Abstract
Background
Although research continues to elucidate the molecular mechanism underlying pituitary tumor pathogenesis, limited information is available on the potential role and expression profile of β-catenin in functional and non-functional pituitary neuroendocrine tumors (PitNETs).
Methods and results
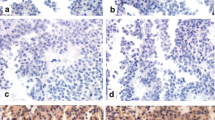
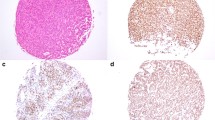
In the current study, 104 pituitary samples (tumors and cadaveric healthy pituitary tissues) were included and the gene and protein expression levels of β-catenin were assessed by Real-Time PCR and immunohistochemistry, respectively. The correlation between expression level of β-catenin and tumor invasive feature and size as well as patient age, gender, and hormonal level was measured. The data showed that PitNET samples expressed higher levels of the β-catenin gene and protein compared to healthy pituitary tissues. Although there was no difference in β-catenin expression level between non-functioning (NF-PitNETs) and growth hormone-producing tumors (GH-PitNETs), both tumor types showed significantly elevated β-catenin levels compared to healthy pituitary tissues. The high level of β-catenin in the invasive functional and non-functional tumors is indicative of the association of β-catenin with PitNETs invasion. The expression pattern of the β-catenin gene and protein was consistently and significantly associated with these tumor types. The correlation between β-catenin and insulin-like growth factor 1 (IGF-1) in GH-PitNETs indicates the potential relevance of β-catenin and IGF-1 for GH-PitNETs.
Conclusions
The simultaneous increase in the expression of β-catenin gene and protein level in PitNET tissues and their relationship to the tumor severity indicates the possible contributing role of β-catenin and its underlying signaling mediators in PitNET pathogenesis.


Similar content being viewed by others
Abbreviations
- GSK-3β:
-
Glycogen synthase kinase-3β
- APC:
-
Adenomatous polyposis coli
- TCF/Lef1:
-
T cell factor/lymphoid enhancer factor-1
- NF-PitNETs:
-
Non-functioning pituitary tumors
- GH- PitNETs:
-
Growth hormone producing pituitary tumors
- ETSS:
-
Endoscopic transnasal transphenoidal surgery
- LMO:
-
Legal Medicine Organization
- GH:
-
Growth hormone
- IGF-1:
-
Insulin-like growth factor 1
- SEM:
-
Standard error mean
- GSK:
-
Glycogen synthase kinase
- EMT:
-
Epithelial-mesenchymal transition.
References
Alatzoglou KS, Gregory LC, Dattani MT (2020) Dev Pituit Gland Compr Physiol 10(2):389–413
Cohen LE, Radovick S (2002) Molecular basis of combined pituitary hormone deficiencies. Endocr Rev 23(4):431–442
Melmed S (2015) Pituitary tumors. Endocrinol Metab Clin North Am 44(1):1–9
Nie D et al (2021) Immune Checkpoints: Therapeutic Targets for Pituitary Tumors Dis Markers, 2021: p. 5300381
Asa SL, Mete O, Ezzat S (2021) Genomics and Epigenomics of Pituitary Tumors: what do pathologists need to know? Endocr Pathol 32(1):3–16
Pai SG et al (2017) Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol 10(1):101
Katoh M, Katoh M (2007) WNT signaling pathway and stem cell signaling network. Clin Cancer Res 13(14):4042–4045
Zhang Y, Wang X (2020) Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol 13(1):165
Valenta T, Hausmann G, Basler K (2012) The many faces and functions of β-catenin. Embo j 31(12):2714–2736
Shang S, Hua F, Hu ZW (2017) The regulation of β-catenin activity and function in cancer: therapeutic opportunities. Oncotarget 8(20):33972–33989
Björklund P, Akerström G, Westin G (2007) Accumulation of nonphosphorylated beta-catenin and c-myc in primary and uremic secondary hyperparathyroid tumors. J Clin Endocrinol Metab 92(1):338–344
Chiang JM et al (2002) Nuclear beta-catenin expression is closely related to ulcerative growth of colorectal carcinoma. Br J Cancer 86(7):1124–1129
Wang W et al (2015) Involvement of Wnt/β-catenin signaling in the mesenchymal stem cells promote metastatic growth and chemoresistance of cholangiocarcinoma. Oncotarget 6(39):42276–42289
Shen T et al (2016) Prognostic value of E-Cadherin and β-Catenin in Triple-Negative breast Cancer. Am J Clin Pathol 146(5):603–610
Lee Y et al (2016) WNT signaling in glioblastoma and therapeutic opportunities. Lab Invest 96(2):137–150
Hoang BH et al (2004) Expression of LDL receptor-related protein 5 (LRP5) as a novel marker for disease progression in high-grade osteosarcoma. Int J Cancer 109(1):106–111
Barooni AB et al (2019) Up-regulation of 15-lipoxygenase enzymes and products in functional and non-functional pituitary adenomas. Lipids Health Dis 18(1):152
