Abstract
Aims
Rural-urban dynamics are leading to agricultural intensification practices, which affect microbial ecosystem functions in a soil-specific way. This study aimed to investigate what effects agricultural intensification has on soil microbial communities.
Methods
The effects of N fertilization level (low and high) and crop type (maize and finger millet) on microbial communities were investigated, using a two-factorial split-plot design, at two fields (irrigated and rainfed) on typical soil types (Nitisol and Acrisol) mimicking an intensification gradient in the rural-urban interface of the Indian Megacity Bangalore.
Results
The Nitisol had higher pH and clay content than the Acrisol. In combination with irrigation, this led to higher aboveground plant biomass (APB), soil organic carbon (SOC), microbial biomass (MB), fungal ergosterol and microbial necromass. High APB resulted in low total P content, due to P export in APB and high soil C/P and MB-C/P ratios in the Nitisol. Crop type and N fertilization level did not affect microbial parameters in the irrigated Nitisol, whereas crop type affected ergosterol and MBP and N fertilization level affected basal respiration in the rainfed Acrisol. Particulate organic matter (POM) was a major explanatory factor for most microbial parameters in both soils. In the Acrisol, drought reduced metabolic demand, which counteracted negative effects of low pH and clay on the MB. This was indicated by similar metabolic quotients and MBC/SOC ratios in both soils.
Conclusions
These results indicate the current need for water and high-quality fresh plant inputs to improve the microbial contribution to soil fertility at Bangalore.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanization of rural areas leads to a higher resource demand and agricultural intensification, altering soil microbial dynamics globally, especially in the tropics (Elmqvist et al. 2013; Steinhübel and von Cramon-Taubadel 2021). In Bangalore (India), in particular, rural-urban dynamics have led to the introduction of irrigation in originally rainfed agriculture, depletion of groundwater sources, intensified fertilization, especially with N, and changes in crop selection (Prasad et al. 2016; Patil et al. 2019; Prasad et al. 2019). In addition, aboveground biomass from crops is removed from the fields for energy or fodder production, and even the resulting manure is used as fuel for domestic activities elsewhere, reducing the C return to the soil (Prasad et al. 2016; Prasad et al. 2019).
Intensified N fertilization and irrigation increase plant primary production and the input of plant residues to the soil (Yue et al. 2016; Oldfield et al. 2019; Araya et al. 2021). In systems where aboveground crop residues are not retained, roots are an important C supply to soil, which illustrates the importance of crops with a higher root/shoot ratio for increasing C input in soils. Particulate organic matter (POM) is an indicator of the labile soil C fraction mainly composed of recent plant residues (Fontaine et al. 2007; Kauer et al. 2021). POM is strongly affected by management and highly correlated with annual root productivity (Ontl et al. 2015; de Freitas Iwata et al. 2021). However, a higher C input may not promote C storage per se. It is the soil microbial activity and demand for C and nutrients that drives soil organic matter (SOM) decomposition or stabilization (Sinsabaugh et al. 2009; Manzoni et al. 2010; Stone et al. 2013; Vidal et al. 2021). This highlights the importance of microbial stoichiometry (element ratios) and limitations in the soil, which determine the requirements of the soil microbial biomass for maintenance and growth (Sinsabaugh et al. 2009; Manzoni et al. 2010; Buchkowski et al. 2015; Schleuss et al. 2021). In general, N and P are considered the most limiting nutrients in temperate and tropical soils, respectively (Elser et al. 2007; Joergensen 2010; Vitousek et al. 2010). In terms of soil microbial community structure, growth and activity, there is evidence to suggest contrasting N addition effects (Wallenstein et al. 2006; Treseder 2008; Padbhushan et al. 2021; Wang et al. 2021; Huang et al. 2008; Prasad et al. 2016; Sathish et al. 2016).
Both fields were cultivated with lablab (Lablab purpureus (L.) Sweet), maize (Zea mays L.), and finger millet (Eleusine coracana Gaertn.) as main crops (Table 1) during the rainy season, and only the irrigated Nitisol was used for vegetable cultivation of cabbage (Brassica oleracea cv.), eggplant (Solanum melongena L.), and tomato (Solanum lycopersicum L.), respectively, during the dry season (Dayananda et al. 2019). Both field experiments, established in 2016, had the same factorial randomized split-plot design, consisting of crop type and N fertilization level as factors. Maize and finger millet were randomly assigned to the plots, in which subplots for low and high N levels were established, with four replicates per treatment (Fig. 1). The high N level treatments received 100% of the recommended urea rate, split into two halves, i.e., at the time of sowing and 4 weeks after sowing (Dayananda et al. 2019). The low N level plots received no N fertilizer input in the sampling year (Table 1). In addition, single super phosphate and potassium were applied to low and high N treatments equally during sowing, but the amounts were adapted to expected yields for crops at each field.
