Abstract
Key message
Root-specific expression of a cytokinin-degrading CKX gene in maize roots causes formation of a larger root system leading to higher element content in shoot organs.
Abstract
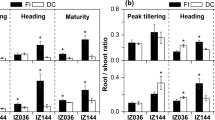
The size and architecture of the root system is functionally relevant for the access to water and soil nutrients. A great number of mostly unknown genes are involved in regulating root architecture complicating targeted breeding of plants with a larger root system. Here, we have explored whether root-specific degradation of the hormone cytokinin, which is a negative regulator of root growth, can be used to genetically engineer maize (Zea mays L.) plants with a larger root system. Root-specific expression of a CYTOKININ OXIDASE/DEHYDROGENASE (CKX) gene of Arabidopsis caused the formation of up to 46% more root dry weight while shoot growth of these transgenic lines was similar as in non-transgenic control plants. The concentration of several elements, in particular of those with low soil mobility (K, P, Mo, Zn), was increased in leaves of transgenic lines. In kernels, the changes in concentration of most elements were less pronounced, but the concentrations of Cu, Mn and Zn were significantly increased in at least one of the three independent lines. Our data illustrate the potential of an increased root system as part of efforts towards achieving biofortification. Taken together, this work has shown that root-specific expression of a CKX gene can be used to engineer the root system of maize and alter shoot element composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Roots fulfil important functions for plants, including anchoring in the soil and providing access to soil nutrients and water. Plant roots are known to be an important factor determining the agricultural performance of crop plants. However, because root traits are difficult to assess and select for, their potential for crop plant improvement has as yet not been fully exploited and numerous details of factors and genes controlling root system traits remain underexplored (Lynch and Brown 2012; White et al. 2013; Rogers and Benfey 2015; Hochholdinger 2016; Koevoets et al. 2016; Bray and Topp 2018).
Root traits are also of vital importance for maize (Zea mays L.), which is one of the most important cereal grains grown worldwide (Shiferaw et al. 2011). A recent study using a machine learning program for trait analysis of 57 commercial maize hybrids concluded that root traits were most important for predicting yield (Tucker et al. 2020). Several root traits have been identified in maize that are relevant for the exploration of soil resources, particularly in resource-poor environments (Lynch and Brown, 2012; York et al. 2013). For example, drought tolerance is associated with an increase in rooting depth and water acquisition from the subsoil (Gao and Lynch 2016). Plants with improved root traits may contribute to relieve a major constrain for the production of maize in develo** countries, which is low soil fertility and high water requirement (Rusinamhodzi et al. 2011; Reynolds et al. 2015; ten Berge et al. 2019).
It is thought that selection for yield has indirectly selected also for root traits contributing to enhance maize yield (Hammer et al. 2009; Bray and Topp 2018). However, the genes regulating these traits are mostly unknown although a large number of QTLs associated with root traits have been identified in maize (Hund et al. 2011; Bray and Topp, 2018). However, so far only eight maize genes were identified that are involved in regulating root growth and development but their individual relevance for plant performance is as yet not clear (Hochholdinger et al. 2018). To understand and fully exploit the potential of roots for crop improvement, it would be important to compare near isogenic lines that differ principally in their root system while shoot growth and development should not be altered. To achieve this, we explore here an experimental approach based on changing the endogenous content of the plant hormone cytokinin by genetic engineering.
