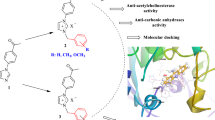
Abstract
To discover alternative substances to compounds used to treat many diseases, especially treating Alzheimer’s disease (AD) and Parkinson’s disease targeting carbonic anhydrase (hCA) and acetylcholinesterase (AChE) enzymes, is important. For this purpose, a series of novel bis-ureido-substituted sulfaguanidine (SG1–4) and sulfisoxazole (SO1–4) derivatives were synthesized, and their inhibitory capacities were screened against hCA isoenzymes (hCA I and II) and AChE. Possible binding mechanisms of inhibitors to the active site were elucidated by in silico studies, and the results were supported by in vitro results. Moreover, the percent radical scavenging capacities of the derivatives were also evaluated. The derivatives (SG1–4 and SO1–4) were more effective against hCAs compared to standard drug acetazolamide (KI values of 98.28–439.17 nM for hCA I and II, respectively) and exhibited the highest inhibition with the KIs in the ranges of 2.54 ± 0.50–41.02 ± 7.52 nM for hCA I, 11.20 ± 2.97–67.14 ± 13.58 nM for hCA II, and 257.60 ± 27.84–442.60 ± 52.13 nM for AChE. Also, compounds SG1 and SO1 also showed ABTS radical scavenging activity at the rate of 70% and 78%, respectively. These results will contribute to the literature for the rational design and synthesis of new potent and selective inhibitors targeting hCAs and AChE with multifunctional effects such as radical scavenging as well as inhibition.
Graphical abstract
This study focused on the synthesis and inhibitory effects of bis-ureido-substituted sulfaguanidine (SG1–4) and sulfisoxazole (SO1–4) derivatives against human hCA I and II isoforms and AChE. In order to test synthesized derivatives’ free radical scavenging potentials were the DPPH and ABTS assays. In silico studies elucidated possible binding mechanisms of inhibitors to the active site.





Similar content being viewed by others
References
Ferreira A, Proença C, Serralheiro M, Araujo M (2006) The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J Ethnopharmacol 108(1):31–37. https://doi.org/10.1016/j.jep.2006.04.010
Heinrich M, Teoh HL (2004) Galanthamine from snowdrop—the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J Ethnopharmacol 92(2–3):147–162. https://doi.org/10.1016/j.jep.2004.02.012
Işık M, Beydemir Ş (2019) AChE mRNA expression as a possible novel biomarker for the diagnosis of coronary artery disease and Alzheimer’s disease, and its association with oxidative stress. Arch Physiol Biochem. https://doi.org/10.1080/13813455.2019.1683584
Voet D, Voet J, John W, Jhon S (1995) Serine proteases. Biochemistry 390:1–7
Tavares L, Fortalezas S, Tyagi M, Barata D, Serra AT, Martins Duarte CM et al (2012) Bioactive compounds from endemic plants of Southwest Portugal: Inhibition of acetylcholinesterase and radical scavenging activities. Pharm Biol 50(2):239–246. https://doi.org/10.3109/13880209.2011.596209
Knapp MJ, Knopman DS, Solomon PR, Pendlebury WW, Davis CS, Gracon SI et al (1994) A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer’s disease. JAMA 271(13):985–991
Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol 8(2):101–112. https://doi.org/10.1038/nrm2101
Stuchbury G, Münch G (2005) Alzheimer’s associated inflammation, potential drug targets and future therapies. J Neural Transm 112(3):429–453. https://doi.org/10.1007/s00702-004-0188-x
Işık M (2019) The binding mechanisms and inhibitory effect of intravenous anesthetics on AChE in vitro and in vivo: kinetic analysis and molecular docking. Neurochem Res 44(9):2147–2155. https://doi.org/10.1007/s11064-019-02852-y
