Abstract
Head and neck squamous cell carcinoma (HNSCC) arises from the epithelial lining of the oral cavity, hypopharynx, oropharynx, and larynx. There are several potential risk factors that cause the generation of HNSCC, including cigarette smoking, alcohol consumption, betel quid chewing, inadequate nutrition, poor oral hygiene, HPV and Epstein–Barr virus, and Candida albicans infections. HNSCC has causative links to both environmental factors and genetic mutations, with the latter playing a more critical role in cancer progression. These molecular changes to epithelial cells include the inactivation of cancer suppressor genes and proto-oncogenes overexpression, resulting in tumour cell proliferation and distant metastasis. HNSCC patients have impaired dendritic cell (DC) and natural killer (NK) cell functions, increased production of higher immune-suppressive molecules, loss of regulatory T cells and co-stimulatory molecules and major histocompatibility complex (MHC) class Ι molecules, lower number of lymphocyte subsets, and a poor response to antigen-presenting cells. At present, the standard treatment modalities for HNSCC patients include surgery, chemotherapy and radiotherapy, and combinatorial therapy. Despite advances in the development of novel treatment modalities over the last few decades, survival rates of HNSCC patients have not increased. To establish effective immunotherapies, a greater understanding of interactions between the immune system and HNSCC is required, and there is a particular need to develop novel therapeutic options. A therapeutic cancer vaccine has been proposed as a promising method to improve outcome by inducing a powerful adaptive immune response that leads to cancer cell elimination. Compared with other vaccines, peptide cancer vaccines are more robust and specific. In the past few years, there have been remarkable achievements in peptide-based vaccines for HNSCC patients. Here, we summarize the latest molecular alterations in HNSCC, explore the immune response to HNSCC, and discuss the latest developments in peptide-based cancer vaccine strategies. This review highlights areas for valuable future research focusing on peptide-based cancer vaccines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tumours of the head and neck are amongst the six most common cancers worldwide, with approximately 377,700 new cancer cases and 177,800 cancer deaths in 2020 (Ferlay et al. 2015; Sung et al. 2021). In the United States in 2021, it is predicted that 54,010 new cases of head and neck cancer will be diagnosed, with approximately 10,850 deaths related to these diseases (Siegel et al. 2021). More than 65% of these cancers occur in high risk geographical area include France, Eastern Europe, Brazil, Uruguay and some South Asia countries such as Taiwan, India and Pakistan (Warnakulasuriya 2009).
Although the incidence rate was slightly decreased in the United States and Canada due to reduced cigarette consumption and alcohol use, with the increased human papillomavirus (HPV) infection, the incidence and mortality rates of head and neck cancer are increasing in Taiwan and some European countries (Sturgis and Cinciripini 2007; Hwang et al. 2015; Simard et al. 2014). The incidence and mortality rates are vary from gender, with the incidence and mortality rates of males generally 2:1 higher occurrence than female in most countries (Syrjanen 2005). Despite advances in the diagnosis, surgical techniques, chemotherapy and radiotherapy, the 5-year survival rates of head and neck cancer throughout the world have not improved in the past 30 years. This is mainly owing to cancer recurrence, distant metastases, progression of second primary cancers and resistance to chemo-radiotherapy (Gilbert et al. 2013; Bonner et al. 2010; Schuler et al. 2014). These figures show that head and neck tumours continue to pose a serious threat to public health.
Head and neck squamous cell carcinoma (HNSCC), which arises from the epithelial linings of the oral cavity, hypopharynx, oropharynx and larynx, account for over 90% of head and neck cancers (Tuttle et al. 2016; Mehanna 2010; Shibata et al. 2021; Johnson et al. 2020). There are various forms of HNSCC, including oral squamous cell carcinoma (OSCC), oropharyngeal squamous cell carcinomas (OPSCC), laryngeal squamous cell carcinoma (LSCC) and hypopharyngeal squamous cell carcinoma (HSCC). These various forms of HNSCC have diverse molecular characteristics, clinical progressions, treatment methods and results (Mao et al. 2004). OSCC is one of major types of HNSCC, accounting for more than half of HNSCC, which develops in the squamous epithelium of the lip or oral cavity (Rivera 2015; Noorlag et al. 2015). Most OSCC (60%) has a poor prognosis, as they are detected at stages III and IV, and the 5-year survival rate is only 30% (Omar 2015). OPSCC occur on the crypt epithelium of the palatine, lingual tonsils and posterior pharyngeal wall (Monsjou et al. 2010). Currently, the incidence rates of OPSCC is increasing and it is estimated that these will continue to rise in many countries (Rodrigo et al. 2014; Rietbergen et al. 2013).
In this review, the risk factors, stages, molecular alterations and the immune response to HNSCC will be systematically reviewed. Additionally, the latest developments in peptide-based cancer vaccine strategies are discussed.
