Abstract
Purpose
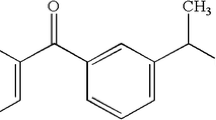
Iontophoresis is a noninvasive method that enhances drug delivery using an electric field. This method can improve drug delivery to the tissues in the oral cavity. The effects of iontophoresis on gingival drug delivery have not been investigated. The objectives of this study were to (a) determine the flux enhancement of model permeants across porcine and human gingiva during iontophoresis, (b) examine the transport mechanisms of gingival iontophoresis, and (c) evaluate the potential of iontophoretically enhanced delivery for three model drugs lidocaine, ketorolac, and chlorhexidine.
Methods
Passive and iontophoretic fluxes were determined with porcine and human gingiva using a modified Franz diffusion cell and model drugs and permeants. To investigate the transport mechanisms of iontophoresis, the enhancement from the direct-field effect was determined by positively and negatively charged model permeants. The electroosmosis enhancement effect was determined with neutral permeants of different molecular weight. The alteration of the gingival barrier due to electropermeabilization was evaluated using electrical resistance measurements.
Results
Significant flux enhancement was observed during gingival iontophoresis. The direct-field effect was the major mechanism governing the iontophoretic transport of the charged permeants. Electroosmosis was from anode to cathode. The effective pore radius of the iontophoretic transport pathways in the porcine gingiva was ~0.68 nm. Irreversible electropermeabilization was observed after 2 and 4 h of iontophoresis under the conditions studied.
Conclusion
Iontophoresis could enhance drug delivery and reduce transport lag time, showing promise for gingival drug delivery.



Similar content being viewed by others
References
Majid OW, Ahmed AM. The anesthetic efficacy of articaine and lidocaine in equivalent doses as buccal and non-palatal infiltration for maxillary molar extraction: a randomized, double-blinded, placebo-controlled clinical trial. J Oral Maxillofac Surg. 2018;76(4):737–43.
Gholami M, Banihashemrad A, Mohammadzadeh A, Ahrari F. The efficacy of 4% articaine versus 2% lidocaine in inducing palatal anesthesia for tooth extraction in different maxillary regions. J Oral Maxillofac Surg. 2021;79(8):1643–9.
Wiswall AT, Bowles WR, Lunos S, McClanahan SB, Harris S. Palatal anesthesia: comparison of four techniques for decreasing injection discomfort. Northwest Dent. 2014;93(4):25–9.
Lim SY, Dafydd M, Ong J, Ord-McDermott LA, Board-Davies E, Sands K, Williams D, Sloan AJ, Heard CM. Mucoadhesive thin films for the simultaneous delivery of microbicide and anti-inflammatory drugs in the treatment of periodontal diseases. Int J Pharm. 2020;573:118860.
Cetin EO, Buduneli N, Atlihan E, Kirilmaz L. In vitro studies on controlled-release cellulose acetate films for local delivery of chlorhexidine, indomethacin, and meloxicam. J Clin Periodontol. 2004;31(12):1117–21.
Xu J, Strandman S, Zhu JX, Barralet J, Cerruti M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials. 2015;37:395–404.
Antimisiaris SG, Marazioti A, Kannavou M, Natsaridis E, Gkartziou F, Kogkos G, Mourtas S. Overcoming barriers by local drug delivery with liposomes. Adv Drug Deliv Rev. 2021;174:53–86.
Wanasathop A, Li SK. Iontophoretic drug delivery in the oral cavity. Pharmaceutics. 2018;10(3):121.
Kalia YN, Naik A, Garrison J, Guy RH. Iontophoretic drug delivery. Adv Drug Deliv Rev. 2004;56(5):619–58.
Cubayachi C, Couto RO, de Gaitani CM, Pedrazzi V, Freitas O, Lopez RF. Needle-free buccal anesthesia using iontophoresis and amino amide salts combined in a mucoadhesive formulation. Colloids Surf B Biointerfaces. 2015;136:1193–201.
Campisi G, Giannola LI, Florena AM, De Caro V, Schumacher A, Gottsche T, Paderni C, Wolff A. Bioavailability in vivo of naltrexone following transbuccal administration by an electronically-controlled intraoral device: a trial on pigs. J Control Release. 2010;145(3):214–20.
Telo I, Tratta E, Guasconi B, Nicoli S, Pescina S, Govoni P, Santi P, Padula C. In-vitro characterization of buccal iontophoresis: the case of sumatriptan succinate. Int J Pharm. 2016;506(1-2):420–8.
Li SK, Hao J. Drug delivery using electrophoresis. In: Swarbrick J, editor. Encyclopedia of Pharmaceutical Science and Technology. 4th ed. New York: Taylor & Francis; 2013. Ch. 354.
Paderni C, Campisi G, Schumacher A, Gottsche T, Giannola LI, De Caro V, Wolff A. Controlled delivery of naltrexone by an intraoral device: in vivo study on human subjects. Int J Pharm. 2013;452(1-2):128–34.