Calabrese V, Scapagnini G, Colombrita C, Ravagna A, Pennisi G, Stella AG et al (2003) Redox regulation of heat shock protein expression in aging and neurodegenerative disorders associated with oxidative stress: a nutritional approach. Amino Acids 25(3–4):437–444. https://doi.org/10.1007/s00726-003-0048-2
Gibson GE, Huang H-M (2005) Oxidative stress in Alzheimer’s disease. Neurobiol Aging 5(26):575–578. https://doi.org/10.1016/j.neurobiolaging.2004.07.017
Işık M, Beydemir Ş, Yılmaz A, Naldan ME, Aslan HE, Gülçin İ (2017) Oxidative stress and mRNA expression of acetylcholinesterase in the leukocytes of ischemic patients. Biomed Pharmacotherapy. https://doi.org/10.1016/j.biopha.2017.01.003
Işık M, Demir Y, Kırıcı M, Demir R, Şimşek F, Beydemir Ş (2015) Changes in the anti-oxidant system in adult epilepsy patients receiving anti-epileptic drugs. Arch Physiol Biochem 121(3):97–102. https://doi.org/10.3109/13813455.2015.1026912
Mossa A, Nawwar G (2011) Free radical scavenging and antiacetylcholinesterase activities of Origanum majorana L. essential oil. Hum Experimental Toxicol 30(10):1501–1513. https://doi.org/10.1177/0960327110391686
Halliwell B, Gutteridge JM (2015) Free radicals in biology and medicine. Oxford University Press, Oxford
Tohma H, Isik M, Korkmaz M, Bursal E, Gulcin I, Koksal E (2015) Determination of antioxidant properties of Gypsophila bitlisensis bark. Int J Pharmacol 100(4):366–437. https://doi.org/10.3923/ijp.2015.366.371
Supuran CT (2008) Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 7(2):168–181. https://doi.org/10.1038/nrd2467
Capasso C, Supuran CT (2015) Bacterial, fungal and protozoan carbonic anhydrases as drug targets. Expert Opin Ther Targets 19(12):1689–1704. https://doi.org/10.1517/14728222.2015.1067685
Sarikaya SBÖ, Topal F, Şentürk M, Gülçin I, Supuran CT (2011) In vitro inhibition of α-carbonic anhydrase isozymes by some phenolic compounds. Bioorg Med Chem Lett 21(14):4259–4262. https://doi.org/10.1016/j.bmcl.2011.05.071
Alterio V, Di Fiore A, D’Ambrosio K, Supuran CT, De Simone G (2012) Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 112(8):4421–4468. https://doi.org/10.1021/cr200176r
Carta F, Scozzafava A, Supuran CT (2012) Sulfonamides: a patent review (2008–2012). Expert Opin Ther Pat 22(7):747–758. https://doi.org/10.1517/13543776.2012.698264
Carta F, Vullo D, Osman SM, AlOthman Z, Supuran CT (2017) Synthesis and carbonic anhydrase inhibition of a series of SLC-0111 analogs. Bioorg Med Chem 25(9):2569–2576. https://doi.org/10.1016/j.bmc.2017.03.027
Akocak S, Alam MR, Shabana AM, Sanku RKK, Vullo D, Thompson H et al (2016) PEGylated bis-sulfonamide carbonic anhydrase inhibitors can efficiently control the growth of several carbonic anhydrase IX-expressing carcinomas. J Med Chem 59(10):5077–5088. https://doi.org/10.1021/acs.jmedchem.6b00492
Akocak S, Lolak N, Nocentini A, Karakoc G, Tufan A, Supuran CT (2017) Synthesis and biological evaluation of novel aromatic and heterocyclic bis-sulfonamide Schiff bases as carbonic anhydrase I, II, VII and IX inhibitors. Bioorg Med Chem 25(12):3093–3097. https://doi.org/10.1016/j.bmc.2017.03.063
Akocak S, Lolak N, Bua S, Nocentini A, Supuran CT (2019) Activation of human α-carbonic anhydrase isoforms I, II, IV and VII with bis-histamine schiff bases and bis-spinaceamine substituted derivatives. J Enzyme Inhib Med Chem 34(1):1193–1198. https://doi.org/10.1080/14756366.2019.1630616
Yapar G, Duran HE, Lolak N, Akocak S, Türkeş C, Durgun M et al (2021) Biological effects of bis-hydrazone compounds bearing isovanillin moiety on the aldose reductase. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2021.105473