The hormone cytokinin is a well-known inhibitor of root elongation and branching (Werner et al. 2001, 2003; Chang et al. 2013, 2015). Cytokinin is degraded by cytokinin oxidases/dehydrogenases (CKX), which are encoded by small gene families in plants including maize (Schmülling et al. 2003). Enhanced degradation of cytokinin by enhanced expression of a CKX gene in roots caused the formation of a larger root system in Arabidopsis thaliana (Werner et al. 2010), barley (Ramireddy et al. 2018a,b), oilseed rape (Nehnevajova et al. 2019), rice (Gao et al. 2014) and chickpea (Khandal et al. 2020). Thus, it was shown repeatedly that a single dominant gene may be used to regulate a complex trait such as root system size. CKX transgenic plants with a larger root system were shown to respond less sensitive than the cognate wild-type plants to drought (Werner et al. 2010; Ramireddy et al. 2018a) underpinning the beneficial effect of a larger root system under water deficit (Comas et al. 2013; Gao and Lynch 2016; Klein et al. 2020). A surprising common feature of these plants had been the higher content of distinct micro- and macro-elements in their shoots. In particular, the concentration on zinc (Zn), a microelement missing in the diet of about 2 billion people, was found to be significantly increased in the seeds of CKX transgenic barley plants grown in the greenhouse and in the field (Ramireddy et al. 2018a,b). Consequently, it has been proposed that root enhancement might contribute to a sustainable solution for nutrient deficiencies (Werner et al. 2010; Ramireddy et al. 2018a; Gao et al. 2014). Supplemental Table S3 lists the primer sequences used in this study. The cDNA samples were used to determine CKX1 transgene expression and cytokinin primary response genes ZmRR1 (gene ID Zm00001d001865) and ZmRR2 (gene ID Zm00001d026594) levels by quantitative real-time PCR according to Cortleven et al. (2014).
Quantification of root system size and biomass
Maize seeds were germinated on soil and three days after germination seedlings were carefully lifted from the soil and cautiously washed to remove bound soil particles. Seedlings of similar size were transferred to a hydroponic system and cultivated for another seven days for root system size analysis, and 15 d for biomass quantification. For the hydroponic system 0.1 × Hoagland solution (1 mM KH2PO4, 0.5 mM KNO3, 0.4 mM Ca(NO3), 0.2 mM MgSO4, 0.1 mM FeNaEDTA, 0.01 mM H3BO3, 2 μM MnSO4, 0.2 μM ZnSO4, 0.2 μM CuSO4, 0.1 μM Na2MoO4 and 0.02 mM NaCI) was used (Krämer et al. 1996). 12 L nutrient solution per box was properly aerated and changed every second day. After harvest, roots and shoots were separated and their fresh weights were determined. Thereafter, samples were dried in an oven at 80 °C for 68 h and the dry weight was recorded. For root system size analysis by WinRHIZO™, roots were carefully lifted from the box and spread out in a root-positioning tray (20 × 30 cm) to minimize root overlap and scanned with a flatbed scanner (EPSON, EU-88, Japan). Greyscale images obtained in tiff format were analysed with WinRHIZO™ (Pro Version 2005a; Regent Instruments Inc., Canada). For quantification of root system size of soil-grown transgenic plants, soil-filled 30 cm diameter pots were used. After five weeks of growth in soil, plants were harvested and separated into shoots and roots. Roots were carefully washed to remove bound soil particles and aggregates. Samples were dried in an oven at 80 °C for 68 h and the dry weight was recorded.
Quantification of leaf and seed element content
Quantification and analysis of leaf and seed elements was performed as described in Ramireddy et al (2018a). Briefly, seeds of three independent transgenic lines and the NTC were germinated on filter paper in vitro. Three-day-old seedlings were transferred to the greenhouse into an unfertilized (type 0) soil supplied by the company Einheitserde (Sinntal-Altengronau, Germany). Composition of unfertilized soil was tested and certified by Institut Koldingen GmbH (Sarstedt, Germany) as described by Drechsler et al. (2015). Plants were grown further for four weeks by supplementing equal amounts of fertilizer solution every second or third day depending on soil moisture. The fertilizer solution was based on the composition of modified Hoagland solution (2 M KNO3, 1 M NH4NO3, 1 M KH2PO4, 2 M Ca(NO3)2 4 H2O,,2 M MgSO4 7 H2O, 100 μM Na-Fe-EDTA, 50 μM H3BO3, 50 μM MnSO4, 18.5 μM ZnSO4, 50 nM CuSO4, 50 nM CoCl2, 0.5 μM NaMoO4 and 2 mM MES). The solution was adjusted to pH 5.7 with 1 M KOH. Total leaves from four-week-old plants were dried for 72 h at 80 °C and grounded carefully. Then equal amounts of powder (1 g) were weighed into polytetrafluoroethylene tubes and digested with a HNO3 + H2O2 mixture in a pressurized microwave digestion system (MARS from CEM GmbH; Kamp-Lintfort, Germany). The concentrations of macro- and microelements were analyzed by inductively-coupled plasma optical emission spectrometry (ICP-OES, iCAP 6500 dual OES spectrometer; Thermo Fischer Scientific) with certified standard reference samples as control. The element content from seed samples was determined in a similar way as outlined above.
