018.02194
Gröngröft A (2013) Mashare - Soils. Biodiversity and Ecology 5. doi: https://doi.org/10.7809/b-e.00259
Hamdi YA (1971) Soil-water tension and the movement of rhizobia. Soil Biol Biochem 3:121–126. https://doi.org/10.1016/0038-0717(71)90004-6
Hamid S, Muzaffar S, Wani IA, Masoodi FA, Bhat MM (2016) Physical and cooking characteristics of two cowpea cultivars grown in temperate Indian climate. J Saudi Soc Agric Sci 15:127–134. https://doi.org/10.1016/j.jssas.2014.08.002
Hassen A, Talore DG, Tesfamariam EH, Friend MA, Mpanza TDE (2017) Potential use of forage-legume intercrop** technologies to adapt to climate-change impacts on mixed crop-livestock systems in Africa: a review. Reg Environ Change 17:1713–1724. https://doi.org/10.1007/s10113-017-1131-7
He M, Dijkstra FA (2014) Drought effect on plant nitrogen and phosphorus: a meta-analysis. New Phytol 204:924–931. https://doi.org/10.1111/nph.12952
Henneron L, Kardol P, Wardle DA, Cros C, Fontaine S (2020) Rhizosphere control of soil nitrogen cycling: a key component of plant economic strategies. New Phytol 228:1269–1282. https://doi.org/10.1111/nph.16760
Herridge DF, Peoples MB, Boddey RM (2008) Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 311:1–18. https://doi.org/10.1007/s11104-008-9668-3
Horn LN, Shimelis H (2020) Production constraints and breeding approaches for cowpea improvement for drought prone agro-ecologies in Sub-Saharan Africa. Ann Agri Sci 65:83–91. https://doi.org/10.1016/j.aoas.2020.03.002
IPCC (2018) Global warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. In: PZ V. Masson-Delmotte, H. O. Pörtner, D. Roberts, J. Skea, P.R. Shukla, A. Pirani, W., CP Moufouma-Okia, R. Pidcock, S. Connors, J. B. R. Matthews, Y. Chen, X. Zhou, M. I. Gomis, E. Lonnoy,, MT T. Maycock, T. Waterfield (eds).
Irisarri P, Cardozo G, Tartaglia C, Reyno R, Gutierrez P, Lattanzi FA, Rebuffo M, Monza J (2019) Selection of Competitive and Efficient Rhizobia Strains for White Clover. Front Microbiol 10:768. https://doi.org/10.3389/fmicb.2019.00768
IUSS Working Group WRB (2015) World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Resources Reports. FAO, Rome
Jürgens N, Schmiedel U, Haarmeyer DH, Dengler J, Finckh M, Goetze D, Grongroft A, Hahn K, Koulibaly A, Luther-Mosebach J, Muche G, Oldeland J, Petersen A, Porembski S, Rutherford MC, Schmidt M, Sinsin B, Strohbach BJ, Thiombiano A, Wittig R, Zizka G (2012) The BIOTA Biodiversity Observatories in Africa–a standardized framework for large-scale environmental monitoring. Environ Monit Assess 184:655–678. https://doi.org/10.1007/s10661-011-1993-y
Kasper S, Christoffersen B, Soti P, Racelis A (2019) Abiotic and Biotic Limitations to Nodulation by Leguminous Cover Crops in South Texas. Agriculture 9. doi: https://doi.org/10.3390/agriculture9100209.
Kebede E (2021) Competency of Rhizobial Inoculation in Sustainable Agricultural Production and Biocontrol of Plant Diseases. Frontiers in Sustainable Food Systems 5. doi: https://doi.org/10.3389/fsufs.2021.728014.