Kalaycı M, Türkeş C, Arslan M, Demir Y, Beydemir Ş (2021) Novel benzoic acid derivatives: synthesis and biological evaluation as multi-target acetylcholinesterase and carbonic anhydrase inhibitors. Arch Pharm (Weinheim, Ger) 354(3):e2000282. https://doi.org/10.1002/ardp.202000282
Türkeş C, Demir Y, Beydemir Ş (2021) Calcium channel blockers: molecular docking and inhibition studies on carbonic anhydrase I and II isoenzymes. J Biomol Struct Dyn 39(5):1672–1680. https://doi.org/10.1080/07391102.2020.1736631
Gündoğdu S, Türkeş C, Arslan M, Demir Y, Beydemir Ş (2019) New isoindole-1, 3-dione substituted sulfonamides as potent inhibitors of carbonic anhydrase and acetylcholinesterase: design, synthesis, and biological evaluation. ChemistrySelect 4(45):13347–13355. https://doi.org/10.1002/slct.201903458
Istrefi Q, Türkeş C, Arslan M, Demir Y, Nixha AR, Beydemir Ş et al (2020) Sulfonamides incorporating ketene N, S-acetal bioisosteres as potent carbonic anhydrase and acetylcholinesterase inhibitors. Arch Pharm (Weinheim, Ger) 353(6):e1900383. https://doi.org/10.1002/ardp.201900383
Sever B, Türkeş C, Altıntop MD, Demir Y, Çiftçi GA, Beydemir Ş (2021) Novel metabolic enzyme inhibitors designed through the molecular hybridization of thiazole and pyrazoline scaffolds. Arch Pharm (Weinheim, Ger) 354(12):e2100294. https://doi.org/10.1002/ardp.202100294
Yaşar Ü, Gönül İ, Türkeş C, Demir Y, Beydemir Ş (2021) Transition–metal complexes of bidentate schiff-base ligands: in vitro and in silico evaluation as non-classical carbonic anhydrase and potential acetylcholinesterase inhibitors. ChemistrySelect 29(6):7278–7284. https://doi.org/10.1002/slct.202102082
Lolak N, Akocak S, Türkeş C, Taslimi P, Işık M, Beydemir Ş et al (2020) Synthesis, characterization, inhibition effects, and molecular docking studies as acetylcholinesterase, α-glycosidase, and carbonic anhydrase inhibitors of novel benzenesulfonamides incorporating 1, 3, 5-triazine structural motifs. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2020.103897
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Akocak S, Taslimi P, Lolak N, Işık M, Durgun M, Budak Y et al (2021) Synthesis, characterization, and inhibition study of novel substituted phenylureido sulfaguanidine derivatives as α-glycosidase and cholinesterase inhibitors. Chem Biodivers 18(4):e2000958. https://doi.org/10.1002/cbdv.202000958
Taslimi P, Işık M, Türkan F, Durgun M, Türkeş C, Gülçin İ et al (2021) Benzenesulfonamide derivatives as potent acetylcholinesterase, α-glycosidase, and glutathione S-transferase inhibitors: biological evaluation and molecular docking studies. J Biomol Struct Dyn 39(15):5449–5460. https://doi.org/10.1080/07391102.2020.1790422
Türkeş C, Akocak S, Işık M, Lolak N, Taslimi P, Durgun M et al (2021) Novel inhibitors with sulfamethazine backbone: synthesis and biological study of multi-target cholinesterases and α-glucosidase inhibitors. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2021.1916599
Çalışkan B, Demir Y, Türkeş C (2021) Ophthalmic Drugs: In vitro paraoxonase 1 inhibition and molecular docking studies. Biotechnol Appl Biochem. https://doi.org/10.1002/bab.2284
Tokalı FS, Demir Y, Demircioğlu İH, Türkeş C, Kalay E, Şendil K et al (2022) Synthesis, biological evaluation, and in silico study of novel library sulfonates containing quinazolin-4 (3H)-one derivatives as potential aldose reductase inhibitors. Drug Dev Res 83(3):586–604. https://doi.org/10.1002/ddr.21887
Türkeş C, Demir Y, Beydemir Ş (2021) Infection medications: assessment in-vitro glutathione S-transferase inhibition and molecular docking study. ChemistrySelect 6(43):11915–11924. https://doi.org/10.1002/slct.202103197
