Abstract
Epilepsy is a neurological disease characterized by repeated seizures. Despite of that the brain-derived neurotrophic factor (BDNF) is implicated in the pathogenesis of epileptogenesis and epilepsy, BDNF may have a neuroprotective effect against epilepsy. Thus, the goal of the present review was to highlight the protective and detrimental roles of BDNF in epilepsy. In this review, we also try to find the relation of BDNF with other signaling pathways and cellular processes including autophagy, mTOR pathway, progranulin (PGN), and α-Synuclein (α-Syn) which negatively and positively regulate BDNF/tyrosine kinase receptor B (TrkB) signaling pathway. Therefore, the assessment of BDNF levels in epilepsy should be related to other neuronal signaling pathways and types of epilepsy in both preclinical and clinical studies. In conclusion, there is a strong controversy concerning the potential role of BDNF in epilepsy. Therefore, preclinical, molecular, and clinical studies are warranted in this regard.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is a protracted neurological disease characterized by repetitive seizures which is hypersynchronous neuronal discharge from explicit brain regions [1]. Epilepsy affects about 1% of the general population worldwide [2]. It has been reported that 80% of epilepsy is more common in develo** countries. Epilepsy is more common in the elderly, as 5–10% of old people have seizures at the age of 80 years which augment the chance of a second seizure by more than 40% [3]. One attack of seizure is not epilepsy, but investigations are sensible to detect the underlying causes of the seizure [4]. A history of more than two seizures is diagnostic for epilepsy [1].
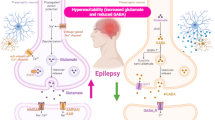
The fundamental mechanism of epileptic seizure is due to the development and progression of the epileptogenesis process, and the imbalance between inhibitory and excitatory neurotransmitters and pathways [5]. Reducing inhibitory gamma-aminobutyric acid (GABA) and/or augmentation of excitatory glutamate neurotransmission prompt epileptogenesis [5]. Epileptogenesis is the genesis of a chronic hyperexcitable epileptic state. Epileptogenesis is one of the most dramatic examples of neuronal plasticity, as can be seen by the development of a normal, non-hyperexcitable nervous system into one capable of producing seizures. Epileptogenesis also has many mechanistic similarities with long-term potentiation (LTP) [5]. The causes of epileptogenesis are due to the mutation of voltage-gated Na+, Ca2+, and K+ channels which augment neuronal hyper-excitability and decrease seizure threshold [6]. Mutation of the Na+ channel gene SCN8A is accompanied by the progress of epileptogenesis (Fig. 1) [6].
According to etiopathology, two types of epilepsy are known, primary, or idiopathic epilepsy without identified causes. However, secondary epilepsy is caused by diverse causes such as head trauma, tumors, brain infection and neurodegenerative disorders [7]. In this state, different studies indicated that dysregulation of brain-derived neurotrophic factor (BDNF) is concerned with the pathogenesis of epilepsy [55]. In chronic epilepsy mainly TLE, BDNF is up-regulated leading to disruption the inhibitory and excitatory neuronal signaling pathway causing seizure [54, 55]. BDNF increases excitatory glutamate and reduces inhibitory GABA with the induction of seizure [54]. KCC2 expression is reduced in patients with TLE due to dysregulation of pro-BDNF/BDNF ratio [76]. In addition, epileptic seizure and SE induces dysregulation of pro-BDNF/BDNF axis with subsequent downregulation regulation of KCC2 expression leading to recurrent seizure [62] (Fig. 2).
Of note, BDNF gene expression in the temporal cortex and hippocampus is augmented in patients with TLE [68]. However, it is still unclear whether the stimulatory effect of BDNF is through presynaptic activation releases of glutamate or phosphorylation of postsynaptic GABA receptors [43]. These clinical findings indicated that an exaggerated BDNF level is associated with epilepsy. However, there is no clinical study that measures BDNF levels in both serum and CSF at the time of seizure due to ethical limitations.
Protective Effect of BDNF in Epilepsy: YES
Preclinical Findings
It has been displayed that BDNF may be beneficial against seizure progression by enhancing the inhibitory GABAergic neurotransmission [77, 78]. Chronic treatment with BDNF inhibits seizure severity and frequency following induction of SE in the animal model study [77]. BDNF can induce LTP of GABAergic neurons, prevent internalization of GABA receptors via activation of protein kinase and inhibit the interaction with phosphatase 2 A complex downstream of protein kinase [78]. BDNF decreases neuronal excitability by increasing NPY [43]. NPY is considered an endogenous anti-seizure via activation of Y2-Y5 receptors expressed in neurons [79]. Therefore, NPY-based gene therapy may be a new anti-epileptic agent for the management of resistance epilepsy. BDNF is reduced in adult epileptic patients [80]. Indeed, TrkB agonists prevent post-traumatic epilepsy by inhibiting epileptogenesis [81]. Chronic infusion of BDNF in mice reduces neuronal excitability by downregulating TrkB and increases the expression of neuroprotective NPY [43].
Certainly, BDNF/TrkB role is differed according to specific brain regions, it reduces neuronal excitability in the neocortex but augments neuronal excitability in the hippocampus [77]. Furthermore, continuous administration of BDNF by a bio-delivery system attenuates generalized epilepsy in rats [82]. Thus, BDNF/TrkB signaling seems to be beneficial rather than harmful in epilepsy, and increasing BDNF level in epilepsy could be a compensatory mechanism to prevent seizure-induced neuronal injury [83]. It has been shown that BDNF which binds TrkB and pro-BDNF which binds p75NTR receptors are involved in the repair of neuronal injury and regulation of synaptic plasticity [38]. Various preclinical studies highlighted the protective role of epileptogenesis and the development of epilepsy. BDNF inhibits epileptogenesis by reducing neuronal excitability in the pyramidal neurons [84]. BDNF improves GABAergic neurotransmission in rats with experimental epilepsy through the phosphorylation of different subunits [85]. Deletion of BDNF/TrkB induces hyper-reactivity of interneurons with the development of seizures in mice [86]. TrkB through different molecular effects improves the maturation of GABAergic and inhibitory interneurons, and loss of BDNF disturbs the balance of the inhibitory/excitatory axis [87]. Furthermore, BDNF can reduce epileptogenesis-induced inflammation through the improvement of BBB integrity in rats with experimental epilepsy [88]. BDNF has a neuroprotective effect and inhibits epileptogenesis thereby reducing epilepsy induced neuronal injury in rats [89]. Supporting this notion, an experimental study illustrated that a selective α-2 agonist dexmedetomidine attenuates kainic acid-induced seizure in rats model of TLE by increasing the expression of TrkB and release of BDNF [90]. Dexmedetomidine inhibits neuronal glutamate release and can reduce excitotoxicity-induced neuronal injury by modulating inflammatory signaling pathways and BDNF [90]. Therefore, dexmedetomidine could be a potential anti-epileptic agent. Likewise, a proton-pump inhibitor pantoprazole has been recently shown to attenuate PTZ-induced seizure in rats by increasing the expression of brain BDNF [91]. Moreover, a recent experimental study demonstrated that probiotics can attenuate PTZ-induced seizure in rats by increasing the expression of BDNF [91]. In vitro study demonstrated that pantoprazole reduces PTZ-induced neurotoxicity in the SH-SY5Y cell line by decreasing oxidative stress and apoptosis with increasing the expression of BDNF [91]. Furthermore, hesperidin protects from PTZ-induced neurotoxicity and epilepsy via stimulation of the cAMP response element binding protein (CREB)/BDNF pathway [92]. TrkB activates the expression of CREB which improves the expression of BDNF that promotes TrkB signaling [82]. Further preclinical studies indicated that the reduction of BDNF increases the risk of epilepsy [82]. Therefore, increasing the expression or delivery of BDNF into the hippocampus which is involved in epileptic activity can decrease the frequency of seizure and reverses different structural neuronal changes linked with chronic epilepsy [82]. In this state, augmentation of BDNF/TrkB could be beneficial in the management of chronic epilepsy. However, there is no solid clinical evidence support that increasing of BDNF above normal physiological level to inhibits epileptogenesis. In addition, the molecular mechanism behind the suppressant effect of BDNF on epileptogenesis still unidentified.