a Description of the field experiments with a randomized split-plot design for crop type and N fertilization level treatments at the irrigated Nitisol and rainfed Acrisol. Finger millet and Maize plots are indicated in different colours, and subplots with low and high N fertilization level are indicated with L and H letters, respectively. b Location of Bangalore within India is indicated with a star
Soil sampling, soil characteristics and plant yields
In October 2018, soil samples were collected before harvest. In each treatment replicate, three soil cores (diameter: 4.2 cm) were randomly taken at 0–10 cm depth and mixed to a composite sample. The soil bulk density was 1.65 and 1.72 g cm3−1 at 15 cm depth for the irrigated Nitisol and rainfed Acrisol, respectively (Stephan Peth, personal communication). Soil samples were sieved to 2 mm and divided into two portions, one fresh portion (approx. 11% and 5% water content after sieving for the irrigated and rainfed soils, respectively) was stored frozen at −18 °C for biological analysis, the other was oven-dried at 105 °C and ground for chemical analysis. Soil pH was measured in water at a ratio of 1:2.5. Total C and total N were determined by gas chromatography, using a Vario MAX (Elementar, Hanau, Germany) elemental analyzer. Total C was considered equivalent to SOC, after verifying the absence of carbonate C. Total P and Total S were determined by HNO3/microwave digestion, followed by inductively coupled plasma atomic emission spectroscopy (ICP-AES) at 213.618 nm wavelength (Vista-Pro radial, Palo Alto, USA). For soil texture, the sand fraction was determined by wet sieving after destruction of the organic matter with hydrogen peroxide, and dispersion with sodium metaphosphate. Clay fraction was quantified by the pipette method. Silt fraction was calculated as the difference of the sum of sand and clay to 100% (Stephan Peth, personal communication). Sampling of aboveground plant biomass (APB) and data of the specific treatments were performed and provided by Dayananda et al. (2019).
Microbial biomass indices
Microbial biomass C (MBC), N (MBN), and P (MBP) were determined by chloroform fumigation extraction, using soil samples adjusted to 50% of their water holding capacity after thawing for 5 d at 4 °C. For MBC (Vance et al. 1987) and MBN (Brookes et al. 1985), fumigated and non-fumigated samples were extracted from 5 g moist soil with 20 ml 0.5 M K2SO4, followed by measuring organic C and total N in the extracts with a multi C/N 2100S automatic analyzer (Analytik Jena, Germany). MBC was calculated as EC/kEC, where EC = (organic C extracted from fumigated soil) − (organic C extracted from non-fumigated soil) and kEC = 0.45 (Wu et al. 1990). MBN was calculated as EN/kEN, where EN = (total N extracted from fumigated soil) − (total N extracted from non-fumigated soil) and kEN = 0.54 (Brookes et al. 1985).
MBP was extracted from 2 g soil (on an oven dry basis) with 40 ml Bray I solution (0.025 M HCl + 0.03 M NH4F) at pH 2.6 (Khan and Joergensen 2012). Phosphate was analyzed by a modified ammonium molybdate-ascorbic acid method (Olsen and Sommers 1982). MBP was calculated as EP/kEP/recovery, were EP = (PO4-P from fumigated soil) – (PO4-P from non-fumigated soil) and kEP = 0.40 (Brookes et al. 1982).
The fungal-cell membrane component ergosterol was extracted from 2 g moist soil with 100 ml ethanol by 30 min oscillating shaking at 250 rev. min−1 (Djajakirana et al. 1996), followed by reversed-phase high-performance liquid chromatography (HPLC) with 100% methanol as the mobile phase and detection at 282 nm.
Basal respiration was measured for one incubation week at 22 °C, after adjusting the water holding capacity of the soil to 40% and pre-incubating the soil for one week. The CO2 was trapped with NaOH solution and then precipitated with 5 ml of a saturated BaCl2 solution. The NaOH not consumed was back titrated with 0.25 M HCl, using a TITRONIC 500 (Xylem Analytics, Weilheim, Germany) system to the transition point of phenolphthalein at a pH of 8.3.
Amino sugars
Glucosamine (GlcN), galactosamine (GalN) and muramic acid (MurN) were measured after hydrolyzing 0.5 dry soil samples with 10 ml 6 M HCl for 6 h at 105 °C (Appuhn et al. 2004) as described by Indorf et al. (2011). Amino sugars were measured, using ortho-phthalaldehyde derivatization, by HPLC in a Dionex (Germering, Germany) Ultimate 3000 pump, a Dionex Ultimate WPS-3000TSL analytical autosampler with in-line split-loop injection and thermostat and an Ultimate 3000 fluorescence detector set at 445 nm emission and 330 nm excitation wavelength. Fungal GlcN was calculated by subtracting bacterial GlcN from total GlcN as an index for fungal residues, assuming that MurN and GlcN occur at a 1 to 2 M ratio in bacteria, with the formula: fungal GlcN (mg g−1soil) = (mmol GlcN −2 × mmol MurN) × 179.17 mg mmol−1, where GlcN is the total GlcN and 179.17 is the molecular weight of GlcN (Engelking et al. 2007; Joergensen 2018). Fungal C and bacterial C for calculating the ratio of fungal to bacterial necromass were obtained by multiplying the contents of fungal GlcN and bacterial MurN by their conversion factors 9 and 45, respectively (Appuhn and Joergensen 2006).
Particulate organic matter
Particulate organic matter (POM) was obtained from 400 g of air-dried soil by wet sieving and flotation-decantation (Magid and Kjærgaard 2001; Muhammad et al. 2006) for the 400–2000 μm size-fraction. POM was dried at 40 °C for 48 h, weighed, ground, and analyzed for total C and N contents. The 13C/12C ratio of SOC and POM-C was measured by elemental analyser – isotope ratio mass spectrometry (Finnigan MAT, Bremen, Germany) and is expressed in δ-notation relative to the Vienna Pee Dee Belemnite (VPDB). To obtain estimates of the C contribution of C3 and C4 plants (%) to POM, δ13C natural labelling was used in a two pooled-mixing model (Balesdent and Mariotti 1996), with the following equation:
where δ13CPOM represents the average signature of POM from all maize and millet plots in the irrigated Nitisol (−23.6 ± 0.7‰) and the rainfed Acrisol (−21.6 ± 0.7‰); δ13Cc4 is the average signature of pure maize and millet litter (−12.8 ± 0.4 ‰ and 12.6 ± 0.6 ‰ in irrigated and rainfed fields, respectively); δ13Ccontrol is a representative signature for tropical C3 plants (−27.6 ± 0.8 ‰) obtained by averaging the value provided by Diels et al. (2004) from a C3 plant and the average value from Swap et al. (2004) for C3 plants under an annual precipitation range between 650 and 970 mm.