Korkmaz IN, Türkeş C, Demir Y, Öztekin A, Özdemir H, Beydemir Ş (2022) Biological evaluation and in silico study of benzohydrazide derivatives as paraoxonase 1 inhibitors. J Biochem Mol Toxicol. https://doi.org/10.1002/jbt.23180
Korkmaz IN, Türkeş C, Demir Y, Özdemir H, Beydemir Ş (2022) Methyl benzoate derivatives: in vitro paraoxonase 1 inhibition and in silico studies. J Biochem Mol Toxicol. https://doi.org/10.1002/jbt.23152
Türkeş C, Demir Y, Beydemir Ş (2019) Anti-diabetic properties of calcium channel blockers: inhibition effects on aldose reductase enzyme activity. Appl Biochem Biotechnol 189(1):318–329. https://doi.org/10.1007/s12010-019-03009-x
Türkeş C, Kesebir Öztürk A, Demir Y, Küfrevioğlu Öİ, Beydemir Ş (2021) Calcium channel blockers: the effect of glutathione S-transferase enzyme activity and molecular docking studies. ChemistrySelect 6(40):11137–11143. https://doi.org/10.1002/slct.202103100
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181(4617):1199–1200. https://doi.org/10.1038/1811199a0
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med 26(9–10):1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Türkeş C, Beydemir Ş, Küfrevioğlu Öİ (2019) In vitro and in silico studies on the toxic effects of antibacterial drugs as human serum paraoxonase 1 inhibitor. ChemistrySelect 4(33):9731–9736. https://doi.org/10.1002/slct.201902424
Beydemir Ş, Türkeş C, Yalçın A (2021) Gadolinium-based contrast agents: in vitro paraoxonase 1 inhibition, in silico studies. Drug Chem Toxicol 44(5):508–517. https://doi.org/10.1080/01480545.2019.1620266
Işık M, Akocak S, Lolak N, Taslimi P, Türkeş C, Gülçin İ et al (2020) Synthesis, characterization, biological evaluation, and in silico studies of novel 1,3-diaryltriazene-substituted sulfathiazole derivatives. Arch Pharm (Weinheim, Ger) 353(9):e2000102. https://doi.org/10.1002/ardp.202000102
Bozdag M, Carta F, Ceruso M, Ferraroni M, McDonald PC, Dedhar S et al (2018) Discovery of 4-hydroxy-3-(3-(phenylureido) benzenesulfonamides as SLC-0111 analogues for the treatment of hypoxic tumors overexpressing carbonic anhydrase IX. J Med Chem 61(14):6328–6338. https://doi.org/10.1021/acs.jmedchem.8b00770
Buemi MR, Di Fiore A, De Luca L, Angeli A, Mancuso F, Ferro S et al (2019) Exploring structural properties of potent human carbonic anhydrase inhibitors bearing a 4-(cycloalkylamino-1-carbonyl) benzenesulfonamide moiety. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2018.11.073
Nachon F, Carletti E, Ronco C, Trovaslet M, Nicolet Y, Jean L et al (2013) Crystal structures of human cholinesterases in complex with huprine W and tacrine: elements of specificity for anti-Alzheimer’s drugs targeting acetyl-and butyryl-cholinesterase. Biochem J 453(3):393–399. https://doi.org/10.1042/BJ20130013
Türkeş C, Beydemir Ş (2020) Inhibition of human serum paraoxonase-I with antimycotic drugs: in vitro and in silico studies. Appl Biochem Biotechnol 190(1):252–269. https://doi.org/10.1007/s12010-019-03073-3
Askin S, Tahtaci H, Türkeş C, Demir Y, Ece A, Çiftçi GA et al (2021) Design, synthesis, characterization, in vitro and in silico evaluation of novel imidazo [2, 1-b][1, 3, 4] thiadiazoles as highly potent acetylcholinesterase and non-classical carbonic anhydrase inhibitors. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2021.105009
Türkeş C, Demir Y, Beydemir Ş (2022) Some calcium-channel blockers: kinetic and in silico studies on paraoxonase-I. J Biomol Struct Dyn 40(1):77–85. https://doi.org/10.1080/07391102.2020.1806927
Türkeş C (2019) Investigation of potential paraoxonase-I inhibitors by kinetic and molecular docking studies: chemotherapeutic drugs. Protein Pept Lett 26(6):392–402. https://doi.org/10.2174/0929866526666190226162225