Clinical Findings
Diverse clinical studies confirmed that BDNF plays a critical role in preventing epileptic seizures. Of note, BDNF serum level was reduced in patients with TLE as compared to healthy controls [93]. A case-control study on 12 patients with psychogenic non-epileptic seizure (PNES), 15 patients with an epileptic seizure, and 17 healthy controls revealed that BDNF level serum was reduced in patients with epileptic seizure compared to other patients and healthy controls [80]. This study had a small sample size which affects the causal relationship between epilepsy and BDNF serum level. A systematic review demonstrated that BDNF serum levels in epileptic patients was not different compared to the general population [77]. In addition, BDNF serum level is reduced in patients with partial epilepsy [77].
BDNF is mainly expressed in the hippocampus a site of epileptic seizure in TLE. Therefore, decreasing of BDNF circulating levels in patients with TLE indicates impairment of brain white matter and associated cognitive dysfunction [93]. However, this small sample size does not give concrete clinical confirmation concerning the association between low levels of BDNF in patients with TLE. Similarly, AEDs can downregulate BDNF expression leading to the reduction of BDNF serum levels in epileptic patients [94]. A case-control study on 143 epileptic patients compared to 48 healthy control subjects exposed that BDNF serum level was reduced in epileptic patients compared to controls [95]. BDNF serum level was reduced rapidly within 1 h in epileptic patients following acute seizure [95]. The underlying cause for the reduction of BDNF after acute epileptic seizure is not fully elucidated. It has been observed that epileptic seizure induced oxidative stress which causes hippocampal injury and inhibition of neurogenesis [96]. Herein, progressive neuronal injury and dysfunction of hippocampal synaptic plasticity are associated with the reduction of BDNF following seizure [97]. Also, the BDNF gene is downregulated during epileptic seizures [98]. Inhibition of machinery cleavage of pro-BDNF to BDNF and reduction of plasminogen proteolytic activity following seizure and SE could be a possible mechanism for decreasing BDNF in epilepsy [44, 59]. In addition, BDNF gene polymorphism affects the release and functional activity in patients with TLE [99]. Moreover, the BDNF signaling pathway inhibits epileptogenesis by modulating the expression of miR124 which induces neuronal excitability [100]. Induction the release of BDNF by exercise can reduce seizure frequency by promoting cellular signaling pathways which involves reducing neuronal excitability and improving synaptic plasticity [101].
These verdicts indicated that BDNF is highly reduced in epileptic patients. In sum, there is a strong controversy concerning whether BDNF serum levels in epilepsy could be protective or detrimental.
Discussion
The present review highlighted that BDNF has detrimental and protective effects in relation to the development of epileptogenesis and the progression of epilepsy. In 1995, it was reported that over-expression of BDNF was linked with epileptogenesis [102]. This foundation excited many researchers to illustrate the link between BDNF and epilepsy. Croll and coworkers in 1999 found that BDNF triggered in vitro hyper-excitability and increased seizure severity in mice [103]. In transgenic mice, over-expression of TrkB is associated with seizure frequency and severity [104]. However, the underlying mechanism for BDNF-induced seizure is controversial since both high and low BDNF can interrupt the neuronal inhibitory/excitatory axis through disruption of neuronal LTP [105]. Preclinical studies that measure BDNF in epileptogenesis and induced epilepsy may not accurately reflect the level of endogenous BDNF as it is affected by different factors including age, sex and diurnal variations [106]. Moreover, little is recognized about normal concentration and cut-off values of BDNF and pro-BDNF in healthy and epileptic patients. For example, antibodies against BDNF to localize its main site in the neurons showed controversial findings, as it is highly dense in synapses or transported from soma to the dendritic during seizure [107]. Andreska et al. [108] confirmed by an experimental study that BDNF expression is highly abundant at hippocampal glutamatergic presynapses. Therefore, the excitability of hippocampal glutamatergic neurons in epileptogenesis may be linked with the release of BDNF [109]. Thus, elevation of BDNF following seizure could be a compensatory mechanism to reduce the excitability of hippocampal glutamatergic neurons [110]. BDNF-mediated activation of TrkB exerts different effects on epileptogenesis depending on the types of epilepsy model, natural history of experimental epilepsy, time of administration and TrkB inhibition or activation [111, 112]. BDNF is reduced in partial epilepsy but increased in generalized epilepsy [94]. However, BDNF was found to decrease desensitization of GABA receptors in humans and animals [113, 114]. However, the administration of BDNF in rats’ hippocampus did not affect seizure frequency and severity [115]. Activation of the TrkB receptor in animals with post-traumatic epilepsy hinders epileptogenesis through the modulation of parvalbumin interneurons [81]. However, inhibition of TrkB after SE in animal model studies can attenuate the development of TLE [77]. TrkB antagonists and agonists are not available in clinical practice to modulate the BDNF-induced epileptogenesis. In this manner, BDNF mimetics could be effective in the management of epilepsy by reducing hippocampal neuronal injury [116]. These outcomes indicated conflicting results regarding BDNF effects which might be pro-epileptogenic or anti-epileptogenic.
To understand the exact effect of BDNF in epilepsy, should revise the molecular signaling associated with epilepsy concerning BDNF expression.
Autophagy and BDNF in Epilepsy
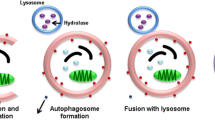
Autophagy is a precise cellular process to eliminate different cytoplasmic misfolded proteins, lipid and damaged organelles to the lysosomes for degradation and clearance [117]. Autophagy role in epilepsy has been lately considered as autophagy inducer rapamycin plays a critical role in the attenuation of induced seizure in animal model studies [118]. Induced autophagy in response to oxidative stress contributes to neuronal cell deaths after seizure in animal model study [119]. Inhibition of oxidative stress by antioxidants significantly attenuates autophagic response in pilocarpine-induced epilepsy [119]. It has been recommended that autophagy prevents the development and progression of epilepsy through regulation the balance between inhibitory GABA and excitatory glutamate [120]. Autophagy is intricate in synaptic homeostasis and the regulation of neurotransmitters, thus imperfect autophagy is associated with reduction the activity of certain neurotransmitters mainly GABAergic ones [121]. In addition, the heterogeneity of GABAergic interneurons affects epileptogenesis and hyper-excitability in epilepsy [122]. Consequently, defective autophagy promotes epileptic seizure in animal and human studies [123, 124]. Induction of autophagy and autophagy-related proteins like Atg7, LC3, and Beclin-1 by endothelial progenitor cells could be a novel therapeutic strategy in the management of epilepsy [9].