Statistical analysis
Results were presented as arithmetic means on a soil dry mass basis. Variance homogeneity and normal distribution of the residuals were tested with the Levene test and Shapiro-Wilk test, respectively. The effect of treatments and their interactions on soil and microbial parameters were evaluated with a two-way analysis of variance employing the ‘aov’-function in the ‘stats’ R package v. 3.5.3 in the R environment (R Core Team 2019), except for MBP at the irrigated Nitisol, the residuals of which were not normally distributed and was tested with the non-parametric Kruskall-Wallis test (‘kruskal.test’-function, ‘stats’ R package v. 3.5.3, R environment, R Core Team 2019). Multiple linear regression models were calculated for relevant microbial parameters as dependent variables and selected soil properties as independent factors, using the ‘lm’-function in the ‘stats’ R package v. 3.5.3 in the R environment (R Core Team 2019). ANOVA and regression model simplification were applied in sequential steps to remove non-significant interactions and/or factors until the minimal adequate model was obtained. To test the relationship between MBC and ergosterol in the irrigated Nitisol and the relationship between the MB-C/N and ergosterol (%MBC) in the rainfed Acrisol, Pearson correlation was used. Pearson correlation was also used to test collinearity between soil properties prior to their selection for regression analysis.
Results
Soil properties of both soils differed, with higher pH and clay, SOC, total N, total S and POM-C contents of the irrigated Nitisol, but total P content 50% below that of the Acrisol (Table 2). The APB of the irrigated Nitisol exceeded that of the rainfed Acrisol by more than three times. In both fields, higher N levels significantly increased APB. Maize yield was higher than millet yield at the irrigated Nitisol. In both fields, soil pH and total P were higher in plots cropped with finger millet, regardless of the N fertilization level.
The irrigated Nitisol contained between 20% (MurN) and 80% (MBC, MBN, fungal GlcN, and GalN) more of the microbial biomass and necromass markers than the rainfed Acrisol (Table 3). MBP varied in a large range around a similar mean of 6.6 μg g−1 soil in both fields. N fertilization level did not affect any soil biological property at the irrigated Nitisol and increased only basal respiration at the rainfed Acrisol. MBP and ergosterol were the two soil biological properties that showed a significant positive response to millet crop**, but only at the rainfed Acrisol.
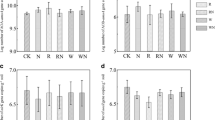
Soil C/N and MB-C/N ratios varied around 10.5 and 6.5, respectively, at both fields, whereas soil C/P and MB-C/P ratios were four and three times larger, respectively, at the irrigated than at the rainfed field (Table 4). The MBC/SOC ratio varied around 1.8% at both fields and was not affected by any treatments, although there was a tendency that high N fertilization level increased this ratio at the rainfed Acrisol (Fig. 2). The metabolic quotient qCO2 varied around 43 μg CO2-C mg−1 MBC d−1 in both fields, with a tendency that millet crop** reduced the qCO2 values at the rainfed Acrisol (Fig. 2). The ergosterol/MBC and ergosterol/fungal GlcN ratios varied around 0.29% and 0.75 mg g−1, respectively, at both fields, exhibiting a considerably larger variation at the rainfed Acrisol (Fig. 3).The fungal C/bacterial C ratio varied around 3.3 at the irrigated Nitisol and around 2.4 at the rainfed Acrisol, without treatment effects (Fig. 3).
Boxplots of the microbial biomass C (MBC)/SOC ratio and metabolic quotient qCO2 at an irrigated Nitisol and a rainfed Acrisol, cropped with maize and finger millet under low and high N fertilization rates (P > 0.05); dots indicate the data of all replicates per treatment. Crop type is indicated by different shape, and N level is indicated by different colour
Boxplots of the ergosterol/microbial biomass C (MBC) ratio, the ergosterol/fungal glucosamine (GlcN) ratio, and the fungal C/bacterial C ratio at an irrigated Nitisol and a rainfed Acrisol, cropped with maize and finger millet under low and high N fertilization rates (P > 0.05); dots indicate the data of all replicates per treatment. Crop type is indicated by different shape, and N level is indicated by different colour
At the irrigated Nitisol, MBC was mainly explained by the soil C/N ratio but also by the soil C/S ratio (Table 5). Ergosterol as well as the ergosterol/MBC and ergosterol/fungal GlcN ratios were all positively affected by POM-C, whereas the necromass markers fungal GlcN and bacterial MurN were both negatively affected by the POM-C/N ratio. At the rainfed Acrisol (Table 5), MBC was mainly explained by the clay content and POM-C, and ergosterol and the ergosterol/fungal GlcN ratio revealed a strong positive influence of POM-C. In contrast, soil C/N was the only predictor found for the fungal C/bacterial C ratio. APB was negatively related to the ergosterol/fungal GlcN and to GalN, while the ergosterol/MBC ratio was negatively influenced by pH and showed a negative relationship to the MB-C/N ratio (Fig. 4). The MB-C/P ratio was influenced by clay content and the MB-C/N ratio by soil pH (Table 5).