Demir Y, Türkeş C, Beydemir Ş (2020) Molecular docking studies and inhibition properties of some antineoplastic agents against paraoxonase-I. Anti-Cancer Agents Med Chem 20(7):887–896. https://doi.org/10.2174/1871520620666200218110645
Türkeş C, Arslan M, Demir Y, Cocaj L, Nixha AR, Beydemir Ş (2019) Synthesis, biological evaluation and in silico studies of novel N-substituted phthalazine sulfonamide compounds as potent carbonic anhydrase and acetylcholinesterase inhibitors. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2019.103004
Işık M, Beydemir Ş, Demir Y, Durgun M, Türkeş C, Nasır A et al (2020) Benzenesulfonamide derivatives containing imine and amine groups: Inhibition on human paraoxonase and molecular docking studies. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2019.09.237
Türkeş C (2019) A potential risk factor for paraoxonase 1: in silico and in-vitro analysis of the biological activity of proton-pump inhibitors. J Pharm Pharmacol 71(10):1553–1564. https://doi.org/10.1111/jphp.13141
Kilic A, Beyazsakal L, Işık M, Türkeş C, Necip A, Takım K et al (2020) Mannich reaction derived novel boron complexes with amine-bis(phenolate) ligands: synthesis, spectroscopy and in vitro/in silico biological studies. J Organomet Chem. https://doi.org/10.1016/j.jorganchem.2020.121542
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT et al (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47(7):1739–1749. https://doi.org/10.1021/jm0306430
Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT et al (2004) Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem 47(7):1750–1759. https://doi.org/10.1021/jm030644s
Sever B, Türkeş C, Altıntop MD, Demir Y, Beydemir Ş (2020) Thiazolyl-pyrazoline derivatives: in vitro and in silico evaluation as potential acetylcholinesterase and carbonic anhydrase inhibitors. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2020.09.043
Sever B, Altıntop MD, Demir Y, Türkeş C, Özbaş K, Çiftçi GA et al (2021) A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity. Open Chem. https://doi.org/10.1515/chem-2021-0032
Demir Y, Ceylan H, Türkeş C, Beydemir Ş (2021) Molecular docking and inhibition studies of vulpinic, carnosic and usnic acids on polyol pathway enzymes. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2021.1967195
Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA et al (2006) Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein–ligand complexes. J Med Chem 49(21):6177–6196. https://doi.org/10.1021/jm051256o
Türkeş C, Arslan M, Demir Y, Çoçaj L, Nixha AR, Beydemir Ş (2022) N-substituted phthalazine sulfonamide derivatives as non-classical aldose reductase inhibitors. J Mol Recognit. https://doi.org/10.1002/JMR.2991
Osmaniye D, Türkeş C, Demir Y, Özkay Y, Beydemir Ş, Kaplancıklı ZA (2022) Design, synthesis, and biological activity of novel dithiocarbamate-methylsulfonyl hybrides as carbonic anhydrase inhibitors. Arch Pharm (Weinheim, Ger). https://doi.org/10.1002/ardp.202200132
Güleç Ö, Türkeş C, Arslan M, Demir Y, Yeni Y, Hacımüftüoğlu A et al (2022) Cytotoxic effect, enzyme inhibition, and in silico studies of some novel N-substituted sulfonyl amides incorporating 1,3,4-oxadiazol structural motif. Mol Divers. https://doi.org/10.1007/s11030-022-10422-8
Pacchiano F, Carta F, McDonald PC, Lou Y, Vullo D, Scozzafava A et al (2011) Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem 54(6):1896–1902. https://doi.org/10.1021/jm101541x
Lou Y, McDonald PC, Oloumi A, Chia S, Ostlund C, Ahmadi A et al (2011) Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res 71(9):3364–3376. https://doi.org/10.1158/0008-5472.CAN-10-4261