It has been reported that BDNF improves synaptic plasticity by inhibiting autophagy which is implicated in the degradation of synaptic proteins [125]. Under the starvation condition, BDNF is activated leading to the activation of the PI3K/Akt pathway which inhibits expression of autophagic protein and the formation of autophagosomes [125]. Conversely, an in vitro study demonstrated that BDNF promotes autophagy by inhibiting PI3K/Akt pathway [126]. Furthermore, an experimental study showed that corticosterone-induced depression is mediated by autophagy hyperactivation and associated reduction of BDNF by excessive lysosomal degradation [127]. Despite these conflicting findings regarding the relationship between autophagy and BDNF, a recent preclinical study confirmed that autophagy improves BDNF signaling [128]. Stress-induced autophagy promotes the release of matrix metalloproteinase 9 (MMP9) which enhances the cleavage of pro-BDNF to BDNF [128]. It has been reported that MMP9 increases availability and optimizes the functional activity of BDNF to facilitate synaptic plasticity and activation of cortical neurons [129]. Notably, MMP9 activity is augmented in the epileptic foci as observed in preclinical and clinical studies [130]. MMP9 triggers BBB injury and neuroinflammation in epilepsy [130]. Therefore, exaggerated autophagy and release of BDNF in epilepsy could explain acceleration of BDNF in relation to epileptogenesis. However, this effect could beneficial rather than detrimental since autophagy inducers like metformin an insulin sensitizing drug used as a first-line in the management of diabetes can reduce seizure severity [131]. Furthermore, metformin attenuates the development of SE by inducing autophagy [131]. A cohort study involved 18 patients with Lafora disease, 8 treated with metformin, and 10 untreated showed that metformin was effective in reducing seizure severity and frequency [132]. Similarly, a macrolide antibiotic rapamycin is effective in patients with tuberous sclerosis complex and could be as an adjuvant treatment with AEDs [133]. Both metformin and rapamycin promote expression of BDNF [134, 135]. Thus, autophagy/BDNF pathway is an essential pathway to maintain neuronal integrity and attenuate epileptogenesis and the development of epilepsy.
Mechanistic Target of Rapamycin (mTOR) and BDNF in Epilepsy
Importantly, the mTOR pathway is an integral pathway intricate in the regulation of neurogenesis, synaptic plasticity, neuronal development and excitability [136]. It has been perceived that mTOR/autophagy axis controls synaptic plasticity, vesicular release, and clustering of GABA receptors with regulation of inhibitory/excitatory balance in the brain [137]. Overstated mTOR activity is related to the progress of TLE, genetic and acquired epilepsy, experimental epilepsy and Lafora disease [138]. Inhibition of the mTOR pathway reduces seizure severity through the activation of autophagy [139]. Inhibition of mTORpathy according to the findings from preclinical and clinical trials may be effective in the management of genetic and acquired epilepsies [139]. Of note, the mTOR pathway is regarded as a negative regulator of autophagy. Inhibition of the mTOR pathway by metformin and rapamycin may explain the protective role of these agents against epilepsy [140]. It has been observed that BDNF improves memory consolidation through the induction of the mTOR pathway in mice [141]. Inhibition of mTOR pathway by rapamycin in mice with TLE induces activation of BDNF [142]. Likewise, six-week exercise reduces seizure frequency in rats through regulation of the BDNF/ mTOR pathway [143]. An elegant experimental study observed that BDNF activates autophagy by inhibiting the mTOR pathway in rats with hypoxic-ischemic encephalopathy [8]. Furthermore, an exaggerated mTOR pathway in diabetes inhibits BDNF signaling leading to neuroinflammation and synaptic dysfunction in diabetic encephalopathy [144]. Thus, BDNF through inhibition of the mTOR pathway and induction of autophagy could be an effective strategy against epileptogenesis.
Progranulin and BDNF in Epilepsy
Progranulin (PGN) is a preserved secreted protein expressed by diverse cell types in the CNS and peripheral tissues [145]. PGN switches cell growth and inflammation, lysosomal function and microglial response [145]. In the CNS, PGN is mostly expressed by microglia and induces uptake of synaptophysin by microglia [146]. It has been shown that mutation of PGN is linked with the development of frontotemporal dementia and other neurodegenerative disorders [147]. It has been shown that PGN expression is increased in the hippocampus after status epilepticus in mice as a compensatory mechanism [148]. PGN expression is augmented by macrophages and microglia in the hippocampus, cerebral cortex and thalamus within 48 h following pilocarpine-induced SE [149]. Besides, CSF PGN level was documented to be increased in epileptic patients following SE as compared to control [149]. A cohort study on patients with resistance epilepsy (n = 56) exposed that CSF PGN level was increased as compared to healthy (n = 36) [150]. Importantly, metformin activates the expression of neuroprotective and anti-inflammatory PGN [138]. Findings from an experimental study showed that pre-treatment with metformin increases PGN which improves anti-inflammatory cytokines and reduces reactive astrogliosis [151]. Deficiency of neuronal PGN due to mutation promotes complement activation which enhances the engulfment of inhibitory synapses by microglia [152]. Consequently, increasing PGN in epilepsy mainly after SE could be a compensatory mechanism to protect inhibitory synapses from injury by microglia. It has been reported that PGN is co-secreted with BDNF, and PGN activates the release of BDNF [153]. PGN acts on specific receptor sortilin-1 which also mediates the function of BDNF. Sortilin-1 regulates BDNF by modulating lysosomal trafficking and anterograde transport. Sortilin-1 forms a complex with pro-BDNF and p75 to promote cell death. As well, sortilin-1 enhances the expression of TrBk on the neuronal terminals [154]. Reduction of PGN is in parallel with reduction of BDNF in different neurodegenerative diseases [155]. Therefore, these findings suggest that increasing of BDNF in epilepsy may due to augmentation the effect of PGN.
Alpha-synuclein and BDNF in Epilepsy
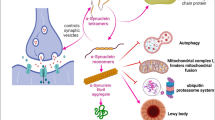
Synucleins (Syns) are extremely ample proteins in the CNS, that control synaptic vesicle trafficking and neurotransmitter release [156]. α-Syn is intricate in the formation of Lewy bodies a hallmark of PD and other neurodegenerative diseases such as AD [157]. The mechanism of α-Syn-induced neurodegeneration is not thriving assumed [158]. However, the formation of neurotoxic α-Syn filaments could be the conceivable mechanism [158]. Epilepsy and neurodegenerative diseases share a common underlying mechanism [159]. Released α-Syn from injured neurons triggers astrocytes and microglia leading to neuroinflammation and degeneration of inhibitory neurotransmitters with subsequent induction of epileptogenesis [160]. Indeed, α-Syn expression is increased in the hippocampus in rats with PTZ-induced seizure [161]. In addition, α-Syn expression is advanced in epileptic brains as compared to normal brains and is associated with disease severity [161]. Likewise, pilocarin-induced seizure in mice triggers expression of α-Syn in the brain within 4 weeks from induction of epilepsy [162]. In the clinical background, it has been stated that α-Syn expression in the brain of patients with TLE was increased [163]. Serum α-Syn level is augmented in epileptic children interrelated with disease severity and cognitive dysfunction [164]. Relevant, serum α-Syn level is linked with CSF α-Syn level and IL-6 [160]. Serum and CSF α-Syn levels are increased in patients with refractory epilepsy [165]. These outcomes provide evidence that epilepsy is linked with neurodegenerative disorders, and α-Syn serum level could be a diagnostic and prognostic biomarker of refractory epilepsy. Therefore, targeting of α-Syn may reduce epileptogenesis in patients with neurodegenerative disorders and epilepsy [159].
Regarding the relationship between α-Syn and BDNF, it has been observed that α-Syn inhibits the expression of BDNF through inhibition of cAMP and CREB [166]. A preclinical study conducted by Feng et al. [167] revealed that accumulation of α-Syn in mice PD model causes neuronal injury and reduction of circulating BDNF. Activation of BDNF attenuates the accumulation of α-Syn in mice [168]. Of interest, a wild-type α-Syn triggers activation of BDNF, though mutant α-Syn suppresses BDNF [21]. Furthermore, α-Syn attenuates the functional activity of TrkB in the PD model [169]. Of note, an MAO-B inhibitor rasagilin prevents the interaction between α-Syn and TrkB in the PD model with subsequent rescuing of the BDNF/TrkB signaling pathway [169]. Higher expression of α-Syn in intractable epilepsy [165] could explain the reduction of circulating BDNF in patients with severe and resistant epilepsy.
Taken together, the potential role of BDNF in epilepsy could detrimental by interrupting the neuronal inhibitory/excitatory axis, or beneficial by inhibiting the excitability of hippocampal glutamatergic neurons. However, dysregulation of BDNF in epilepsy is not a single entity but is related to the dysregulation of autophagy, mTOR pathway, PGN and α-Syn which negatively and positively regulate the BDNF/TrkB signaling pathway. Therefore, the measurement of BDNF in epilepsy should be related to other neuronal signaling pathways and types of epilepsy in both preclinical and clinical studies.