Relationships between microbial biomass C (MBC) and ergosterol at the irrigated Nitisol (y = 154.1 × x + 87.9, R2 = 0.52, P < 0.01) and between the microbial biomass C/N ratio (MB-C/N) and ergosterol/MBC ratio at the rainfed Acrisol (y = −8.11 × x + 8.59, R2 = 0.63, P < 0.001), cropped with maize and finger millet under low and high N fertilization rates. Crop type is indicated by different shape, and N level is indicated by different colour
The mean soil δ13C values varied around −21.9‰ and − 19.3‰ at the irrigated and rainfed field, respectively (Table 6). These mean values were 2.0‰ lower in the POM-C recovered at both fields. The C contribution from maize and millet residues to POM-C was on average only 27% in the irrigated Nitisol and 40% in the rainfed Acrisol, thus the contribution of former C3 plant material represented the larger contribution in the POM fraction.
Discussion
Soil and treatment effects on general microbial properties
The effects of soil type and water management considerably exceeded the experimental treatment effects on soil microorganisms. Nevertheless, the MBC and SOC contents, and the pH of the rainfed Acrisol were in line with those obtained previously on Nitisols and Acrisols under rainfed conditions in or around Bangalore, summarized as Alfisols (Vineela et al. 2008; Prasad et al. 2016; Sathish et al. 2016). At the irrigated, more fertile Nitisol, none of the microbial properties responded to the crop type or N fertilization level. In this soil, the negative effect of the soil C/N and soil C/S ratios on MBC indicate nutrient limitation. However, the fact that this effect was not reflected by an increased MB-C/N ratio suggests an additional limitation of C inputs, which were reduced under lower N availability. Effects of N fertilization level and crop type were observed in the acidic rainfed Acrisol. The higher total P content in millet plots was reflected by higher MBP. Similar amounts of P fertilizer applied in millet and maize plots but lower millet yields resulted in higher P uptake in microbial biomass under millet. In the rainfed Acrisol, microorganisms responded more sensitively to basic properties, i.e., variations in clay content and soil pH.
It was a striking feature of the current results that lower clay content and lower soil pH of the rainfed Acrisol did not result in much higher qCO2 values and lower MBC/SOC ratios in comparison with the irrigated Nitisol. This contrasts other studies, in which a lower pH (Anderson and Domsch 1993, 2010; Hartman and Richardson 2013) or lower clay content (Müller and Höper 2004) significantly increased the qCO2, due to an increased demand for maintenance energy and reduced protection of SOM, respectively. However, such effects may have been counteracted by a drought-reduced microbial turnover in this study.
The irrigated Nitisol was characterized by extremely low total P contents, but the total P contents of the rainfed Acrisol were also low in comparison with other soils from India (Paul et al. 2018). The reason for the three times lower total P content of the irrigated Nitisol in comparison with the rainfed Acrisol could not be fully explained by the current study. Most likely, a similar P fertilization, but considerably lower yield resulted in less P uptake by crops and finally in a higher total P content of the rainfed Acrisol. This view is supported by the observation that the lower yield of millet crop** generally increased total P content and soil pH in comparison with maize, with a positive correlation between soil C/P and APB (r = 0.64) in the irrigated Nitisol. This indicates a rapid soil response to changes in the crop cultivated.
It should be considered that millet cultivation followed maize after lablab. Maize received more N from the preceding legume crop and from the higher urea fertilization than millet, which led to a small but significant decrease in soil pH at both fields. Consequently, the observed crop effects on soil properties were larger than those of the N fertilization level. This was especially true at the rainfed Acrisol, where millet crop** increased the contents of saprotrophic fungi by approximately 50% in comparison with maize, suggesting a larger C input by millet roots. On the basis of the current APB results and the calculated root/shoot ratios of 0.57 for millet (Goron et al. 2015) and of 0.16 for maize (Amos and Walters 2006), the resulting belowground root biomass at maturity was higher (P < 0.05) for millet (1.4 kg and 0.4 kg m−2 at the irrigated and rainfed field, respectively) compared to maize (0.6 kg and 0.2 kg m−2 at the irrigated and rainfed field, respectively). Although the rhizodeposits of the two crop species may be very similar, acid phosphatase and dehydrogenase activities are higher in finger millet than in maize crop** systems (Dotaniya et al. 2014). Acid phosphatases are also excreted by fungi (Dotaniya et al. 2019), which may additionally support the observed interactions between roots, the increase in saprotrophic fungi, and MBP under millet plots.
Specific effects on microbial stoichiometry
The strong P deficiency of the irrigated Nitisol led to wide MB-C/P ratios in comparison with other tropical soils (Joergensen 2010; Tischer et al. 2014). Large MB-C/P ratios were often observed in situations of low P availability in combination with relatively high C availability (Anderson and Domsch 1980; Kapoor and Haider 1982; He et al. 1997). The higher yield level at the current irrigated Nitisol aggravated P deficiency. In contrast to other studies (Heuck et al. 2015), high MB-C/P ratios were not related to high MB-C/N ratios, probably due to the combination of sufficient N fertilization and N2 fixing lablab in the crop rotation.
At the rainfed Acrisol, clay positively affected the MB-C/P ratio, probably due to an increase in MBC, although no correlation was found between MB-C/P and MBC, both positively benefited from higher clay content. With increasing soil pH, the MB-C/N ratio decreased but the ergosterol/MBC ratio increased, indicating an increasing contribution of saprotrophic fungi to the microbial community. The MB-C/N ratio reflected the complex interaction with SOC, total N and P and not the ratio of fungi to bacteria suggested by Khan et al. (2016).
Specific soil and treatments effects on fungi
Irrigation led to a strong increase in crop yield, and most likely also in root input in comparison with the rainfed Acrisol. This increased MBC, MBN, and especially the ergosterol content, an indicator of saprotrophic fungi in arable soils (Joergensen and Wichern 2008). The positive relationship of ergosterol with POM-C was in line with the observation that fungal biomass is strongly dependent upon POM in soils with low carbon contents (Wachendorf et al. 2014).