Supuran CT, Winum J-Y (2015) Designing carbonic anhydrase inhibitors for the treatment of breast cancer. Expert Opin Drug Discov 10(6):591–597. https://doi.org/10.1517/17460441.2015.1038235
Kosak U, Brus B, Knez D, Zakelj S, Trontelj J, Pislar A et al (2018) The magic of crystal structure-based inhibitor optimization: development of a butyrylcholinesterase inhibitor with picomolar affinity and in vivo activity. J Med Chem 61(1):119–139. https://doi.org/10.1021/acs.jmedchem.7b01086
Khanfar MA, AbuKhader MM, Alqtaishat S, Taha MO (2013) Pharmacophore modeling, homology modeling, and in silico screening reveal mammalian target of rapamycin inhibitory activities for sotalol, glyburide, metipranolol, sulfamethizole, glipizide, and pioglitazone. J Mol Graph Model. https://doi.org/10.1016/j.jmgm.2013.02.009
Riaz S, Khan IU, Bajda M, Ashraf M, Shaukat A, Rehman TU et al (2015) Pyridine sulfonamide as a small key organic molecule for the potential treatment of type-II diabetes mellitus and Alzheimer’s disease: in vitro studies against yeast α-glucosidase, acetylcholinesterase and butyrylcholinesterase. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2015.09.008
Supuran CT (2016) Structure and function of carbonic anhydrases. Biochem J 473(14):2023–2032. https://doi.org/10.1042/BCJ20160115
Neri D, Supuran CT (2011) Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 10(10):767–777. https://doi.org/10.1038/nrd3554
De Simone G, Alterio V, Supuran CT (2013) Exploiting the hydrophobic and hydrophilic binding sites for designing carbonic anhydrase inhibitors. Expert Opin Drug Discov 8(7):793–810. https://doi.org/10.1517/17460441.2013.795145
Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, Chaves PH et al (2014) Vitamin D and the risk of dementia and Alzheimer disease. Neurology 83(10):920–928. https://doi.org/10.1212/WNL.0000000000000755
Takeda M, Tanaka T, Okochi M (2011) New drugs for Alzheimer’s disease in Japan. Psychiatry Clin Neurosci 65(5):399–404. https://doi.org/10.1111/j.1440-1819.2011.02253.x
Tugrak M, Gul HI, Demir Y, Levent S, Gulcin I (2021) Synthesis and in vitro carbonic anhydrases and acetylcholinesterase inhibitory activities of novel imidazolinone-based benzenesulfonamides. Arch Pharm (Weinheim, Ger) 354(4):2000375. https://doi.org/10.1002/ardp.202000375
Alyar S, Şen C, Alyar H, Adem Ş, Kalkanci A, Ozdemir UO (2018) Synthesis, characterization, antimicrobial activity, carbonic anhydrase enzyme inhibitor effects, and computational studies on new Schiff bases of Sulfa drugs and their Pd(II), Cu(II) complexes. J Mol Struct. https://doi.org/10.1016/j.molstruc.2018.06.004
Mert S, Alım Z, İşgör MM, Anıl B, Kasımoğulları R, Beydemir Ş (2019) Novel pyrazole-3, 4-dicarboxamides bearing biologically active sulfonamide moiety as potential carbonic anhydrase inhibitors. Arab J Chem 12(8):2740–2748. https://doi.org/10.1016/j.arabjc.2015.05.020
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biol Med 20(7):933–956. https://doi.org/10.1016/0891-5849(95)02227-9
Durgun M, Türkeş C, Işık M, Demir Y, Saklı A, Kuru A et al (2020) Synthesis, characterisation, biological evaluation and in silico studies of sulphonamide Schiff bases. J Enzyme Inhib Med Chem 35(1):950–962. https://doi.org/10.1080/14756366.2020.1746784
Acknowledgements
This work was supported by the Research Fund of Anadolu University (Grant Number 2102S003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lolak, N., Akocak, S., Durgun, M. et al. Novel bis-ureido-substituted sulfaguanidines and sulfisoxazoles as carbonic anhydrase and acetylcholinesterase inhibitors. Mol Divers 27, 1735–1749 (2023). https://doi.org/10.1007/s11030-022-10527-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10527-0