Risk Factors Associated with HNSCC
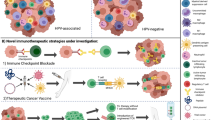
There are several potential risk factors that are associated with the generation of HNSCC, including cigarette smoking, alcohol consumption, betel quid chewing, inadequate nutrition, poor oral hygiene, HPV and Epstein–Barr virus and Candida albicans infections (Johnson et al. 2020; Canning, et al. 2019) (Fig. 1).
People with a long history of tobacco smoking and alcohol drinking frequently develop HNSCC. Approximately 80% of HNSCC patients were correlated with an increased risk due to tobacco smoking or alcohol drinking (Vigneswaran and Williams 2014). The carcinogenic influence of cigarette smoking has been recognised as the primary cause of various types of cancers, including HNSCC. The various types of tobacco (including electronic cigarette and water pipe smoking) all have detrimental, long-term effects on health, particularly in the region of head and neck (McQueen et al. 2016; Munshi et al. 2015).
There has been an increase in the incidence of OPSCC during the last three decades, especially in younger patients, which is strongly correlated with increased risk of HPV (Nasman et al. 2015; Mehanna et al. 2013). It has been reported that the incidence of HPV-positive OPSCC increased in the USA from less than 20% to 80% in the 2000s (Fakhry and D’Souza 2013). There are over 200 serotypes of HPV and 15 of them are closely associated with HNSCC (Marur et al. 2010). In one study, scientists found that more than 90% of HPV positive OPSCC patients tested positive for HPV16 serotype infection (D'Souza et al. 2007). The mechanistic link between HPV infection and increased risk of OPSCC development is related to the E6 and E7 oncoproteins. The E6 and E7 proteins are viral oncoproteins that accelerate the degradation of the tumour suppressor protein p53 and retinoblastoma protein (pRb), resulting in uncontrolled cancer cell growth and contributing to oncogenesis (Münger and Howley 2002; Estêvão et al. 2019). These proteins also have an important effect on cell transformation and immortalisation (Ruttkay-Nedecky et al. 2013). E7 tends to increase the expression of the Rad51 protein, which inhibits homologous recombination of DNA double strand breaks, resulting in further DNA damage and genomic instability (Park et al. 2014; Klein 2008). More recently, HPV-associated OPSCC is considered as a distinct subgroup of HNSCC entity due to its characteristic molecular alterations and therapeutic responses (Vigneswaran and Williams 2014). However, the mechanisms and disease progression of OPSCC HPV infection is still not fully understood nor is the best clinical therapy for this disease clear (Marur et al. 2010).
The role of HPV infection in OSCC development is unclear (Hubbers and Akgul 2015). In China, research has indicated that HPV infection may increase the risk of OSCC carcinogenesis (Zhou et al. 2015). High-risk HPV serotypes, especially HPV16, is positively correlated with OSCC tumourigenesis in young patients (Kaminagakura et al. 2012). Oral tongue squamous cell carcinoma (SCC) was also closely linked with HPV infection (Elango et al. 2011). In contrast, research in Japan and southern Germany did not find a relationship between HPV infection and OSCC patients (Rushatamukayanunt et al. 2014; Reuschenbach et al. 2013). Interestingly, some research found that by increasing the expression of miR-20a, a small RNA involved in cancer development and carcinogenesis, HPV-16 E7 protein may inhibit the development of OSCC (Hu et al. 2016). Further investigations are required to define the relationship between HPV infection and OSCC progression.
Infection with pathogens other than HPV is also correlated with the development of HNSCC. Candida albicans is the most common fungal pathogen in the oral cavity and is also closely correlated with the development of oral cancer. It has been suggested that oral Candida colonisation may be correlated with oral cancer development (Alnuaimi et al. 2015). Infection with Epstein–Barr virus has also been shown to be significantly associated with the carcinogenesis of nasopharyngeal carcinoma, NPC (Hettmann 2015).
Poor oral hygiene and inadequate dental care, such as irregular dental visits and not brushing teeth, play a key role in the occurrence of HNSCC, particularly when combined with cigarette smoking and alcohol consumption (Chang et al. 2013). Betel quid chewing is positively associated with oral cancer development. With or without tobacco consumption, betel quid has been shown as an independent risk factor for HNSCC (Amtha et al. 2014; Guha et al. 2014). Betel quid chewing is the main risk factor for HNSCC in an East Asian population (Lee et al. 2019). Approximately 50% of HNSCC patients are malnourished when they were diagnosed. Nutritional deficiencies, such as a lack of minerals and vitamins, might hasten the development of oral cancer. Consequently, in order to obtain optimal treatment outcomes proper nutrition should be provided to patients in addition to standard cancer treatment methodologies (Alshadwi et al. 2013). This is supported by reports that increased fruit and vegetable consumption may reduce the risk of oral cancer (Pavia et al. 2006).