At the irrigated Nitisol, the ergosterol/MBC ratio was negatively affected by the soil C/P ratio, suggesting that P availability specifically controls saprotrophic fungi. The current ergosterol/MBC ratios were generally in the range of other tropical arable soils (Joergensen and Castillo 2001; Joergensen 2010; Murugan and Kumar 2013). However, the strong differences between the two fields in clay content and in soil pH had only minor effects on the ergosterol/MBC ratio in comparison with the results of others (Joergensen and Castillo 2001; Rousk et al. 2009; Wentzel et al. 2015).
The ergosterol/fungal GlcN ratio was relatively consistent in all treatments of the two fields, which supports the view that there is a strong relationship between fungal biomass and fungal necromass (Khan et al. 2016). In contrast, the fungal C/bacterial C ratio was markedly higher at the irrigated Nitisol, in line with the lack of response of bacterial biomass but positive response of fungi to soil moisture (Frey et al. 1999). Under rainfed conditions, GalN and fungal GlcN were more than 43% lower than in the irrigated Nitisol, whereas MurN only decreased by 25%. Drought apparently promoted the specific accumulation of bacterial residues. For this reason, Amelung et al. (1999) observed a relative increase in MurN in comparison with GlcN with decreasing mean annual precipitation. The markedly higher fungal C/bacterial C ratio at the neutral Nitisol in comparison with the acidic Acrisol contrasted other studies from India (Murugan and Kumar 2013) and many other regions (Rousk et al. 2011; Khan et al. 2016), suggesting that drought effects override pH effects.
Relevance of particulate organic matter at the two fields
Drought also promoted the accumulation of POM-C, which contributed 3.7% to SOC at the irrigated Nitisol and 5.0% at the rainfed Acrisol. POM contained only 27–40% C4 plant material, indicating that maize and millet were not main contributors to POM. Therefore, there was a lack of correlation between POM-C and APB. This result is surprising, considering that the C4 plants were the actual main crops for the study year in maize plots and for two consecutive years in millet plots, neglecting the small root input of vegetables during the dry period at the irrigated Nitisol. Hence, POM mainly consisted of plant residues not decomposed for several years. This contrasts the view that POM-C is always a very labile and readily bio-available pool, derived from less decomposed plant material of the previous crop (Benbi and Richter 2002; Wang et al. 2004; Heitkamp et al. 2009). The current results are even more surprising, considering the observation that POM-C generally had dominating positive effects on saprotrophic soil fungi, which were apparently unable to decompose these plant residues under the environmental conditions of the two fields. However, it is important to highlight that the crop yield in 2018 was particularly low compared to the previous year (Dayananda et al. 2019). This probably reduced the contribution of the current crop residues to POM and the correlation between the two variables, thus reducing the influence of APB on microbial indices.
The generally positive effects of POM-C on the ergosterol/fungal GlcN ratio indicate that this C-fraction promoted especially fungal biomass in comparison with fungal necromass. The negative effects of the POM-C/N ratio at the irrigated Nitisol on fungal GlcN and bacterial MurN suggest that not the amount but the quality of plant residues controls microbial necromass accumulation. APB at the rainfed Acrisol had negative effects on the accumulation of GalN, an indicator of microbial extracellular polymeric substances (Joergensen 2018). This suggests that microorganisms have to invest more energy in this fraction under drought conditions with a lower input of root and harvest residues.
Conclusions
Microorganisms in typical soil types at Bangalore are C and nutrient limited under major cereal crop** systems. The microbial response to intensification varied depending on soil pH and water availability. More favorable abiotic soil conditions and higher SOM of the irrigated Nitisol resulted in higher APB and MB in comparison with the rainfed Acrisol. N fertilization generally led to higher APB but had only minor effects on microbial biomass in both soil types. Crop effects were probably strengthened by indirect effects of higher N-fertilization of maize than millet, inducing a decrease of soil pH under maize. A low percentage of particulate organic matter from recent plant material indicates the additional importance of crop residues from previous rotations for soil microorganisms under tropical conditions. Fungal biomass and necromass were reduced in the rainfed Acrisol in comparison with the irrigated Nitisol. However, it was not possible to differentiate irrigation effects from land use history and soil effects, i.e., irrigation and rainfed treatments should be carried out on both soil types. To address the entire rural-urban dynamics taking place in the soils at Bangalore, additional crops should be studied. In particular, the effects of vegetables, fruits and non-food crops, which are being increasingly cultivated due to increasing urban demand, have been largely unexplored.