Conclusions
Epilepsy is a neurological disease characterized by repeated seizures. BDNF is increased or decreased in epilepsy, and depending on these findings, BDNF is implicated in epileptogenesis and epilepsy. However, BDNF may have a neuroprotective effect against epilepsy. Thus, the goal of the present review was to highlight the protective and detrimental roles of BDNF. Preclinical and clinical data illustrated that BDNF has detrimental effects by enhancing epileptogenesis. However, other findings indicated that BDNF has protective effects against epileptogenesis. The autophagy/BDNF pathway is an essential pathway to maintain neuronal integrity and attenuate epileptogenesis and the development of epilepsy. BDNF through inhibition of the mTOR pathway and induction of autophagy could be an effective strategy against epileptogenesis. The increasing BDNF in epilepsy may be due to augmentation of the effect of PGN. Furthermore, higher expression of α-Syn in intractable epilepsy could explain the reduction of circulating BDNF in patients with severe and resistant epilepsy. These outcomes excite many researchers to illustrate the link between BDNF and epilepsy by preclinical and clinical studies.
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Change history
06 January 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11064-023-04092-7
References
Thijs RD, Surges R, O’Brien TJ, Sander JW (2019) Epilepsy in adults. The Lancet 393(10172):689–701
Miller WR, Von Gaudecker J, Tanner A, Buelow JM (2020) Epilepsy self-management during a pandemic: experiences of people with Epilepsy. Epilepsy Behav 111:107238
Cretin B (2021) Treatment of seizures in older patients with Dementia. Drugs Aging 38(3):181–192
Fisher RS, Acharya JN, Baumer FM, French JA, Parisi P, Solodar JH et al (2022) Visually sensitive seizures: an updated review by the Epilepsy Foundation. Epilepsia 63(4):739–768
Li RJ, Liu Y, Liu HQ, Li J (2020) Ketogenic diets and protective mechanisms in Epilepsy, metabolic disorders, cancer, neuronal loss, and muscle and nerve degeneration. J Food Biochem 44(3):e13140
Rubio C, Piñón E, Molina-García J, Portilla A, Osornio MR (2023) Participation of na + channels in Epilepsy: a bibliometric analysis of the Scientific production in the World. Adv Bioeng Biomedical Sci Res 6(3):33–41
Steriade C, Britton J, Dale RC, Gadoth A, Irani SR, Linnoila J et al (2020) Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune-associated Epilepsy: conceptual definitions. Epilepsia 61(7):1341–1351
Zheng Z, Zhang L, Qu Y, **ao G, Li S, Bao S et al (2018) Mesenchymal stem cells protect against hypoxia-ischemia brain damage by enhancing autophagy through brain derived neurotrophic factor/mammalin target of rapamycin signaling pathway. Stem Cells 36(7):1109–1121
Ali SO, Shahin NN, Safar MM, Rizk SM (2019) Therapeutic potential of endothelial progenitor cells in a rat model of Epilepsy: role of autophagy. J Adv Res 18:101–112
Castrén E, Monteggia LM (2021) Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol Psychiatry 90(2):128–136
Gao L, Zhang Y, Sterling K, Song W (2022) Brain-derived neurotrophic factor in Alzheimer’s Disease and its pharmaceutical potential. Translational Neurodegeneration 11(1):1–34
McGregor CE, English AW (2019) The role of BDNF in peripheral nerve regeneration: activity-dependent treatments and Val66Met. Front Cell Neurosci 12:522
Chaldarov GN, Tonchev AB, Aloe L (2009) NGF and BDNF: from nerves to adipose tissue, from neurokines to metabokines. Rivista Di Psichiatria 44(2):79–87
Lu B, Nagappan G, Guan X, Nathan PJ, Wren P (2013) BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative Diseases. Nat Rev Neurosci 14(6):401–416
Guerrera CS, Furneri G, Grasso M, Caruso G, Castellano S, Drago F et al (2020) Antidepressant Drugs and physical activity: a possible synergism in the treatment of major depression? Front Psychol 11:857
Miao Z, Wang Y, Sun Z (2020) The relationships between stress, mental disorders, and epigenetic regulation of BDNF. Int J Mol Sci 21(4):1375
Goldhardt MG, Andreia A, Dorneles GP, da Silva IR, Pochmann D, Peres A et al (2019) Does a single bout of exercise impacts BDNF, oxidative stress and epigenetic markers in spinal cord injury patients. Funct Neurol 34:158–166
Ventriglia M, Zanardini R, Bonomini C, Zanetti O, Volpe D, Pasqualetti P et al (2013) Serum brain-derived neurotrophic factor levels in different neurological diseases. BioMed research international 2013
Diniz BS, Teixeira AL (2011) Brain-derived neurotrophic factor and Alzheimer’s Disease: physiopathology and beyond. Neuromolecular Med 13:217–222
Murer M, Boissiere F, Yan Q, Hunot S, Villares J, Faucheux B et al (1999) An immunohistochemical study of the distribution of brain-derived neurotrophic factor in the adult human brain, with particular reference to Alzheimer’s Disease. Neuroscience 88(4):1015–1032
Kohno R, Sawada H, Kawamoto Y, Uemura K, Shibasaki H, Shimohama S (2004) BDNF is induced by wild-type α-synuclein but not by the two mutants, A30P or A53T, in glioma cell line. Biochem Biophys Res Commun 318(1):113–118
Zuccato C, Cattaneo E (2009) Brain-derived neurotrophic factor in neurodegenerative Diseases. Nat Reviews Neurol 5(6):311–322
Fusar-Poli L, Aguglia A, Amerio A, Orsolini L, Salvi V, Serafini G et al (2021) Peripheral BDNF levels in psychiatric patients with and without a history of Suicide attempt: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 111:110342
El Ouaamari Y, Van den Bos J, Willekens B, Cools N, Wens I (2023) Neurotrophic factors as regenerative therapy for neurodegenerative Diseases: current status, challenges and Future perspectives. Int J Mol Sci 24(4):3866
Lang UE, Hellweg R, Seifert F, Schubert F, Gallinat J (2007) Correlation between serum brain-derived neurotrophic factor level and an in vivo marker of cortical integrity. Biol Psychiatry 62(5):530–535
Gunstad J, Benitez A, Smith J, Glickman E, Spitznagel MB, Alexander T et al (2008) Serum brain-derived neurotrophic factor is associated with cognitive function in healthy older adults. J Geriatr Psychiatry Neurol 21(3):166–170
Bocchio-Chiavetto L, Bagnardi V, Zanardini R, Molteni R, Gabriela Nielsen M, Placentino A et al (2010) Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. World J Biol Psychiatry 11(6):763–773
Castrén E, Rantamäki T (2010) The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol 70(5):289–297
Nishino S, Ohtomo K, Numata Y, Sato T, Nakahata N, Kurita M (2012) Divergent effects of lithium and sodium valproate on brain-derived neurotrophic factor (BDNF) production in human astrocytoma cells at therapeutic concentrations. Prog Neuropsychopharmacol Biol Psychiatry 39(1):17–22