Kermah M, Franke AC, Adjei-Nsiah S, Ahiabor BDK, Abaidoo RC, Giller KE (2018) N2-fixation and N contribution by grain legumes under different soil fertility status and crop** systems in the Guinea savanna of northern Ghana. Agric Ecosyst Environ 261:201–210. https://doi.org/10.1016/j.agee.2017.08.028
Kerr RB, Snapp S, Chirwa M, Shumba L, Msachi R (2007) Participatory research on legume diversification with malawian smallholder farmers for improved human nutrition and soil fertility. Exp Agr 43:437–453. https://doi.org/10.1017/S0014479707005339
Khadka J, Tatsumi J (2015) Difference in δ15N Signatures among Plant Parts of PerennialSpecies Subjected to Drought Stress with Special Referenceto the Contribution of Symbiotic N2-fixation to Plant N. Plant Production Science 9:115–122. https://doi.org/10.1626/pps.9.115
Kokkoris V, Li Y, Hamel C, Hanson K, Hart M (2019) Site specificity in establishment of a commercial arbuscular mycorrhizal fungal inoculant. Sci Total Environ 660:1135–1143. https://doi.org/10.1016/j.scitotenv.2019.01.100
Kumar S, Meena RS, Lal R, Singh Yadav G, Mitran T, Meena BL, Dotaniya ML, El-Sabagh A (2018) Role of Legumes in Soil Carbon Sequestration. In: RS Meena, A Das, GS Yadav, R Lal (eds) Legumes for Soil Health and Sustainable Management. Springer Singapore, Singapore
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Kurdali F, Al-Ain F, Al-Shamma M (2006) Nodulation, Dry Matter Production, and N2fixation by Fababean and Chickpea as Affected by Soil Moisture and Potassium Fertilizer. J Plant Nutr 25:355–368. https://doi.org/10.1081/pln-100108841
Laguerre G, Mavingui P, Allard MR, Charnay MP, Louvrier P, Mazurier SI, Rigottier-Gois L, Amarger N (1996) Ty** of rhizobia by PCR DNA fingerprinting and PCR-restriction fragment length polymorphism analysis of chromosomal and symbiotic gene regions: application to Rhizobium leguminosarum and its different biovars. Appl Environ Microbiol 62:2029–2036. https://doi.org/10.1128/aem.62.6.2029-2036.1996
Lal R (2015a) Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 7:5875–5895. https://doi.org/10.3390/su7055875
Lal R (2015b) Soil carbon sequestration and aggregation by cover crop**. J Soil Water Conserv 70:329–339. https://doi.org/10.2489/jswc.70.6.329
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. https://doi.org/10.1093/bioinformatics/btm404
Leenaars JGB, Hengl T, Ruiperez Gonzalez M, Mendes de Jesus JS, Heuvelink GBM, Wolf J, van Bussel LGJ, Claessens H, Yang H, Cassman KG (2015) Root zone plant-available water holding capacity of the Sub-Saharan Africa soil, version 1.0. ISRIC - World Soil Information, Wageningen
Liu Y, Evans SE, Friesen ML, Tiemann LK (2022) Root exudates shift how N mineralization and N fixation contribute to the plant-available N supply in low fertility soils. Soil Biology and Biochemistry 165. doi: https://doi.org/10.1016/j.soilbio.2021.108541
Lynch JM, Leij F (2012) Rhizosphere. eLS
Makgato MJ, Araya HT, du Plooy CP, Mokgehle SN, Mudau FN (2020) Effects of Rhizobium Inoculation on N2 Fixation, Phytochemical Profiles and Rhizosphere Soil Microbes of Cancer Bush Lessertia frutescens (L.). Agronomy 10. doi: https://doi.org/10.3390/agronomy10111675
Maltais-Landry G (2015) Legumes have a greater effect on rhizosphere properties (pH, organic acids and enzyme activity) but a smaller impact on soil P compared to other cover crops. Plant Soil 394:139–154. https://doi.org/10.1007/s11104-015-2518-1
Manzoni S, Schimel JP, Porporato A (2012) Responses of soil microbial communities to water stress: results from a meta-analysis. Ecology 93:930–938. https://doi.org/10.1890/11-0026.1
Meena BL, Fagodiya RK, Prajapat K, Dotaniya ML, Kaledhonkar MJ, Sharma PC, Meena RS, Mitran T, Kumar S (2018) Legume Green Manuring: An Option for Soil Sustainability. Legumes for Soil Health and Sustainable Management