References
Amelung W, Zhang X, Flach KW, Zech W (1999) Amino sugars in native grassland soils along a climosequence in North America. Soil Sci Soc Am J 63:86–92
Amos B, Walters DT (2006) Maize root biomass and net rhizodeposited carbon. Soil Sci Soc Am J 70:1489–1503
Anderson JPE, Domsch KH (1980) Quantities of plant nutrients in the microbial biomass of selected soils. Soil Sci 171:106–111
Anderson TH, Domsch KH (1993) The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol Biochem 25:393–395
Anderson TH, Domsch KH (2010) Soil microbial biomass: the eco-physiological approach. Soil Biol Biochem 42:2039–2043
Appuhn A, Joergensen RG (2006) Microbial colonisation of roots as a function of plant species. Soil Biol Biochem 38:1040–1051
Appuhn A, Joergensen RG, Raubuch M, Scheller E, Wilke B (2004) The automated determination of glucosamine, galactosamine, muramic acid and mannosamine in soil and root hydrolysates by HPLC. J Plant Nutr Soil Sci 167:17–21
Araya A, Gowda PH, Rad MR, Ariyaratne CB, Ciampitti IA, Rice CW, Prasad PVV (2021) Evaluating optimal irrigation for potential yield and economic performance of major crops in southwestern Kansas. Agric Water Manag 244:106536
Balesdent J, Mariotti A (1996) Measurement of soil organic matter turnover using 13C natural abundance. In: Boutton TW, Yamasaki SI (eds) Mass spectrometry of soils. Marcel Dekker, New York, pp 83–111
Banger K, Tian H, Tao B, Lu C, Ren W, Yang J (2015) Magnitude, spatiotemporal patterns, and controls for soil organic carbon stocks in India during 1901–2010. Soil Sci Soc Am J 79:864–875
Benbi DK, Richter J (2002) A critical review of some approaches to modelling nitrogen mineralization. Biol Fertil Soils 35:168–183
Brookes PC, Powlson DS, Jenkinson DS (1982) Measurement of microbial biomass phosphorus in soil. Soil Biol Biochem 14:319–329
Brookes PC, Kragt JF, Powlson DS, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: the effects of fumigation time and temperature. Soil Biol Biochem 17:831–835
Buchkowski RW, Schmitz OJ, Bradford MA (2015) Microbial stoichiometry overrides biomass as a regulator of soil carbon and nitrogen cycling. Ecology 96:1139–1149
Dayananda S, Astor T, Wijesingha J, Chickadibburahalli Thimappa S, Dimba Chowdappa H, Mudalagiriyappa NRR, Nautiyal S, Wachendorf M (2019) Multi-temporal monsoon crop biomass estimation using hyperspectral imaging. Remote Sens 11:1771
de Freitas Iwata B, Brandão MLSM, dos Santos BR, Leite LFC, Costa MCG (2021) Total and particulate contents and vertical stratification of organic carbon in agroforestry system in Caatinga. Revista Caatinga 34:443
Diels J, Vanlauwe B, van der Meersch MK, Sanginga N, Merckx R (2004) Long-term soil organic carbon dynamics in a subhumid tropical climate: 13C data in mixed C3/C4 crop** and modeling with ROTHC. Soil Biol Biochem 36:1739–1750
Djajakirana G, Joergensen RG, Meyer B (1996) Ergosterol and microbial biomass relationship in soil. Biol Fertil Soils 22:299–304
Dotaniya ML, Kushwah SK, Rajendiran S, Coumar MV, Kundu S, Rao AS (2014) Rhizosphere effect of kharif crops on phosphatases and dehydrogenase activities in a Typic Haplustert. Nat Acad Sci Lett 7:103–106
Dotaniya ML, Aparna K, Dotaniya CK, Singh M, Regar KL (2019) Role of soil enzymes in sustainable crop production. In: Kuddus M (ed) Enzymes in food biotechnology. Academic Press, Cambridge, MA, pp 569–589
Elmqvist T, Fragkias M, Goodness J, Güneralp B, Marcotullio PJ, McDonald RI, Parnell S, Schewenius M, Sendstad M, Seto K, Wilkinson C (2013) Urbanization, biodiversity and ecosystem services: challenges and opportunities: a global assessment. Springer, Dordrecht
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142
Engelking B, Flessa H, Joergensen RG (2007) Shifts in amino sugar and ergosterol contents after addition of sucrose and cellulose to soil. Soil Biol Biochem 39:2111–2118
Faust S, Heinze S, Ngosong C, Sradnick A, Oltmanns M, Raupp J, Geisseler D, Joergensen RG (2017) Effect of biodynamic soil amendments on microbial communities in comparison with inorganic fertilization. Appl Soil Ecol 114:82–89
Fontaine S, Barot S, Barré P, Bdioui N, Mary B, Rumpel C (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450:277–280
Frey SD, Elliott ET, Paustian K (1999) Bacterial and fungal abundance and biomass in conventional and no-tillage agroecosystems along two climatic gradients. Soil Biol Biochem 31:573–585
Goron TL, Bhosekar VK, Shearer CR, Watts S, Raizada MN (2015) Whole plant acclimation responses by finger millet to low nitrogen stress. Front Plant Sci 6:652
Hartman WH, Richardson CJ (2013) Differential nutrient limitation of soil microbial biomass and metabolic quotients (qCO2): is there a biological stoichiometry of soil microbes? PLoS One 8:e57127
He ZL, Wu J, O‘Donnell AG, Syers JK (1997) Seasonal responses in microbial biomass carbon, phosphorus and Sulphur in soils under pasture. Biol Fertil Soils 24:421–428
Heitkamp F, Raupp J, Ludwig B (2009) Impact of fertilizer type and rate on carbon and nitrogen pools in a sandy Cambisol. Plant Soil 319:259–275
Heuck C, Weig A, Spohn M (2015) Soil microbial biomass C:N:P stoichiometry and microbial use of organic phosphorus. Soil Biol Biochem 85:119–129
Huang Y, Wang Q, Zhang W, Zhu P, **ao Q, Wang C, Wu L, Tian Y, Xu M, Gunina A (2021) Stoichiometric imbalance of soil carbon and nutrients drives microbial community structure under long-term fertilization. Appl Soil Ecol 168:104119