References
Abdel-Ghani AH, Kumar B, Reyes-Matamoros J, Gonzalez-Portilla PJ, Jansen C, San Martin JP, Lee M, Lübberstedt T (2013) Genotypic variation and relationships between seedling and adult plant traits in maize (Zea mays L.) inbred lines grown under contrasting nitrogen levels. Euphytica 189:123–133. https://doi.org/10.1007/s10681-012-0759-0
Adams E, Shin R (2014) Transport, signaling, and homeostasis of potassium and sodium in plants. J Integr Plant Biol 56:231–249. https://doi.org/10.1111/jipb.12159
Andersen TG, Naseer S, Ursache R, Wybouw B, Smet W, De Rybel B, Vermeer JEM, Geldner N (2018) Diffusible repression of cytokinin signalling produces endodermal symmetry and passage cells. Nature 22:529–533. https://doi.org/10.1038/nature25976
Argyros RD, Mathews DE, Chiang YH, Palmer CM, Thibault DM, Etheridge N, Argyros DA, Mason MG, Kieber JJ, Schaller GE (2008) Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development. Plant Cell 20:2102–2116. https://doi.org/10.1105/tpc.108.059584
Asakura Y, Hagino T, Ohta Y, Aoki K, Yonekura-Sakakibara K, Deji A, Yamaya T, Sugiyama T, Sakakibara H (2003) Molecular characterization of His-Asp phosphorelay signaling factors in maize leaves: implications of the signal divergence by cytokinin-inducible response regulators in the cytosol and the nuclei. Plant Mol Biol 52:331–341. https://doi.org/10.1023/a:1023971315108
Bouis HE, Welch RM (2010) Biofortification – a sustainable agricultural strategy for reducing micronutrient malnutrition in the global South. Crop Sci 50:S20–S32. https://doi.org/10.2135/cropsci2009.09.0531
Bray AL, Topp CN (2018) The quantitative genetic control of root architecture in maize. Plant Cell Physiol 59:1919–1930. https://doi.org/10.1093/pcp/pcy141
Chang L, Ramireddy E, Schmülling T (2013) Lateral root formation and growth of Arabidopsis is redundantly regulated by cytokinin metabolism and signalling genes. J Exp Bot 64:5021–5032. https://doi.org/10.1093/jxb/ert291
Chang L, Ramireddy E, Schmülling T (2015) Cytokinin as a positional cue regulating lateral root spacing in Arabidopsis. J Exp Bot 66:4759–4768. https://doi.org/10.1093/jxb/erv252
Comas LH, Becker SR, Cruz VMV, Byrne PF, Dierig DA (2013) Root traits contributing to plant productivity under drought. Front Plant Sci 4:442. https://doi.org/10.3389/fpls.2013.00442
Cortleven A, Nitschke S, Klaumünzer M, Abdelgawad H, Asard H, Grimm B, Riefler M, Schmülling T (2014) A novel protective function for cytokinin in the light stress response is mediated by the Arabidopsis histidine kinase2 and Arabidopsis histidine kinase3 receptors. Plant Physiol 164:1470–1483. https://doi.org/10.1104/pp.113.224667
Coussens G, Aesaert G, Verelst W, Demeulenaere M, De Buck S, Njuguna E, Inzé D, Van Lijsebettens M (2012) Brachypodium distachyon promoters as efficient building blocks for transgenic research in maize. J Exp Bot 63:4263–4273. https://doi.org/10.1093/jxb/ers113
Franco-Zorrilla JM, Martín AC, Leyva A, Paz-Ares J (2005) Interaction between phosphate-starvation, sugar, and cytokinin signaling in Arabidopsis and the roles of cytokinin receptors CRE1/AHK4 and AHK3. Plant Physiol 138:847–857. https://doi.org/10.1104/pp.105.060517
Gao Y, Lynch JP (2016) Reduced crown root number improves water acquisition under water deficit stress in maize (Zea mays L.). J Exp Bot 67:4545–4587. https://doi.org/10.1093/jxb/erw243
Gao S, Fang J, Xu F, Wang W, Sun X, Chu J, Cai B, Feng Y, Chu C (2014) CYTOKININ OXIDASE/ DEHYDROGENASE4 integrates cytokinin and auxin signaling to control rice crown root formation. Plant Physiol 165:1035–1046. https://doi.org/10.1104/pp.114.238584