Huang T-L, Hung Y-Y (2009) Lorazepam reduces the serum brain-derived neurotrophic factor level in schizophrenia patients with catatonia. Prog Neuropsychopharmacol Biol Psychiatry 33(1):158–159
Jornada LK, Moretti M, Valvassori SS, Ferreira CL, Padilha PT, Arent CO et al (2010) Effects of mood stabilizers on hippocampus and amygdala BDNF levels in an animal model of mania induced by ouabain. J Psychiatr Res 44(8):506–510
Matrisciano F, Bonaccorso S, Ricciardi A, Scaccianoce S, Panaccione I, Wang L et al (2009) Changes in BDNF serum levels in patients with major depression disorder (MDD) after 6 months treatment with sertraline, escitalopram, or venlafaxine. J Psychiatr Res 43(3):247–254
Ibrahim AM, Chauhan L, Bhardwaj A, Sharma A, Fayaz F, Kumar B et al (2022) Brain-derived neurotropic factor in neurodegenerative disorders. Biomedicines 10(5):1143
Andreska T, Rauskolb S, Schukraft N, Lüningschrör P, Sasi M, Signoret-Genest J et al (2020) Induction of BDNF expression in layer II/III and layer V neurons of the motor cortex is essential for motor learning. J Neurosci 40(33):6289–6308
de Deus JL, Amorim MR, Ribeiro AB, Barcellos-Filho PC, Ceballos CC, Branco LGS et al (2021) Loss of brain-derived neurotrophic factor mediates inhibition of hippocampal long-term potentiation by high-intensity sound. Cell Mol Neurobiol 41:751–763
Del Angel YC, Orfila JE, Herson PS, Brooks-Kayal A, González MI (2021) Down-regulation of AMPA receptors and long-term potentiation during early epileptogenesis. Epilepsy Behav 124:108320
Anderson WW (2020) Epileptogenesis. Cortical Plasticity: Garland Science; p. 149 – 89
Walczak A, Szczepankiewicz AA, Ruszczycki B, Magalska A, Zamlynska K, Dzwonek J et al (2013) Novel higher-order epigenetic regulation of the Bdnf gene upon seizures. J Neurosci 33(6):2507–2511
Geyer PK, Vitalini MW, Wallrath LL (2011) Nuclear organization: taking a position on gene expression. Curr Opin Cell Biol 23(3):354–359
Bandeira IC, Giombelli L, Werlang IC, Abujamra AL, Secchi TL, Brondani R et al (2021) Methylation of BDNF and SLC6A4 gene promoters in Brazilian patients with temporal lobe Epilepsy presenting or not psychiatric comorbidities. Front Integr Neurosci 15:764742
Skupien-Jaroszek A, Walczak A, Czaban I, Pels KK, Szczepankiewicz AA, Krawczyk K et al (2021) The interplay of seizures-induced axonal sprouting and transcription-dependent Bdnf repositioning in the model of temporal lobe Epilepsy. PLoS ONE 16(6):e0239111
Binder DK, Croll SD, Gall CM, Scharfman HE (2001) BDNF and Epilepsy: too much of a good thing? Trends Neurosci 24(1):47–53
Iughetti L, Lucaccioni L, Fugetto F, Predieri B, Berardi A, Ferrari F (2018) Brain-derived neurotrophic factor and Epilepsy: a systematic review. Neuropeptides 72:23–29
Fernández-García S, Sancho-Balsells A, Longueville S, Hervé D, Gruart A, Delgado-García JM et al (2020) Astrocytic BDNF and TrkB regulate severity and neuronal activity in mouse models of temporal lobe Epilepsy. Cell Death Dis 11(6):411
Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S (2005) Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol 192(2):348–356
Koyama R, Yamada MK, Fujisawa S, Katoh-Semba R, Matsuki N, Ikegaya Y (2004) Brain-derived neurotrophic factor induces hyperexcitable reentrant circuits in the dentate gyrus. J Neurosci 24(33):7215–7224
Xu B, Michalski B, Racine R, Fahnestock M (2004) The effects of brain-derived neurotrophic factor (BDNF) administration on kindling induction, trk expression and seizure-related morphological changes. Neuroscience 126(3):521–531
Scharfman HE (2005) Brain-derived neurotrophic factor and epilepsy—a missing link? Epilepsy Curr 5(3):83–88
Yu X, Guan Q, Wang Y, Shen H, Zhai L, Lu X et al (2019) Anticonvulsant and anti-apoptosis effects of salvianolic acid B on pentylenetetrazole-kindled rats via AKT/CREB/BDNF signaling. Epilepsy Res 154:90–96
Zhao T, Ding Y, Li M, Zhou C, Lin W (2019) Silencing lncRNA PVT1 inhibits activation of astrocytes and increases BDNF expression in hippocampus tissues of rats with Epilepsy by downregulating the wnt signaling pathway. J Cell Physiol 234(9):16054–16067
Gu GF, Parada I, Yang T, Longo FM, Prince DA (2022) Chronic partial TrkB activation reduces seizures and mortality in a mouse model of Dravet syndrome. Proc Natl Acad Sci 119(7):e2022726119
Chmielewska N, Wawer A, Maciejak P, Turzyńska D, Sobolewska A, Skórzewska A et al (2020) The role of REST/NRSF, TrkB and BDNF in neurobiological mechanisms of different susceptibility to seizure in a PTZ model of Epilepsy. Brain Res Bull 158:108–115
Wang X, Hu Z, Zhong K (2021) The role of brain-derived neurotrophic factor in epileptogenesis: an update. Front Pharmacol 12:758232
Koyama R, Ikegaya Y (2005) To BDNF or not to BDNF: that is the epileptic hippocampus. Neuroscientist 11(4):282–287
Binder DK (2004) The role of BDNF in Epilepsy and other Diseases of the mature nervous system. Recent Adv Epilepsy Res :34–56
Liu G, Gu B, He X-P, Joshi RB, Wackerle HD, Rodriguiz RM et al (2013) Transient inhibition of TrkB kinase after status epilepticus prevents development of temporal lobe Epilepsy. Neuron 79(1):31–38
Kotloski R, McNamara JO (2010) Reduction of TrkB expression de novo in the adult mouse impairs epileptogenesis in the kindling model. Hippocampus 20(6):713–723
Wake H, Watanabe M, Moorhouse AJ, Kanematsu T, Horibe S, Matsukawa N et al (2007) Early changes in KCC2 phosphorylation in response to neuronal stress result in functional downregulation. J Neurosci 27(7):1642–1650
Kyyriäinen J, Bolkvadze T, Koivisto H, Lipponen A, Perez LO, Ndode-Ekane XE et al (2019) Deficiency of urokinase-type plasminogen activator and its receptor affects social behavior and increases seizure susceptibility. Epilepsy Res 151:67–74
Kourdougli N, Pellegrino C, Renko JM, Khirug S, Chazal G, Kukko-Lukjanov TK et al (2017) Depolarizing γ‐aminobutyric acid contributes to glutamatergic network rewiring in Epilepsy. Ann Neurol 81(2):251–265
Riffault B, Kourdougli N, Dumon C, Ferrand N, Buhler E, Schaller F et al (2018) Pro-brain-derived neurotrophic factor (proBDNF)-mediated p75NTR activation promotes depolarizing actions of GABA and increases susceptibility to epileptic seizures. Cereb Cortex 28(2):510–527
McNamara JO, Scharfman HE (2012) Temporal lobe epilepsy and the BDNF receptor, TrkB
Ghadiri T, Vakilzadeh G, Hajali V, Khodagholi F (2019) Progesterone modulates post-traumatic epileptogenesis through regulation of BDNF-TrkB signaling and cell survival-related pathways in the rat hippocampus. Neurosci Lett 709:134384
Yang N, Guan Q-W, Chen F-H, **a Q-X, Yin X-X, Zhou H-H et al (2020) Antioxidants targeting mitochondrial oxidative stress: promising neuroprotectants for epilepsy. Oxid Med Cell Longev 2020
Quan H, Koltai E, Suzuki K, Aguiar AS Jr, Pinho R, Boldogh I et al (2020) Exercise, redox system and neurodegenerative Diseases. Biochim et Biophys Acta (BBA)-Molecular Basis Disease 1866(10):165778