Indorf C, Dyckmans J, Khan KS, Joergensen RG (2011) Optimisation of amino sugar quantification by HPLC in soil and plant hydrolysates. Biol Fertil Soils 47:387–396
IUSS Working Group WRB (2015) World Reference Base for soil resources 2014, update 2015 international soil classification system for naming soils and creating legends for soil maps. FAO, Rome
Joergensen RG (2010) Organic matter and micro-organisms in tropical soils. In: Dion P (ed) Soil biology and agriculture in the tropics. Springer, Berlin, pp 17–44
Joergensen RG (2018) Amino sugars as specific indices for fungal and bacterial residues in soil. Biol Fertil Soils 54:559–568
Joergensen RG, Castillo X (2001) Interrelationships between microbial and soil properties in young volcanic ash soils of Nicaragua. Soil Biol Biochem 33:1581–1589
Joergensen RG, Wichern F (2008) Quantitative assessment of the fungal contribution to microbial tissue in soil. Soil Biol Biochem 40:2977–2991
Kallenbach CM, Frey SD, Grandy AS (2016) Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat Comm 7:13630
Kapoor KK, Haider K (1982) Mineralization and plant availability of phosphorus from biomass of hyaline and melanic fungi. Soil Sci Soc Am J 46:953–957
Kauer K, Pärnpuu S, Talgre L, Eremeev V, Luik A (2021) Soil particulate and mineral-associated organic matter increases in organic farming under cover crop** and manure addition. Agriculture 11:903
Khan KS, Joergensen RG (2012) Compost and phosphorus amendments for stimulating microorganisms and growth of ryegrass in a Ferralsol and a Luvisol. J Plant Nutr Soil Sci 175:108–114
Khan KS, Mack R, Castillo X, Kaiser M, Joergensen RG (2016) Microbial biomass, fungal and bacterial residues, and their relationships to the soil organic matter C/N/P/S ratios. Geoderma 271:115–123
Liang C, Amelung W, Lehmann J, Kästner M (2019) Quantitative assessment of microbial necromass contribution to soil organic matter. Glob Change Biol 25:3578–3590
Magid J, Kjærgaard C (2001) Recovering decomposing plant residues from the particulate soil organic matter fraction: size versus density separation. Biol Fertil Soils 33:252–257
Malik AA, Chowdhury S, Schlager V, Oliver A, Puissant J, Vazquez PGM, Jehmlich N, von Bergen M, Griffiths RI, Gleixner G (2016) Soil fungal:bacterial ratios are linked to altered carbon cycling. Front Microbiol 7:1247
Manzoni S, Trofymow JA, Jackson RB, Porporato A (2010) Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol Monogr 80:89–106
Muhammad S, Müller T, Joergensen RG (2006) Decomposition of pea and maize straw in Pakistani soils along a gradient in salinity. Biol Fertil Soils 43:93–101
Müller T, Höper H (2004) Soil organic matter turnover as a function of the soil clay content: consequences for model applications. Soil Biol Biochem 36:877–888
Murugan R, Kumar S (2013) Influence of long-term fertilisation and crop rotation on changes in fungal and bacterial residues in a tropical rice-field soil. Biol Fertil Soils 49:847–856
Murugan R, Parama VRR, Madan B, Muthuraju R, Ludwig B (2019) Short-term effect of nitrogen intensification on aggregate size distribution, microbial biomass and enzyme activities in a semi-arid soil under different crop types. Pedosphere 29:483–491
Oldfield EE, Bradford MA, Wood SA (2019) Global meta-analysis of the relationship between soil organic matter and crop yields. Soil 5:15–32
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part 2 chemical and microbiological properties 2. American Society of Agronomy, Madison, pp 403–430
Olsson PA, Larsson L, Bago B, Wallander H, van Aarle IM (2003) Ergosterol and fatty acids for biomass estimation of mycorrhizal fungi. New Phytol 159:1–10
Ontl TA, Cambardella CA, Schulte LA, Kolka RK (2015) Factors influencing soil aggregation and particulate organic matter responses to bioenergy crops across a topographic gradient. Geoderma 255:1–11
Padbhushan R, Sharma S, Kumar U, Rana DS, Kohli A, Kaviraj M, Parmar B, Kumar R, Annapurna K, Sinha AK, Gupta VSR (2021) Meta-analysis approach to measure the effect of integrated nutrient management on crop performance, microbial activity, and carbon stocks in Indian soils. Front Environ Sci 9:724702
Patil VS, Thomas BK, Lele S, Eswar M, Srinivasan V (2019) Adapting or chasing water? Crop choice and farmers' responses to water stress in peri-urban Bangalore, India. Irrig Drain 68:140–151
Paul R, Singh RD, Patra AK, Biswas DR, Bhattacharyya R, Arunkumar K (2018) Phosphorus dynamics and solubilizing microorganisms in acid soils under different land uses of lesser Himalayas of India. Agrofor Syst 92:449–461
Prasad JVNS, Rao CS, Srinivas K, Jyothi CN, Venkateswarlu B, Ramachandrappa BK, Dhanapal GN, Ravichandra K, Mishra PK (2016) Effect of ten years of reduced tillage and recycling of organic matter on crop yields, soil organic C and its fractions in Alfisols of semi arid tropics of southern India. Soil Till Res 156:131–139
Prasad CS, Anandan S, Gowda NKS, Schlecht E, Buerkert A (2019) Managing nutrient flows in Indian urban and peri-urban livestock systems. Nutr Cycl Agroecosyst 115:159–172
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna https://www.R-project.org
Rousk J, Bååth E (2011) Growth of saprotrophic fungi and bacteria in soil. FEMS Microbiol Ecol 78:17–30
Rousk J, Brookes PC, Bååth E (2009) Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl Environ Microbiol 75:1589–1596
Rousk J, Brookes PC, Bååth E (2011) Fungal and bacterial growth responses to N fertilization and pH in the 150-year 'Park Grass' UK grassland experiment. FEMS Microbiol Ecol 76:89–99