Ren W, Baig A, Li SK. Passive and iontophoretic transport of fluorides across enamel in vitro. J Pharm Sci. 2014;103(6):1692–700.
Li SK, Hao J, Liddell MR. Electrotransport across membranes in biological media: Electrokinetic theories and applications in drug delivery. In: Becker SM, Kuznetsov AV, editors. Transport in Biological Media. 1st ed. New York: Elsevier; 2013. Ch. 11.
Zhu H, Peck KD, Li SK, Ghanem AH, Higuchi WI. Quantification of pore induction in human epidermal membrane during iontophoresis: the importance of background electrolyte selection. J Pharm Sci. 2001;90(7):932–42.
Li SK, Ghanem AH, Peck KD, Higuchi WI. Characterization of the transport pathways induced during low to moderate voltage iontophoresis in human epidermal membrane. J Pharm Sci. 1998;87(1):40–8.
Deen WM. Hindered transport of large molecules in liquid-filled pores. AIChE J. 1987;33(9):1409–25.
Hao J, Li SK. Transungual iontophoretic transport of polar neutral and positively charged model permeants: effects of electrophoresis and electroosmosis. J Pharm Sci. 2008;97(2):893–905.
Peck KD, Srinivasan V, Li SK, Higuchi WI, Ghanem AH. Quantitative description of the effect of molecular size upon electroosmotic flux enhancement during iontophoresis for a synthetic membrane and human epidermal membrane. J Pharm Sci. 1996;85(7):781–8.
Wanasathop A, Zhong C, Nimmansophon P, Murawsky M, Li SK. Characterization of porcine gingiva for drug absorption. J Pharm Sci. 2023;112(4):1032–40.
Li SK, Ghanem AH, Peck KD, Higuchi WI. Pore induction in human epidermal membrane during low to moderate voltage iontophoresis: A study using AC iontophoresis. J Pharm Sci. 1999;88(4):419–27.
Ren W, Baig A, White DJ, Li SK. Characterization of cornified oral mucosa for iontophoretically enhanced delivery of chlorhexidine. Eur J Pharm Biopharm. 2016;99:35–44.
Inada H, Ghanem AH, Higuchi WI. Studies on the effects of applied voltage and duration on human epidermal membrane alteration/recovery and the resultant effects upon iontophoresis. Pharm Res. 1994;11(5):687–97.
Wanasathop A, Choi HA, Nimmansophon P, Murawsky M, Krishnan DG, Li SK. Permeability of fresh and frozen porcine and human gingiva and the effect of storage duration. Pharmaceutics. 2023;15(5):1492.
Peck KD, Ghanem AH, Higuchi WI. Hindered diffusion of polar molecules through and effective pore radii estimates of intact and ethanol treated human epidermal membrane. Pharm Res. 1994;11(9):1306–14.
Altamirano M, Martinoya C. The permeability of the gastric mucosa of dog. J Physiol. 1966;184(4):771–90.
Xu Q, Ibrahim SA, Higuchi WI, Li SK. Ion-exchange membrane assisted transdermal iontophoretic delivery of salicylate and acyclovir. Int J Pharm. 2009;369(1-2):105–13.
Mudry B, Carrupt PA, Guy RH, Delgado-Charro MB. Quantitative structure-permeation relationship for iontophoretic transport across the skin. J Control Release. 2007;122(2):165–72.
Li SK, Zhang Y, Zhu H, Higuchi WI, White HS. Influence of asymmetric donor-receiver ion concentration upon transscleral iontophoretic transport. J Pharm Sci. 2005;94(4):847–60.
Zeng P, Rao A, Wiedmann TS, Bowles W. Solubility properties of chlorhexidine salts. Drug Dev Ind Pharm. 2009;35(2):172–6.
Hu L, Silva SM, Damaj BB, Martin R, Michniak-Kohn BB. Transdermal and transbuccal drug delivery systems: enhancement using iontophoretic and chemical approaches. Int J Pharm. 2011;421(1):53–62.
Scholz A, Kuboyama N, Hempelmann G, Vogel W. Complex blockade of TTX-resistant Na+ currents by lidocaine and bupivacaine reduce firing frequency in DRG neurons. J Neurophysiol. 1998;79(4):1746–54.
Sheets PL, Jarecki BW, Cummins TR. Lidocaine reduces the transition to slow inactivation in Nav 1.7 voltage-gated sodium channels. Br J Pharmacol. 2011;164(2b):719–30.
Bennett PB, Valenzuela C, Chen L-Q, Kallen RG. On the molecular nature of the lidocaine receptor of cardiac Na+ channels: modification of block by alterations in the α-subunit III-IV interdomain. Circ Res. 1995;77(3):584–92.
Carabaza A, Cabre F, Rotllan E, Gomez M, Gutierrez M, Garcia ML, Mauleon D. Stereoselective inhibition of inducible cyclooxygenase by chiral nonsteroidal antiinflammatory drugs. J Clin Pharmacol. 1996;36(6):505–12.