Yang T, Nie Z, Shu H, Kuang Y, Chen X, Cheng J et al (2020) The role of BDNF on neural plasticity in depression. Front Cell Neurosci 14:82
Ferrini F, De Koninck Y (2013) Microglia control neuronal network excitability via BDNF signalling. Neural Plast 2013
Martínez-Levy G, Rocha L, Rodríguez-Pineda F, Alonso-Vanegas M, Nani A, Buentello-García R et al (2018) Increased expression of brain-derived neurotrophic factor transcripts I and VI, cAMP response element binding, and glucocorticoid receptor in the cortex of patients with temporal lobe Epilepsy. Mol Neurobiol 55:3698–3708
Katz D (2014) Brain-derived neurotrophic factor and Rett syndrome. Neurotrophic Factors:481 – 95
Sha’ari HM, Haerian BS, Baum L, Tan HJ, Rafia MH, Kwan P et al (2016) Association of BDNF polymorphisms with the risk of Epilepsy: a multicenter study. Mol Neurobiol 53:2869–2877
Demir M, Akarsu EO, Dede HO, Bebek N, Yıldız SO, Baykan B et al (2020) Investigation of the roles of new antiepileptic Drugs and serum BDNF levels in efficacy and safety monitoring and quality of life: a clinical research. Curr Clin Pharmacol 15(1):49–63
Alvim MK, Morita-Sherman ME, Yasuda CL, Rocha NP, Vieira EL, Pimentel‐Silva LR et al (2021) Inflammatory and neurotrophic factor plasma levels are related to Epilepsy independently of etiology. Epilepsia 62(10):2385–2394
Murray KD, Isackson PJ, Eskin TA, King MA, Montesinos SP, Abraham LA et al (2000) Altered mRNA expression for brain-derived neurotrophic factor and type II calcium/calmodulin‐dependent protein kinase in the hippocampus of patients with intractable temporal lobe Epilepsy. J Comp Neurol 418(4):411–422
Takahashi M, Hayashi S, Kakita A, Wakabayashi K, Fukuda M, Kameyama S et al (1999) Patients with temporal lobe Epilepsy show an increase in brain-derived neurotrophic factor protein and its correlation with neuropeptide Y. Brain Res 818(2):579–582
Vinti V, Dell’Isola GB, Tascini G, Mencaroni E, Cara GD, Striano P et al (2021) Temporal lobe Epilepsy and psychiatric comorbidity. Front Neurol 12:775781
Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R et al (2007) Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe Epilepsy. J Neurosci 27(37):9866–9873
Lin TW, Harward SC, Huang YZ, McNamara JO (2020) Targeting BDNF/TrkB pathways for preventing or suppressing Epilepsy. Neuropharmacology 167:107734
Porcher C, Medina I, Gaiarsa J-L (2018) Mechanism of BDNF modulation in GABAergic synaptic transmission in healthy and Disease brains. Front Cell Neurosci 12:273
Cattaneo S, Verlengia G, Marino P, Simonato M, Bettegazzi B (2021) NPY and gene therapy for epilepsy: how, when,... and Y. Front Mol Neurosci 13:608001
LaFrance W, Leaver K, Stopa E, Papandonatos G, Blum A (2010) Decreased serum BDNF levels in patients with epileptic and psychogenic nonepileptic seizures. Neurology 75(14):1285–1291
Gu F, Parada I, Yang T, Longo FM, Prince DA (2018) Partial TrkB receptor activation suppresses cortical epileptogenesis through actions on parvalbumin interneurons. Neurobiol Dis 113:45–58
Falcicchia C, Paolone G, Emerich DF, Lovisari F, Bell WJ, Fradet T et al (2018) Seizure-suppressant and neuroprotective effects of encapsulated BDNF-producing cells in a rat model of temporal lobe Epilepsy. Mol Therapy-Methods Clin Dev 9:211–224
Shetty AK (2014) Hippocampal injury-induced cognitive and mood dysfunction, altered neurogenesis, and Epilepsy: can early neural stem cell grafting intervention provide protection? Epilepsy Behav 38:117–124
Gibon J, Buckley SM, Unsain N, Kaartinen V, Séguéla P, Barker PA (2015) proBDNF and p75NTR control excitability and persistent firing of cortical pyramidal neurons. J Neurosci 35(26):9741–9753
Cifelli P, Palma E, Roseti C, Verlengia G, Simonato M (2013) Changes in the sensitivity of GABAA current rundown to drug treatments in a model of temporal lobe Epilepsy. Front Cell Neurosci 7:108
Maynard KR, Kardian A, Hill JL, Mai Y, Barry B, Hallock HL et al (2020) TrkB signaling influences gene expression in cortistatin-expressing interneurons. Eneuro 7(1)
Bovolenta R, Zucchini S, Paradiso B, Rodi D, Merigo F, Mora GN et al (2010) Hippocampal FGF-2 and BDNF overexpression attenuates epileptogenesis-associated neuroinflammation and reduces spontaneous recurrent seizures. J Neuroinflammation 7:1–6
Soysal H, Doğan Z, Kamışlı Ö (2016) Effects of phenytoin and lamotrigine treatment on serum BDNF levels in offsprings of epileptic rats. Neuropeptides 56:1–8
Chiu K-M, Lin T-Y, Lee M-Y, Lu C-W, Wang M-J, Wang S-J (2019) Dexmedetomidine protects neurons from kainic acid-induced excitotoxicity by activating BDNF signaling. Neurochem Int 129:104493
Taskiran AS, Ergul M, Gunes H, Ozturk A, Sahin B, Ozdemir E (2021) The effects of proton pump inhibitors (pantoprazole) on pentylenetetrazole-induced epileptic seizures in rats and neurotoxicity in the SH-SY5Y human neuroblastoma cell line. Cell Mol Neurobiol 41:173–183
Sharma P, Kumari S, Sharma J, Purohit R, Singh D (2021) Hesperidin interacts with CREB-BDNF signaling pathway to suppress pentylenetetrazole-induced convulsions in zebrafish. Front Pharmacol 11:607797
Carlezon WA, Duman RS, Nestler EJ (2005) The many faces of CREB. Trends Neurosci 28(8):436–445
Chen N-C, Chuang Y-C, Huang C-W, Lui C-C, Lee C-C, Hsu S-W et al (2016) Interictal serum brain-derived neurotrophic factor level reflects white matter integrity, Epilepsy severity, and cognitive dysfunction in chronic temporal lobe Epilepsy. Epilepsy Behav 59:147–154
Nowroozi A, Salehi MA, Mohammadi S (2021) Brain-derived neurotrophic factor in patients with Epilepsy: a systematic review and meta-analysis. Epilepsy Res 178:106794
Poniatowski ŁA, Cudna A, Kurczych K, Bronisz E, Kurkowska-Jastrzębska I (2021) Kinetics of serum brain-derived neurotrophic factor (BDNF) concentration levels in epileptic patients after generalized tonic-clonic seizures. Epilepsy Res 173:106612
Sun H, Li X, Guo Q, Liu S (2022) Research progress on oxidative stress regulating different types of neuronal death caused by epileptic seizures. Neurol Sci 43(11):6279–6298
Li M, **a M, Chen W, Wang J, Yin Y, Guo C et al (2020) Lithium treatment mitigates white matter injury after intracerebral Hemorrhage through brain-derived neurotrophic factor signaling in mice. Translational Res 217:61–74
Liu J, Zhu H-X, Fu W-L, Xu X-W, Yang J-Z, Dai D et al (2019) Downregulated hippocampal expression of brain derived neurotrophic factor and tyrosine kinase B in a rat model of comorbid Epilepsy and depression. Neurol Res 41(5):437–445
Shen N, Zhu X, Lin H, Li J, Li L, Niu F et al (2016) Role of BDNF Val66Met functional polymorphism in temporal lobe Epilepsy. Int J Neurosci 126(5):436–441