Sathish A, Ramachandrappa BK, Shankar MA, Srikanth Babu PN, Srinivasarao C, Sharma KL (2016) Long-term effects of organic manure and manufactured fertilizer additions on soil quality and sustainable productivity of finger millet under a finger millet–groundnut crop** system in southern India. Soil Use Manag 32:311–321
Schleuss PM, Widdig M, Biederman LA, Borer ET, Crawley MJ, Kirkman KP, Spohn M (2021) Microbial substrate stoichiometry governs nutrient effects on nitrogen cycling in grassland soils. Soil Biol Biochem 155:108168
Scott JT, Cotner JB, LaPara TM (2012) Variable stoichiometry and homeostatic regulation of bacterial biomass elemental composition. Front Microbiol 3:42
Sinsabaugh RL, Hill BH, Follstad Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795–798
Srivastava P, Singh R, Bhadouria R, Tripathi S, Raghubanshi AS (2020) Temporal change in soil physicochemical, microbial, aggregate and available C characteristic in dry tropical ecosystem. Catena 190:104553
Steinhübel L, von Cramon-Taubadel S (2021) Somewhere in between towns, markets and jobs–agricultural intensification in the rural–urban interface. J Dev Stud 57:669–694
Stone MM, Plante AF, Casper BB (2013) Plant and nutrient controls on microbial functional characteristics in a tropical Oxisol. Plant Soil 373:893–905
Swap RJ, Aranibar JN, Dowty PR, Gilhooly WP III, Macko SA (2004) Natural abundance of 13C and 15N in C3 and C4 vegetation of southern Africa: patterns and implications. Glob Chang Biol 10:350–358
Tischer A, Potthast K, Hamer U (2014) Land-use and soil depth affect resource and microbial stoichiometry in a tropical mountain rainforest region of southern Ecuador. Oecologia 175:375–393
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Verbruggen E, Pena R, Fernandez CW, Soong JL (2017) Mycorrhizal interactions with saprotrophs and impact on soil C storage. In: Johnson NC, Gehring C, Jansa J (eds) Mycorrhizal mediation of soil. Elsevier, Amsterdam, pp 441–460
Vidal A, Klöffel T, Guigue J, Angst G, Steffens M, Hoeschen C, Mueller CW (2021) Visualizing the transfer of organic matter from decaying plant residues to soil mineral surfaces controlled by microorganisms. Soil Biol Biochem 160:108347
Vineela C, Wani SP, Srinivasarao C, Padmaja B, Vittal KPR (2008) Microbial properties of soils as affected by crop** and nutrient management practices in several long-term manurial experiments in the semi-arid tropics of India. Appl Soil Ecol 40:165–173
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol Appl 20:5–15
Wachendorf C, Potthoff M, Ludwig B, Joergensen RG (2014) Effects of addition of maize litter and earthworms on C mineralization and aggregate formation in single and mixed soils differing in soil organic carbon and clay content. Pedobiologia 57:161–169
Wallenstein MD, McNulty S, Fernandez IJ, Boggs J, Schlesinger WH (2006) Nitrogen fertilization decreases forest soil fungal and bacterial biomass in three long-term experiments. For Ecol Manag 222:459–468
Wang FE, Chen YX, Tian GM, Kumar S, He YF, Fu QL, Lin Q (2004) Microbial biomass carbon, nitrogen and phosphorus in the soil profiles of different vegetation covers established for soil rehabilitation in a red soil region of southeastern China. Nutr Cycl Agroecosyst 68:181–189
Wang F, Chen S, Qin S, Sun R, Zhang Y, Wang S, Hu C, Hu H, Liu B (2021) Long-term nitrogen fertilization alters microbial community structure and denitrifier abundance in the deep vadose zone. J Soils Sediments 21:2394–2403
Wentzel S, Schmidt R, Piepho HP, Semmler-Busch U, Joergensen RG (2015) Response of soil fertility indices to long-term application of biogas and raw slurry under organic farming. Appl Soil Ecol 96:99–107
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of microbial biomass C by fumigation extraction – an automated procedure. Soil Biol Biochem 22:1167–1169
Yue K, Peng Y, Peng C, Yang W, Peng X, Wu F (2016) Stimulation of terrestrial ecosystem C storage by nitrogen addition: a meta-analysis. Sci Reports 6:19895
Zhang J, Elser JJ (2017) Carbon:nitrogen:phosphorus stoichiometry in fungi: a meta-analysis. Front Microbiol 8:1281
Acknowledgements
This project was funded by the graduate program BangaDyn from the University of Kassel. The authors are grateful to Prof. Dr. Stephan Peth, and Dr. Rajasekaran Murugan from the project FOR2432 for providing texture data and litter material for isotopic analysis, to the colleagues from the University of Agricultural Science Bangalore, especially to Prof. Dr. Subbarayappa, Dr. Hanumanthappa and Dr. Mudalagiriyappa for the management of the experimental fields, to Prof. Dr. Sathish and Prof. Dr. Prakash for their support in use of laboratories, for field assistance by Rajanna and Dhananjaya, and especially for the technical assistance of Gabriele Dormann. We would also like to thank Mick Locke for carefully correcting our English.
Funding
Open Access funding enabled and organized by Projekt DEAL. This project was funded by the graduate program BangaDyn, University of Kassel.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Responsible Editor: Manuel Delgado-Baquerizo.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moran-Rodas, V.E., Chavannavar, S.V., Joergensen, R.G. et al. Microbial response of distinct soil types to land-use intensification at a South-Indian rural-urban interface. Plant Soil 473, 389–405 (2022). https://doi.org/10.1007/s11104-021-05292-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-05292-2