Stages of HNSCC Development
In order to assess prognosis and deliver optimal treatment, it is critical for clinicians to accurately determine the stage of tumour in HNSCC patients. From 1943 to 1952, Pierre Denoix first developed the tumour–node–metastasis (TNM) system to define the stage of malignant tumours (Rutkowski 2014). This system is dependent on three fundamental anatomical spread indicators, including the extent of primary lesion (T), regional lymph nodal spread (N) as well as distant metastasis (M) (Mao et al. 2004). It is generally accepted that HNSCC have four stages: stages I and II (early HNSCC), stages III and IV (locally advanced HNSCC) and recurrent or distant metastasis. When people are first diagnosed with head and neck cancer, more than half of them are at stage III or IV, resulting in low 5-year survival rates (Kao and Lim 2015). Although the TNM staging system is already in its 7th iteration and is widely used around the world in clinical and research settings, the current TNM system for HNSCC does not include patient prognostic factors, biological markers and molecular characteristics (Schroeff and Baatenburg de Jong 2009; Patel and Shah 2005). Therefore, a more comprehensive and detailed staging system is required.
Molecular Alterations of HNSCC
HNSCC are caused by both environmental factors and genetic mutations, with the latter playing a more critical role in cancer progression. These molecular changes to DNA include inactivation of cancer suppressor genes and overexpression of proto-oncogenes, resulting in tumour cell proliferation and distant metastasis (Acin et al. 2011; Alsahafi et al. 2019).
Alterations in p53 Expression
Mutations in the p53 tumour suppressor gene can be detected in over half of HNSCC patients (Sittichai 2013). p53 is a transcription factor that inhibits cancer growth and can arrest cell cycle and induce cell apoptosis (Perri et al. 2015a). The p53 mutations impair the ability of the cell to regulate the cell cycle and activate cell death. Mutations in p53 also render the cell unable to terminate DNA replication, which results in uncontrolled growth of cells and consequently the DNA mutation accumulation (Perri et al. 2015a). In addition, p53 mutations are highly linked with worse prognosis in HNSCC patients (Acin et al. 2011). HNSCC patients with p53 mutations respond poorly to traditional therapies, including radiotherapy and chemotherapy, mainly due to p53 alterations suppressing E6 and E7 protein expression (Perri et al. 2015a; Ziemann et al. 2015). A recent study by Amit showed that p53 status was closely linked with nerve density and can promote tumour growth. Loss of p53 stimulates neuron reprogramming in HNSCC (Amit et al. 2020). Therefore, targeting p53 may be an effective anticancer therapy for HNSCC (Amit et al. 2020; Stransky et al. 2011).
Alterations in Angiogenesis
Angiogenesis (blood vessel formation) is the process of vascular network growth and expansion, and is the foundation for the uncontrolled cell proliferation and metastasis of tumours (Guerra 2021). The process of angiogenesis is governed by several different growth factors including vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) (Guerra 2021; Vassilakopoulou et al. 2015; Costache et al. 2015). Impeding angiogenesis has been proposed as an efficient way to control head and neck tumours (Calixto et al. 2014). The most crucial element for blood vessel differentiation and development is the VEGF family and their receptors. More than 90% of HNSCC patients express high levels of VEGF (Costache et al. 2015). Overexpression of VEGF results in uncontrolled cell proliferation and increases radio-resistance. Consequently, the amount of VEGF expression can be considered an indicator of HNSCC, and the subsequent diagnoses, treatment, and prognoses (Costache et al. 2015; Hsu et al. 2014). VEGF significantly inhibits the maturation and differentiation of dendritic cells (DCs) from hemopoietic progenitor cells (HPC) and inhibits the growth of many other hematopoietic lineages, such as macrophages (Gabrilovich et al. 1998). This inhibition is attributed to the stimulation of nuclear factor κB (NF-κB), which is a transcription factor that regulates many immune responses, such as cytokines and immune cell growth factors (Gabrilovich et al. 1998). Immature DCs contribute to immunosuppression through inducing differentiation of regulatory T cells (Tregs) and suppressing CD8+ T cell response, resulting in inhibition of T cell function (Huang et al. 2006; Almand et al. 2001). Moreover, HNSCC highly express several VEGFs on the vascular endothelium, such as VEGF-A, VEGF-C and VEGF-D, and are also positively correlated with lymph node metastasis of OSCC (Karatzanis et al. 2012).