Wang W, Wang X, Chen L, Zhang Y, Xu Z, Liu J et al (2016) The microRNA miR-124 suppresses seizure activity and regulates CREB1 activity. Expert Rev Mol Med 18:e4
Cavalcante BRR, Improta-Caria AC, de Melo VH, De Sousa RAL (2021) Exercise-linked consequences on Epilepsy. Epilepsy Behav 121:108079
Kokaia M, Ernfors P, Kokaia Z, Elmér E, Jaenisch R, Lindvall O (1995) Suppressed epileptogenesis in BDNF mutant mice. Exp Neurol 133(2):215–224
Croll S, Suri C, Compton D, Simmons M, Yancopoulos G, Lindsay R et al (1999) Brain-derived neurotrophic factor transgenic mice exhibit passive avoidance deficits, increased seizure severity and in vitro hyperexcitability in the hippocampus and entorhinal cortex. Neuroscience 93(4):1491–1506
Łukawski K, Czuczwar SJ (2022) Emerging therapeutic targets for Epilepsy: preclinical insights. Expert Opin Ther Targets 26(3):193–206
Na K-S, Won E, Kang J, Chang HS, Yoon H-K, Tae WS et al (2016) Brain-derived neurotrophic factor promoter methylation and cortical thickness in recurrent major depressive disorder. Sci Rep 6(1):21089
Heinrich C, Lähteinen S, Suzuki F, Anne-Marie L, Huber S, Häussler U et al (2011) Increase in BDNF-mediated TrkB signaling promotes epileptogenesis in a mouse model of mesial temporal lobe Epilepsy. Neurobiol Dis 42(1):35–47
Song M, Martinowich K, Lee F (2017) BDNF at the synapse: why location matters. Mol Psychiatry 22(10):1370–1375
Andreska T, Aufmkolk S, Sauer M, Blum R (2014) High abundance of BDNF within glutamatergic presynapses of cultured hippocampal neurons. Front Cell Neurosci 8:107
Green JL, Dos Santos WF, Fontana ACK (2021) Role of glutamate excitotoxicity and glutamate transporter EAAT2 in Epilepsy: opportunities for novel therapeutics development. Biochem Pharmacol 193:114786
Yu Y, Jiang J (2020) COX-2/PGE2 axis regulates hippocampal BDNF/TrkB signaling via EP2 receptor after prolonged seizures. Epilepsia Open 5(3):418–431
Gu B, Huang YZ, He X-P, Joshi RB, Jang W, McNamara JO (2015) A peptide uncoupling BDNF receptor TrkB from phospholipase Cγ1 prevents Epilepsy induced by status epilepticus. Neuron 88(3):484–491
Kuramoto S, Yasuhara T, Agari T, Kondo A, **g M, Kikuchi Y et al (2011) BDNF-secreting capsule exerts neuroprotective effects on Epilepsy model of rats. Brain Res 1368:281–289
Palma E, Torchia G, Limatola C, Trettel F, Arcella A, Cantore G et al (2005) BDNF modulates GABAA receptors microtransplanted from the human epileptic brain to Xenopus oocytes. Proceedings of the National Academy of Sciences 102(5):1667-72
Palma E, Roseti C, Maiolino F, Fucile S, Martinello K, Mazzuferi M et al (2007) GABAA-current rundown of temporal lobe epilepsy is associated with repetitive activation of GABAA “phasic” receptors. Proceedings of the National Academy of Sciences 104(52):20944-8
Paradiso B, Marconi P, Zucchini S, Berto E, Binaschi A, Bozac A et al (2009) Localized delivery of fibroblast growth factor–2 and brain-derived neurotrophic factor reduces spontaneous seizures in an epilepsy model. Proceedings of the National Academy of Sciences 106(17):7191-6
Kipnis PA, Sullivan BJ, Carter BM, Kadam SD (2020) TrkB agonists prevent postischemic emergence of refractory neonatal seizures in mice. JCI Insight 5(12)
Vargas JNS, Hamasaki M, Kawabata T, Youle RJ, Yoshimori T (2023) The mechanisms and roles of selective autophagy in mammals. Nat Rev Mol Cell Biol 24(3):167–185
Giorgi FS, Biagioni F, Lenzi P, Frati A, Fornai F (2015) The role of autophagy in epileptogenesis and in epilepsy-induced neuronal alterations. J Neural Transm 122:849–862
Cao L, Chen R, Xu J, Lin Y, Wang R, Chi Z (2009) Vitamin E inhibits activated chaperone-mediated autophagy in rats with status epilepticus. Neuroscience 161(1):73–77
Bejarano E, Rodríguez-Navarro JA (2015) Autophagy and amino acid metabolism in the brain: implications for Epilepsy. Amino Acids 47(10):2113–2126
Yin Y, Yi M-H, Kim DW (2018) Impaired autophagy of GABAergic interneurons in neuropathic pain. Pain Research and Management 2018
Marafiga JR, Pasquetti MV, Calcagnotto ME (2021) GABAergic interneurons in Epilepsy: more than a simple change in inhibition. Epilepsy Behav 121:106935
Yasin SA, Ali AM, Tata M, Picker SR, Anderson GW, Latimer-Bowman E et al (2013) mTOR-dependent abnormalities in autophagy characterize human malformations of cortical development: evidence from focal cortical dysplasia and tuberous sclerosis. Acta Neuropathol 126:207–218
McMahon J, Huang X, Yang J, Komatsu M, Yue Z, Qian J et al (2012) Impaired autophagy in neurons after disinhibition of mammalian target of rapamycin and its contribution to epileptogenesis. J Neurosci 32(45):15704–15714
Nikoletopoulou V, Sidiropoulou K, Kallergi E, Dalezios Y, Tavernarakis N (2017) Modulation of autophagy by BDNF underlies synaptic plasticity. Cell Metab 26(1):230–242 e5
Chen A, **ong L-J, Tong Y, Mao M (2013) Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol Med Report 8(4):1011–1016
Zhang K, Wang F, Zhai M, He M, Hu Y, Feng L et al (2023) Hyperactive neuronal autophagy depletes BDNF and impairs adult hippocampal neurogenesis in a corticosterone-induced mouse model of depression. Theranostics 13(3):1059
Martinelli S, Anderzhanova EA, Bajaj T, Wiechmann S, Dethloff F, Weckmann K et al (2021) Stress-primed secretory autophagy promotes extracellular BDNF maturation by enhancing MMP9 secretion. Nat Commun 12(1):4643
Kuzniewska B, Rejmak E, Malik AR, Jaworski J, Kaczmarek L, Kalita K (2013) Brain-derived neurotrophic factor induces matrix metalloproteinase 9 expression in neurons via the serum response factor/c-Fos pathway. Mol Cell Biol 33(11):2149–2162
Bronisz E, Kurkowska-Jastrzębska I (2016) Matrix metalloproteinase 9 in epilepsy: the role of neuroinflammation in seizure development. Mediators Inflamm 2016
Mohamed MAE, Abdel-Rahman RF, Mahmoud SS, Khattab MM, Safar MM (2020) Metformin and trimetazidine ameliorate diabetes-induced cognitive impediment in status epileptic rats. Epilepsy Behav 104:106893
Burgos DF, Machío-Castello M, Iglesias-Cabeza N, Giráldez BG, González-Fernández J, Sánchez-Martín G et al (2023) Early treatment with metformin improves neurological outcomes in lafora Disease. Neurotherapeutics 20(1):230–244
Zhao W, **e C, Zhang X, Liu J, Liu J, **a Z (2023) Advances in the mTOR signaling pathway and its inhibitor rapamycin in Epilepsy. Brain and Behavior :e2995
Fang W, Zhang J, Hong L, Huang W, Dai X, Ye Q et al (2020) Metformin ameliorates stress-induced depression-like behaviors via enhancing the expression of BDNF by activating AMPK/CREB-mediated histone acetylation. J Affect Disord 260:302–313
Lee JA (2022) Where is the mechanistic target of Rapamycin Signaling Pathway in Depression? Mood and Emotion 20(2):23–30
Gao J, Yao M, Chang D, Liu J (2023) mTOR (mammalian target of Rapamycin): hitting the bull’s Eye for Enhancing Neurogenesis after. Cereb Ischemia? Stroke 54(1):279–285