Meier IC, Finzi AC, Phillips RP (2017) Root exudates increase N availability by stimulating microbial turnover of fast-cycling N pools. Soil Biol Biochem 106:119–128. https://doi.org/10.1016/j.soilbio.2016.12.004
Mendoza-Suarez M, Andersen SU, Poole PS, Sanchez-Canizares C (2021) Competition, Nodule Occupancy, and Persistence of Inoculant Strains: Key Factors in the Rhizobium-Legume Symbioses. Front Plant Sci 12: 690567. doi: https://doi.org/10.3389/fpls.2021.690567
Naylor D, Coleman-Derr D (2018) Drought Stress and Root-Associated Bacterial Communities. Frontiers in Plant Science 8
Nyaga JW, Njeru EM (2020) Potential of Native Rhizobia to Improve Cowpea Growth and Production in Semiarid Regions of Kenya. Frontiers in Agronomy 2. doi: https://doi.org/10.3389/fagro.2020.606293
O’Dea JK, Jones CA, Zabinski CA, Miller PR, Keren IN (2015) Legume, crop** intensity, and N-fertilization effects on soil attributes and processes from an eight-year-old semiarid wheat system. Nutr Cycl Agroecosyst 102:179–194. https://doi.org/10.1007/s10705-015-9687-4
Pararajasingham S, Knievel DP (1990) Nitrogenase activity of cowpea (Vigna unguiculata (L.) Walp.) during and after drought stress. Can J Plant Sci 70:163–171. https://doi.org/10.4141/cjps90-018
Parkin TB, Kaspar TC, Cambardella C (2002) Plant Soil 243:187–195. https://doi.org/10.1023/a:1019949727575
Parte AC (2014) LPSN–list of prokaryotic names with standing in nomenclature. Nucleic Acids Res 42:D613-616. https://doi.org/10.1093/nar/gkt1111
Paul BK, Koge J, Maass BL, Notenbaert A, Peters M, Groot JCJ, Tittonell P (2020) Tropical forage technologies can deliver multiple benefits in Sub-Saharan Africa. A meta-analysis. Agronomy for Sustainable Development 40. doi: https://doi.org/10.1007/s13593-020-00626-3
Pausch J, Kuzyakov Y (2018) Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob Chang Biol 24:1–12. https://doi.org/10.1111/gcb.13850
R-Core-Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rehman HM, Cooper JW, Lam HM, Yang SH (2019) Legume biofortification is an underexploited strategy for combatting hidden hunger. Plant Cell Environ 42:52–70. https://doi.org/10.1111/pce.13368
Reinhold-Hurek B, Bunger W, Burbano CS, Sabale M, Hurek T (2015) Roots sha** their microbiome: global hotspots for microbial activity. Annu Rev Phytopathol 53:403–424. https://doi.org/10.1146/annurev-phyto-082712-102342
Reinprecht Y, Schram L, Marsolais F, Smith TH, Hill B, Pauls KP (2020) Effects of Nitrogen Application on Nitrogen Fixation in Common Bean Production. Front Plant Sci 11:1172. https://doi.org/10.3389/fpls.2020.01172
Salgado GC, Ambrosano EJ, Rossi F, Otsuk IP, Ambrosano GMB, Santana CA, Muraoka T, Trivelin PCO (2021) Biological N Fixation and N Transfer in an Intercrop** System between Legumes and Organic Cherry Tomatoes in Succession to Green Corn. Agriculture 11. doi: https://doi.org/10.3390/agriculture11080690
Sammauria R, Kumawat S (2018) Legume Plant Growth-Promoting Rhizobacteria (PGPRs): Role in Soil Sustainability. Legumes for Soil Health and Sustainable Management
Smaling E, Roscoe R, Lesschen J, Bouwman A, Comunello E (2008) From forest to waste: Assessment of the Brazilian soybean chain, using nitrogen as a marker☆. Agr Ecosyst Environ 128:185–197. https://doi.org/10.1016/j.agee.2008.06.005
Soumare A, Diedhiou AG, Thuita M, Hafidi M, Ouhdouch Y, Gopalakrishnan S, Kouisni L (2020) Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture. Plants (Basel) 9. doi: https://doi.org/10.3390/plants9081011
Sugiyama A, Yazaki K (2012) Root Exudates of Legume Plants and Their Involvement in Interactions with Soil Microbes. Secretions and Exudates in Biological Systems
Thies JE, Singleton PW, Bohlool BB (1991) Influence of the size of indigenous rhizobial populations on establishment and symbiotic performance of introduced rhizobia on field-grown legumes. Appl Environ Microbiol 57:19–28. https://doi.org/10.1128/aem.57.1.19-28.1991