Akbari N et al (2020) Cyclooxygenase enzyme and PGE2 expression in patients with functional and non-functional pituitary adenomas. BMC Endocr Disord 20(1):39
Shirian FI et al (2021) Up-regulation of sex-determining region Y-box 9 (SOX9) in growth hormone-secreting pituitary adenomas. BMC Endocr Disord 21(1):50
Wang R et al (2022) Editorial: Refractory Pituitary Adenoma-Current Challenges and emerging treatments. Front Endocrinol (Lausanne) 13:868174
Martin E, Agazie YM (2021) SHP2 potentiates the oncogenic activity of β-Catenin to promote triple-negative breast Cancer. Mol Cancer Res 19(11):1946–1956
Wagstaff M et al (2022) Targeting β-catenin in acute myeloid leukaemia: past, present, and future perspectives. Biosci Rep, 42(4)
Mishra J, Das JK, Kumar N (2017) Janus kinase 3 regulates adherens junctions and epithelial mesenchymal transition through β-catenin. J Biol Chem 292(40):16406–16419
Braeuning A, Pavek P (2020) β-catenin signaling, the constitutive androstane receptor and their mutual interactions Arch Toxicol, 94(12): p. 3983–3991
Semba S et al (2001) Frequent nuclear accumulation of beta-catenin in pituitary adenoma. Cancer 91(1):42–48
Howng S-L et al (2002) Differential expression of wnt genes, β-catenin and E-cadherin in human brain tumors. Cancer Lett 183(1):95–101
Cha KB et al (2004) WNT5A signaling affects pituitary gland shape. Mech Dev 121(2):183–194
Elston MS et al (2008) Wnt pathway inhibitors are strongly down-regulated in pituitary tumors. Endocrinology 149(3):1235–1242
Olson LE et al (2006) Homeodomain-mediated beta-catenin-dependent switching events dictate cell-lineage determination. Cell 125(3):593–605
Buslei R et al (2005) Common mutations of β-catenin in adamantinomatous craniopharyngiomas but not in other tumours originating from the sellar region. Acta Neuropathol 109(6):589–597
Ogasawara N et al (2006) Mutations and nuclear accumulation of beta-catenin correlate with intestinal phenotypic expression in human gastric cancer. Histopathology 49(6):612–621
Chen J et al (2014) Cytoplasmic and/or nuclear expression of β-catenin correlate with poor prognosis and unfavorable clinicopathological factors in hepatocellular carcinoma: a meta-analysis. PLoS ONE 9(11):e111885
Tziortzioti V et al (2001) Analysis of beta-catenin mutations and alpha-, beta-, and gamma-catenin expression in normal and neoplastic human pituitary tissues. Endocr Pathol 12(2):125–136
Taciak B et al (2018) Wnt signaling pathway in development and cancer. J Physiol Pharmacol, 69(2)
Kioussi C et al (2002) Identification of a Wnt/Dvl/beta-Catenin --> Pitx2 pathway mediating cell-type-specific proliferation during development. Cell 111(5):673–685
Kalavalapalli S et al (2007) Silent growth hormone secreting pituitary adenomas: IGF-1 is not sufficient to exclude growth hormone excess. Ann Clin Biochem 44(Pt 1):89–93
Desbois-Mouthon C et al (2001) Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3beta inhibition and ras activation. Oncogene 20(2):252–259
Acknowledgements
We really appreciate all the patients who took part in this survey and provided us with their tissue samples. We also thank Dr. Mohammad E. Khamseh for his scientific assistance.
Funding
This work was financially supported by the Iran University of Medical Sciences (Grant Number: 94-04-30- 27725).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [S. Fahimeh Taghavi], [Mohammad Ghorbani], [Mahshid Panahi] ,[ Shima Nazem], [Milad Karimi], [Vahid Salimi]. The first draft of the manuscript was written by [Masoumeh Tavakoli-Yaraki] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
No conflict of interest is declared by authors.
Ethics approval
The ethics committee of the Vice president of research of Iran University of Medical Sciences with ethics committee code: IR.IUMS.FMD.REC 1396.31841 approved this project ethically. All patients were informed about the project process and they consented to have their tumor tissue collected for the present study with written informed consent. The Legal Medicine Organization (LMO) provided healthy pituitary autopsies for the project with written and signed consent forms obtained from the first-degree relatives of deceased individuals [17]. This study was performed based on the guidelines of Helsinki Declaration.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the data in Table 1; Fig. 2.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taghavi, S.F., Ghorbani, M., Panahi, M. et al. Differential expression levels of β-catenin are associated with invasive behavior of both functional and non-functional pituitary neuroendocrine tumor (PitNET). Mol Biol Rep 50, 6425–6434 (2023). https://doi.org/10.1007/s11033-023-08523-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08523-0