Limanaqi F, Biagioni F, Busceti CL, Fabrizi C, Frati A, Fornai F (2020) mTOR-related cell-clearing systems in epileptic seizures, an update. Int J Mol Sci 21(5):1642
Sanz P, Serratosa JM, Sánchez MP (2021) Beneficial effects of metformin on the central nervous system, with a focus on Epilepsy and Lafora Disease. Int J Mol Sci 22(10):5351
Griffith JL, Wong M (2018) The mTOR pathway in treatment of Epilepsy: a clinical update. Future Neurol 13(2):49–58
Singh R, Sarangi SC, Singh S, Tripathi M (2022) A review on role of metformin as a potential drug for epilepsy treatment and modulation of epileptogenesis. Seizure
Slipczuk L, Bekinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH (2009) BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS ONE 4(6):e6007
Shima A, Nitta N, Suzuki F, Laharie AM, Nozaki K, Depaulis A (2015) Activation of mTOR signaling pathway is secondary to neuronal excitability in a mouse model of mesio-temporal lobe Epilepsy. Eur J Neurosci 41(7):976–988
de Almeida AA, Gomes da Silva S, Lopim GM, Vannucci Campos D, Fernandes J, Cabral FR et al (2017) Resistance exercise reduces seizure occurrence, attenuates memory deficits and restores BDNF signaling in rats with chronic Epilepsy. Neurochem Res 42:1230–1239
Xu T, Liu J, Li X-r, Yu Y, Luo X, Zheng X et al (2021) The mTOR/NF-κB pathway mediates neuroinflammation and synaptic plasticity in diabetic encephalopathy. Mol Neurobiol 58:3848–3862
Rhinn H, Tatton N, McCaughey S, Kurnellas M, Rosenthal A (2022) Progranulin as a therapeutic target in neurodegenerative Diseases. Trends Pharmacol Sci
Liu L, Guo H, Song A, Huang J, Zhang Y, ** S et al (2020) Progranulin inhibits LPS-induced macrophage M1 polarization via NF-кB and MAPK pathways. BMC Immunol 21:1–12
Simon MJ, Logan T, DeVos SL, Di Paolo G (2022) Lysosomal functions of progranulin and implications for treatment of frontotemporal Dementia. Trends Cell Biol
Huchtemann T, Körtvélyessy P, Feistner H, Heinze H, Bittner D (2015) Progranulin levels in status epilepticus as a marker of neuronal recovery and neuroprotection. Epilepsy Behav 49:170–172
Zhu S, Tai C, Petkau TL, Zhang S, Liao C, Dong Z et al (2013) Progranulin promotes activation of microglia/macrophage after pilocarpine-induced status epilepticus. Brain Res 1530:54–65
Hanin A, Denis JA, Frazzini V, Cousyn L, Imbert-Bismut F, Rucheton B et al (2022) Neuron Specific Enolase, S100-beta protein and progranulin as diagnostic biomarkers of status epilepticus. J Neurol 269(7):3752–3760
Vazifehkhah S, Khanizadeh AM, Mojarad TB, Nikbakht F (2020) The possible role of progranulin on anti-inflammatory effects of metformin in temporal lobe Epilepsy. J Chem Neuroanat 109:101849
Lui H, Zhang J, Makinson SR, Cahill MK, Kelley KW, Huang H-Y et al (2016) Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell 165(4):921–935
Petoukhov E, Fernando S, Mills F, Shivji F, Hunter D, Krieger C et al (2013) Activity-dependent secretion of progranulin from synapses. J Cell Sci 126(23):5412–5421
Vaegter CB, Jansen P, Fjorback AW, Glerup S, Skeldal S, Kjolby M et al (2011) Sortilin associates with trk receptors to enhance anterograde transport and neurotrophin signaling. Nat Neurosci 14(1):54–61
Zanardini R, Ciani M, Benussi L, Ghidoni R (2016) Molecular pathways bridging frontotemporal lobar degeneration and psychiatric disorders. Front Aging Neurosci 8:10
Sharma M, Burré J (2023) α-Synuclein in synaptic function and dysfunction. Trends Neurosci 46(2):153–166
Henderson MX, Trojanowski JQ, Lee VM-Y (2019) α-Synuclein pathology in Parkinson’s Disease and related α-synucleinopathies. Neurosci Lett 709:134316
Ding J, Hu S, Meng Y, Li C, Huang J, He Y et al (2020) Alpha-synuclein deficiency ameliorates chronic methamphetamine induced neurodegeneration in mice. Toxicology 438:152461
Paudel YN, Angelopoulou E, Piperi C, Othman I, Shaikh MF (2020) Revisiting the impact of neurodegenerative proteins in Epilepsy: focus on alpha-synuclein, beta-amyloid, and tau. Biology 9(6):122
Choi J, Kim SY, Kim H, Lim BC, Hwang H, Chae JH et al (2020) Serum α-synuclein and IL-1β are increased and correlated with measures of Disease severity in children with Epilepsy: potential prognostic biomarkers? BMC Neurol 20:1–11
Hussein AM, Eldosoky M, El-Shafey M, El-Mesery M, Ali AN, Abbas KM et al (2019) Effects of metformin on apoptosis and α-synuclein in a rat model of pentylenetetrazole-induced Epilepsy. Can J Physiol Pharmacol 97(1):37–46
Li A, Choi YS, Dziema H, Cao R, Cho HY, Jung YJ et al (2010) Proteomic profiling of the epileptic dentate gyrus. Brain Pathol 20(6):1077–1089
Yang J, Czech T, Felizardo M, Baumgartner C, Lubec G (2006) Aberrant expression of cytoskeleton proteins in hippocampus from patients with mesial temporal lobe Epilepsy. Amino Acids 30:477–493
van den Berg L, de Weerd A, Reuvekamp M, van der Meere J (2020) Cognitive control deficits in pediatric frontal lobe Epilepsy. Epilepsy Behav 102:106645
Rong H, ** L, Wei W, Wang X, ** Z (2015) Alpha-synuclein is a potential biomarker in the serum and CSF of patients with intractable Epilepsy. Seizure 27:6–9
Yuan Y, Sun J, Zhao M, Hu J, Wang X, Du G et al (2010) Overexpression of α-synuclein down-regulates BDNF expression. Cell Mol Neurobiol 30:939–946
Fang F, Yang W, Florio JB, Rockenstein E, Spencer B, Orain XM et al (2017) Synuclein impairs trafficking and signaling of BDNF in a mouse model of Parkinson’s Disease. Sci Rep 7(1):1–13
Cao Q, Luo S, Yao W, Qu Y, Wang N, Hong J et al (2022) Suppression of abnormal α-synuclein expression by activation of BDNF transcription ameliorates Parkinson’s disease-like pathology. Mol Therapy-Nucleic Acids 29:1–15
Kang SS, Zhang Z, Liu X, Manfredsson FP, Benskey MJ, Cao X et al (2017) TrkB neurotrophic activities are blocked by α-synuclein, triggering dopaminergic cell death in Parkinson’s disease. Proceedings of the National Academy of Sciences 114(40):10773-8
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the University of Witten-Herdecke Germany.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
RA, HMA-K and AIA conceptualized the manuscript, wrote, edited and reviewed the main text and approved the final edition of the manuscript. NHA, AA, MP, HMS and GE-SB prepared the figures, wrote, corrected, amended and approved the final edition of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to changes in affiliation of authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
AlRuwaili, R., Al-kuraishy, H.M., Al-Gareeb, A.I. et al. The Possible Role of Brain-derived Neurotrophic Factor in Epilepsy. Neurochem Res 49, 533–547 (2024). https://doi.org/10.1007/s11064-023-04064-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-04064-x