United Nations (2019) World Population Prospects 2019. United Nations
Vanlauwe B, Hungria M, Kanampiu F, Giller KE (2019) The role of legumes in the sustainable intensification of African smallholder agriculture: Lessons learnt and challenges for the future. Agric Ecosyst Environ 284: 106583. doi: https://doi.org/10.1016/j.agee.2019.106583
Villarino SH, Pinto P, Jackson RB, Piñeiro G (2021) Plant rhizodeposition: A key factor for soil organic matter formation in stable fractions. Science Advances 7: eabd3176. doi: https://doi.org/10.1126/sciadv.abd3176
Virk AL, Lin B-J, Kan Z-R, Qi J-Y, Dang YP, Lal R, Zhao X, Zhang H-L (2022) Chapter Two - Simultaneous effects of legume cultivation on carbon and nitrogen accumulation in soil. In: DL Sparks (ed) Advances in Agronomy. Academic Press
Vlassak KM, Vanderleyden J, Graham PH (2010) Factors Influencing Nodule Occupancy by Inoculant Rhizobia. Crit Rev Plant Sci 16:163–229. https://doi.org/10.1080/07352689709701948
Wall PC, Thierfelder C, Ngwira A, Govaerts B, Nyagumbo I, Baudron F (2014) Conservation Agriculture in Eastern and Southern Africa. Conservation Agriculture: Global Prospects and Challenges: 263–292. doi: Book_Doi https://doi.org/10.1079/9781780642598.0000
Wanek W, Arndt SK (2002) Difference in delta(15)N signatures between nodulated roots and shoots of soybean is indicative of the contribution of symbiotic N(2) fixation to plant N. J Exp Bot 53:1109–1118. https://doi.org/10.1093/jexbot/53.371.1109
Webb J, Bellamy P, Loveland PJ, Goodlass G (2003) Crop Residue Returns and Equilibrium Soil Organic Carbon in England and Wales. Soil Sci Soc Am J 67:928–936. https://doi.org/10.2136/sssaj2003.9280
Willems A, Coopman R, Gillis M (2001) Phylogenetic and DNA-DNA hybridization analyses of Bradyrhizobium species. Int J Syst Evol Microbiol 51:111–117. https://doi.org/10.1099/00207713-51-1-111
Willems A, Munive A, de Lajudie P, Gillis M (2003) In most Bradyrhizobium groups sequence comparison of 16S–23S rDNA internal transcribed spacer regions corroborates DNA-DNA hybridizations. Syst Appl Microbiol 26:203–210. https://doi.org/10.1078/072320203322346056
Wongphatcharachai M, Staley C, Wang P, Moncada KM, Sheaffer CC, Sadowsky MJ (2015) Predominant populations of indigenous soybean-nodulating Bradyrhizobium japonicum strains obtained from organic farming systems in Minnesota. J Appl Microbiol 118:1152–1164. https://doi.org/10.1111/jam.12771
Acknowledgements
We thank Prof. Dr. Percy Chimwamurombe (Namibia University of Science and Technology) and Prof. Dr. Fisseha Itanna (National University of Lesotho) for their expertise and providing resources and facilities for our field sites. Further, we thank Elisa Toth (Universität Hamburg) for hel** with image analyses, as well as Deborah Harms and Sumita Rui for laboratory work. This research was supported by the National Commission on Research, Science and Technology Namibia (Permit RPIV00092018) and funded by the Federal Ministry of Education and Research Germany (grants #01DG17004B and #01DG17004A-1), as well as the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany‘s Excellence Strategy—EXC 2037 ‘CLICCS–Climate, Climatic Change, and Society’ Project Number: 390683824, contribution to the Center for Earth System Research and Sustainability (CEN) of Universität Hamburg.
The authors have no relevant financial or non-financial interests to disclose.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Yinglong Chen
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Becker, J.N., Grozinger, J., Sarkar, A. et al. Effects of cowpea (Vigna unguiculata) inoculation on nodule development and rhizosphere carbon and nitrogen content under simulated drought. Plant Soil (2023). https://doi.org/10.1007/s11104-023-06051-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-023-06051-1