Gao S, **ao Y, Xu F, Gao X, Cao S, Zhang F, Wang G, Sanders D, Chu C (2019) Cytokinin-dependent regulatory module underlies the maintenance of zinc nutrition in rice. New Phytol 224:202–215. https://doi.org/10.1111/nph.15962
García-Mauriño S, Monreal J, Alvarez R, Vidal J, Echevarría C (2003) Characterization of salt stress-enhanced phosphoenolpyruvate carboxylase kinase activity in leaves of Sorghum vulgare: Independence from osmotic stress, involvement of ion toxicity and significance of dark phosphorylation. Planta 216:648–655. https://doi.org/10.1007/s00425-002-0893-3
Hammer GL, Dong Z, McLean G, Doherty A, Messina C, Schussler J, Zinselmeier C, Paszkiewicz S, Cooper M (2009) Can changes in canopy and/or root system architecture explain historical maize yield trends in the U.S. Corn Belt? Crop Sci 49:299–312. https://doi.org/10.2135/cropsci2008.03.0152
Hochholdinger F (2016) Untap** root system architecture for crop improvement. J Exp Bot 67:4431–4433. https://doi.org/10.1093/jxb/erw262
Hochholdinger F, Tuberosa R (2009) Genetic and genomic dissection of maize root development and architecture. Curr Opin Plant Biol 12:172–177. https://doi.org/10.1016/j.pbi.2008.12.002
Hochholdinger F, Yu P, Marcon C (2018) Genetic control of root system development in maize. Trends Plant Sci 23:79–88. https://doi.org/10.1016/j.tplants.2017.10.004
Hund A, Reimer R, Messmer R (2011) A consensus map of QTLs controlling the root length of maize. Plant Soil 344:143–158. https://doi.org/10.1007/s11104-011-0735-9
Jeong JS, Kim YS, Baek KH, Jung H, Ha SH, Choi YD, Kim M, Reuzeau C, Kim JK (2010) Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol 153:185–197. https://doi.org/10.1104/pp.110.154773
Kaiser BN, Gridley KL, Brady JN, Philips T, Tyerman SD (2005) The role of molybdenum in agricultural plant production. Ann Bot 96:745–754. https://doi.org/10.1093/aob/mci226
Kell DB (2011) Breeding crop plants with deep roots: Their role in sustainable carbon, nutrient and water sequestration. Ann Bot 108:407–418. https://doi.org/10.1093/aob/mcr175
Khandal H, Gupta SK, Dwivedi V, Mandal D, Sharma NK, Vishwakarma NK, Pal L, Choudhary M, Francis A, Malakar P, Singh NP, Sharma K, Sinharoy S, Singh NP, Sharma R, Chattopadhyay D (2020) Root-specific expression of chickpea cytokinin oxidase/dehydrogenase 6 leads to enhanced root growth, drought tolerance and yield without compromising nodulation. Plant Biotechnol J. https://doi.org/10.1111/pbi.13378
Kihara J, Bolo P, Kinyua M, Rurinda J, Piikki K (2020) Micronutrient deficiencies in African soils and the human nutritional nexus: opportunities with staple crops. Environ Geochem Health 42(9):3015–3033. https://doi.org/10.1007/s10653-019-00499-w
Klein SP, Schneider HM, Perkins AC, Brown KM, Lynch JP (2020) Multiple integrated root phenotypes are associated with improved drought tolerance. Plant Physiol 183:1011–1025. https://doi.org/10.1104/pp.20.00211
Koevoets IT, Venema JH, Elzenga JTM, Testerink C (2016) Roots withstanding their environment: Exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front Plant Sci 7:1335. https://doi.org/10.3389/fpls.2016.01335
Kovács B, Puskás-Preszner A, Huzsvai L, Lévai L, Bódi E (2015) Effect of molybdenum treatment on molybdenum concentration and nitrate reduction in maize seedlings. Plant Physiol Biochem 96:38–44. https://doi.org/10.1016/j.plaphy.2015.07.013