Waterbury LD, Silliman D, Jolas T. Comparison of cyclooxygenase inhibitory activity and ocular anti-inflammatory effects of ketorolac tromethamine and bromfenac sodium. Curr Med Res Opin. 2006;22(6):1133–40.
Hudson LG, Cook LS, Grimes MM, Muller CY, Adams SF, Wandinger-Ness A. Dual actions of ketorolac in metastatic ovarian cancer. Cancers (Basel). 2019;11(8):1049.
Guo Y, Kenney SR, Cook L, Adams SF, Rutledge T, Romero E, Oprea TI, Sklar LA, Bedrick E, Wiggins CL, Kang H, Lomo L, Muller CY, Wandinger-Ness A, Hudson LG. A novel pharmacologic activity of ketorolac for therapeutic benefit in ovarian cancer patients. Clin Cancer Res. 2015;21(22):5064–72.
Dagash H, McCaffrey S, Mellor K, Roycroft A, Helbling I. Tap water iontophoresis in the treatment of pediatric hyperhidrosis. J Pediatr Surg. 2017;52(2):309–12.
Siah TW, Hampton PJ. The effectiveness of tap water iontophoresis for palmoplantar hyperhidrosis using a Monday, Wednesday, and Friday treatment regime. Dermatol Online J. 2013;19(3):14.
Banga AK. Electrically assisted transdermal and topical drug delivery. CRC Press; 1998.
Parkinson TM, Ferguson E, Febbraro S, Bakhtyari A, King M, Mundasad M. Tolerance of ocular iontophoresis in healthy volunteers. J Ocul Pharmacol Ther. 2003;19(2):145–51.
Patane MA, Cohen A, From S, Torkildsen G, Welch D, Ousler GW 3rd. Ocular iontophoresis of EGP-437 (dexamethasone phosphate) in dry eye patients: results of a randomized clinical trial. Clin Ophthalmol. 2011;5:633–43.
Comeau M, Brummett R. Anesthesia of the human tympanic membrane by iontophoresis of a local anesthetic. Laryngoscope. 1978;88(2 Pt 1):277–85.
Chinnapareddy S. Novel biomedical approaches in health monitoring and oral care. PhD Thesis. Potsdam, NY: Clarkson University; 2012.
Acknowledgements
The porcine tissues were donated by UC Laboratory Animal Medical Services (LAMS). The authors thank Dr. Gerald B. Kasting and Dr. Jerome McMahon for their helpful discussion.
Funding
This research was supported in part by National Institute of Dental & Craniofacial Research (NIDCR) of the National Institutes of Health (NIH), Award Number R15 DE028701. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
For hindered transport across a porous membrane assuming cylindrical pores, the H factor describes transport hindrance for diffusion and the W factor describes transport hindrance for convection of parabolic flow [19].
where \(\lambda =\frac{r_s}{R_p}\), \({K}_t=\frac{9}{4}{\pi}^2\sqrt{2}{\left(1-\lambda \right)}^{-\frac{5}{2}}\left[1+{\sum}_{n=1}^2{a}_n{\left(1-\lambda \right)}^n\right]+{\sum}_{n=0}^4{a}_{n+3}{\lambda}^{\eta }\), and a1 = -1.217, a2 = 1.534, a3 = -22.51, a4 = -5.612, a5 = -0.3363, a6 = -1.216, and a7 = 1.647. rs is the solute hydrodynamic radius and Rp is the effective pore radius of the membrane.
where\({K}_s=\frac{9}{4}{\pi}^2\sqrt{2}{\left(1-\lambda \right)}^{-\frac{5}{2}}\left[1+{\sum}_{n=1}^2{b}_n{\left(1-\lambda \right)}^n\right]+{\sum}_{n=0}^4{b}_{n+3}{\lambda}^{\eta }\) and b1 = 0.11667, b2 = -0.04489, b3 = 4.018, b4 = -3.979, b5 = -1.9215, b6 = 4.392, and b7 = 5.006. Eqs. 10 and 11 are the hindered transport equations used in Eqs. 4, 7, and 15.
To model the changes in porosity due to electropermeabilization, membrane porosity ratio (εratio) is related to the membrane porosity for permeant i during iontophoresis (εtotal,i) which is the total porosity from the newly created pores (εiont,i) and the existing pores before iontophoresis (εpassive,i).
Membrane electrical resistance can be used to evaluate membrane porosity. The ratio of the membrane electrical resistance during iontophoresis to passive transport is related to the ratio of the fluxes of the ions in the background electrolyte (i.e., fluxes of NaCl) during iontophoresis to passive transport.
The membrane porosity ratio (εratio) can be expressed as:
To correct for the difference in transport hindrance on permeant i and NaCl,
Eq. 16 describes the effect of electropermeabilization on iontophoretic delivery using the electrical resistance data (Eq. 4).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wanasathop, A., Nimmansophon, P., Murawsky, M. et al. Iontophoresis on Porcine and Human Gingiva. Pharm Res 40, 1977–1987 (2023). https://doi.org/10.1007/s11095-023-03535-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03535-8