Krämer U, Cotter-Howells JD, Charnock JM, Baker AJM, Smith JAC (1996) Free histidine as a metal chelator in plants that accumulate nickel. Nature 379:635–638. https://doi.org/10.1038/379635a0
Kumar B, Abdel-Ghani AH, Reyes-Matamoros J, Hochholdinger F, Lübberstedt T (2012) Genotypic variation for root architecture traits in seedlings of maize (Zea mays L.) inbred lines. Plant Breed 131:465–478. https://doi.org/10.1111/j.1439-0523.2012.01980.x
Lancashire P, Bleiholder H, Boom TPL, Strauss R, Weber E, Witzenberger A (1991) A uniform decimal code for growth stages of crops and weeds. Ann Appl Biol 119:561–601. https://doi.org/10.1111/j.1744-7348.1991.tb04895.x
Li R, Zeng Y, Xu J, Wang Q, Wu F, Cao M, Lan H, Liu Y, Lu Y (2015) Genetic variation for maize root architecture in response to drought stress at the seedling stage. Breed Sci 65:298–307. https://doi.org/10.1270/jsbbs.65.298
Li Y, Liu X, Chen R, Tian J, Fan Y, Zhou X (2019) Genome-scale mining of root-preferential genes from maize and characterization of their promoter activity. BMC Plant Biol 19:584. https://doi.org/10.1186/s12870-019-2198-8
Lin F, Jiang L, Liu Y, Lv Y, Dai H, Zhao H (2014) Genome-wide identification of housekee** genes in maize. Plant Mol Biol 86:543–554. https://doi.org/10.1007/s11103-014-0246-1
Lynch JP (2011) Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol 156:1041–1049. https://doi.org/10.1104/pp.111.175414
Lynch JP (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot 112:347–357. https://doi.org/10.1093/aob/mcs293
Lynch JP, Brown KM (2012) New roots for agriculture: exploiting the root phenome. Philos Trans R Soc Lond B Biol Sci 367:1598–1604. https://doi.org/10.1098/rstb.2011.0243
Maqbool MA, Beshir AR (2019) Zinc biofortification of maize (Zea mays L.): Status and challenges. Plant Breed 138:1–28. https://doi.org/10.1111/pbr.12658
McCay-Buis TS, Huber DM, Graham RD, Phillips JD, Miskin KE (1995) Manganese seed content and take-all of cereals. J Plant Nutr 18:1711–1721. https://doi.org/10.1080/01904169509365016
Menguer PK, Vincent T, Miller AJ, Brown JKM, Vincze E, Borg S, Holm PB, Sanders D, Podar D (2018) Improving zinc accumulation in cereal endosperm using HvMTP1, a transition metal transporter. Plant Biotechnol J 16:63–71. https://doi.org/10.1111/pbi.12749
Moussavi-Nik M, Rengel Z, Hollamby GJ, Ascher JS (1997) Seed manganese (Mn) content is more important than Mn fertilisation for wheat growth under Mn deficient conditions. In: Ando T, Fujita K, Mae T, Matsumoto H, Mori S, Sekiya J (eds) Plant nutrition for sustainable food production and environment. Developments in plant and Soil sciences, vol 78. Springer, Dordrecht . https://doi.org/10.1007/978-94-009-0047-9_74
Nam YJ, Tran LS, Kojima M, Sakakibara H, Nishiyama R, Shin R (2012) Regulatory roles of cytokinins and cytokinin signaling in response to potassium deficiency in Arabidopsis. PLoS ONE 7:e47797. https://doi.org/10.1371/journal.pone.0047797
Nehnevajova E, Ramireddy E, Stolz A, Gerdemann-Knörck M, Novák O, Strnad M, Schmülling T (2019) Root enhancement in cytokinin-deficient oilseed rape causes leaf mineral enrichment, increases the chlorophyll concentration under nutrient limitation and enhances the phytoremediation capacity. BMC Plant Biol 19:83. https://doi.org/10.1186/s12870-019-1657-6
Niemann MCE, Weber H, Hluska T, Leonte G, Anderson SM, Novák O, Senes A, Werner T (2018) The cytokinin oxidase/dehydrogenase CKX1 is a membrane-bound protein requiring homooligomerization in the endoplasmic reticulum for its cellular activity. Plant Physiol 176:2024–2039. https://doi.org/10.1104/pp.17.00925
Prasad AS (2013) Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr 4:176–190. https://doi.org/10.3945/an.112.003210
Ramireddy E, Hosseini SA, Eggert K, Gillandt S, Gnad H, von Wiren N, Schmülling T (2018a) Root engineering in barley: increasing cytokinin degradation produces a larger root system, mineral enrichment in the shoot and improved drought tolerance. Plant Physiol 177:1078–1095. https://doi.org/10.1104/pp.18.00199
Ramireddy E, Galuszka P, Schmülling T (2018b) Zn-fortified cereal grains in field-grown barley by enhanced root cytokinin breakdown. Plant Signal Behav 13:e1530023. https://doi.org/10.1080/15592324.2018.1530023
Reynolds TW, Waddington SR, Anderson CL, Chew A, True Z, Cullen A (2015) Environmental impacts and constraints associated with the production of major food crops in Sub-Saharan Africa and South Asia. Food Sec 7:795–822. https://doi.org/10.1007/s12571-015-0478-1
Rogers ED, Benfey PN (2015) Regulation of plant root system architecture: implications for crop advancement. Curr Opin Biotechnol 32:93–98. https://doi.org/10.1016/j.copbio.2014.11.015
Rosa AT, Diaz DAR, Hansel FD, Sebastian JSV, Adee EA (2019) Genotypic variation on root growth and nutrient uptake in corn and soybean. Agrosyst Geosci Environm 2:1–12. https://doi.org/10.2134/age2019.03.0018
Rusinamhodzi L, Corbeels M, van Wijk MT, Rufino MC, Nyamangara J, Giller KE (2011) A meta-analysis of long-term effects of conservation agriculture on maize grain yield under rain-fed conditions. Agron Sustain Dev 31:657. https://doi.org/10.1007/s13593-011-0040-2
Sakakibara H, Hayakawa A, Deji A, Gawronski SW, Sugiyama T (1999) His-Asp phosphotransfer possibly involved in the nitrogen signal transduction mediated by cytokinin in maize: molecular cloning of cDNAs for two-component regulatory factors and demonstration of phosphotransfer activity in vitro. Plant Mol Biol 41:563–573. https://doi.org/10.1023/a:1006391304881
Sanguineti MC, Giuliani MM, Govi G, Tuberosa R, Landi P (1998) Root and shoot traits of maize inbred lines grown in the field and in hydroponic culture and their relationships with root lodging. Maydica 43:211–216
Schmidt SB, Husted S (2019) The biochemical properties of manganese in plants. Plants 8:381. https://doi.org/10.3390/plants8100381
Schmülling T, Werner T, Riefler M, Krupková E, Bartrina y Manns I, (2003) Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species. Journal Plant Res 116:241–252. https://doi.org/10.1007/s10265-003-0096-4
Sekhon RS, Lin H, Childs KL, Hansey CN, Buell CR, de Leon N, Kaeppler SM (2011) Genome-wide atlas of transcription during maize development. Plant J 66:553–563. https://doi.org/10.1111/j.1365-313X.2011.04527.x
Shiferaw B, Prasanna BM, Hellin J, Bänziger M (2011) Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Sec 3:307. https://doi.org/10.1007/s12571-011-0140-5
Singh DK, Bharti S (1985) Seed manganese content and its relationship with the growth characteristics of wheat cultivars. New Phytol 101:387–391. https://doi.org/10.1111/j.1469-8137.1985.tb02845.x
ten Berge HFM, Hijbeek R, van Loon MP, Rurinda J, Tesfaye K, Zingore S, Craufurd P, van Heerwaarden J, Brentrup F, Schröder JJ, Boogaard HL (2019) Maize crop nutrient input requirements for food security in sub-Saharan Africa. Glob Food Sec 23:9–21. https://doi.org/10.1016/j.gfs.2019.02.001
Tucker SL, Dohleman FG, Grapov D, Flagel L, Yang S, Wegener KM, Kosola K, Swarup S, Rapp RA, Bedair M, Halls SC, Glenn KC, Hall MA, Allen E, Rice EA (2020) Evaluating maize phenotypic variance, heritability, and yield relationships at multiple biological scales across agronomically relevant environments. Plant Cell Environ 43:880–902. https://doi.org/10.1111/pce.13681
Vercruyssen L, Gonzalez N, Werner T, Schmülling T, Inzé D (2011) Combining enhanced root and shoot growth reveals cross talk between pathways that control plant organ size in Arabidopsis. Plant Physiol 155:1339–1352. https://doi.org/10.1104/pp.110.167049
Werner T, Motyka V, Strnad M, Schmülling T (2001) Regulation of plant growth by cytokinin. Proc Natl Acad Sci USA 98:10487–10492. https://doi.org/10.1073/pnas.171304098
Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T (2003) Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15:2532–2550. https://doi.org/10.1105/tpc.014928
Werner T, Nehnevajova E, Köllmer I, Novák O, Strnad M, Krämer U, Schmülling T (2010) Root-Specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 22:3905–3920. https://doi.org/10.1105/tpc.109.072694
White PJ, Broadley MR (2009) Biofortification of crops with seven mineral elements often lacking in human diets - iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol 182:49–84. https://doi.org/10.1111/j.1469-8137.2008.02738.x
White PJ, George TS, Gregory PJ, Bengough AG, Hallett PD, McKenzie BM (2013) Matching roots to their environment. Ann Bot 112:207–222. https://doi.org/10.1093/aob/mct123
Worthen JM, Yamburenko MV, Lim J, Nimchuk ZL, Kieber JJ, Schaller GE (2019) Type-B response regulators of rice play key roles in growth, development and cytokinin signaling. Development. https://doi.org/10.1242/dev.174870
Xu Y, Buchholz WG, DeRose RT, Hall TC (1995) Characterization of a rice gene family encoding root-specific proteins. Plant Mol Biol 27:237–248. https://doi.org/10.1007/BF00020180
York LM, Nord EA, Lynch JP (2013) Integration of root phenes for soil resource acquisition. Front Plant Sci 4:355. https://doi.org/10.3389/fpls.2013.00355
Zhang H, Liu J, ** T, Huang Y, Chen J, Zhu L, Zhao Y, Guo J (2017) Identification of quantitative trait locus and prediction of candidate genes for grain mineral concentration in maize across multiple environments. Euphytica 213:90. https://doi.org/10.1007/s10681-017-1875-7
Zhao QY, Xu SJ, Zhang WS, Zhang Z, Yao Z, Chen XP, Zou CQ (2020) Identifying key drivers for geospatial variation of grain micronutrient concentrations in major maize production regions of China. Environ Pollut 266:115114. https://doi.org/10.1016/j.envpol.2020.115114
Acknowledgements
We thank the late Sabine Gillandt for technical help with qPCR and Kirin Demuynck for assistance with the leaf growth assays, Griet Coussens and Stijn Aesaert for the maize transformation.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
ER and TS have conceived and coordinated the project; ER, HN, DI and TS designed experiments; MVL produced transgenic maize plants and HN measured leaf growth; ER carried out molecular and phenotypic analysis of maize plants; JEL analyzed gene expression; ER and TS wrote the article with contributions of all authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramireddy, E., Nelissen, H., Leuendorf, J.E. et al. Root engineering in maize by increasing cytokinin degradation causes enhanced root growth and leaf mineral enrichment. Plant Mol Biol 106, 555–567 (2021). https://doi.org/10.1007/s11103-021-01173-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-021-01173-5