Another important factor that contributes to HNSCC tumourigenesis and progression is the EGFR, which is greatly expressed in most HNSCC patients (Perri et al. 2015b). Like VEGF, high levels of EGFR are strongly correlated with poor response to treatment, and a high rate of HNSCC recurrence (Rabinowits and Haddad 2012; Machiels and Schmitz 2015). Moreover, EGFR may promote angiogenesis in HNSCC patients through the hypoxia-inducing factor-1α (HIF-1α) and Notch1 signalling pathways. HIF-1α is a significant molecular mediator for blood vessel formation and the interaction with Notch1 can affect blood vessel formation (Wang et al. 2013). The mechanism by which EGFR influences angiogenesis in HNSCC patients through these pathways is not fully understood. As the suppression of HIF-1α and Notch1 pathways may provide a promising and new therapy options for HNSCC further studies about these mechanisms are necessary (Wang 2015a).
Alterations in Telomerase
Telomerase has a significant influence on cellular immortality and tumourigenesis, as a result, telomerase activity is a tumour diagnostic marker (Chen and Chen 2011). Normal somatic cells do not express telomerase, however, high telomerase activity is found in the majority of human carcinomas (Ruttkay-Nedecky et al. 2013; Takahashi et al. 2014). Telomerase activation is tightly associated with poor clinical outcome in locally advanced cancer (Chen and Chen 2011). The activity of telomerase is strongly correlated with human telomerase reverse transcriptase (hTERT) expression, which plays a key role in unlimited cell proliferation (Takenaka and Sato 2015). In OSCC cells, the level of hTERT was substantially higher than normal oral epithelium cells (Takenaka and Sato 2015). Therefore, increased telomerase activity may contribute to the occurrence of head and neck malignancy (Takahashi et al. 2014; Yuji et al. 2015). However, the mechanism of activation of telomerase is still unclear.
Other Molecular Interactions
Apart from these factors, a variety of other molecular changes have been studied in the growth and development of HNSCC. High levels of signal transducers and activators of transcription (STATs) expression can be detected in the majority of HNSCC patients (Vangara and Grandis 2014). STATs, a family of cytoplasmic proteins, are responsible for signal transduction in the normal cell response to growth factors such as fibroblast growth factor and the cytokine receptor-kinase complex. Activation of STATs, especially STAT3 in cancer cells, could inhibit tumour cells apoptosis and promote cancel cell proliferation (Mali 2015). STAT3 can indirectly induce the expression of VEGF by upregulating the levels of HIF-1α, which contributes to tumour angiogenesis (Vangara and Grandis 2014; Mali 2015). As a result, the levels of STAT3 provide a novel clinical outcome factor for HNSCC patients (Masuda et al. 2002).
STAT3 also plays a key role in immune system evasion through inducing increased expression of matrix metalloproteinases (MMPs) (Mali 2015). High expressions of MMPs have been observed in OSCC patients. MMPs are a family of zinc-dependent endopeptidases that degrade the basement membranes (BM) and the extracellular matrix (ECM) (Vilen 2013). Overexpression of MMPs results in the degradation of the ECM. MMPs also release numerous substances such as cytokines from their cryptic locations. By influencing angiogenesis, proliferation, and invasion, these factors affect cell behaviour and aid cancer growth (Vilen 2013; Liu 2012).
Aberrant expression of proteins that regulate the cell cycle is commonly associated with tumourigenesis. Cyclin D1 is overexpressed during OSCC, and is a prognostic factor for OSCC patients (Hanken et al. 2014). Cyclin D1, a 45-kDa protein encoded on chromosome 11q13, is known to regulate the transition of the cell cycle from G1 phase to S phase, giving rise to cellular replication (Miyamoto et al. 2003). Increased abnormal cell proliferation is a key indicator for the aggressiveness of tumour. Overexpression of cyclin D1 in OSCC patients is closely related to worse clinical outcome and lymph node metastasis (Miyamoto et al. 2003). Similarly, overexpression of Cdk6 was also observed in more than 90% of HNSCC patients. Cdk6 is a cyclin-dependent kinase, an important regulator that drives the cell cycle transition from G1 stage to S stage, a process which plays a vital role in enhancing cell proliferation (Poomsawat et al. 2016). High expression of Cdk6 is an unfavourable factor for cancer patients, as it is indicative of an advanced tumour status (Poomsawat et al. 2016). Research has demonstrated that decreased Cdk6 expression may inhibit the development of OSCC, and that consequently Cdk6 might be regarded as a therapeutic target in this tumour (Shao et al. 2013).
Yet another cell-cycle protein that is important for tumourigenesis is p16 (Patel et al. 2020). p16 is a tumour suppressor gene and cyclin-dependent kinase 4 (CDK4) inhibitor, and normally functions as a checkpoint regulator of G1/S cell cycle stage (Thomas and Primeaux 2012). In normal cells it is expressed at a very low level. However, it highly expressed in HNSCC patients, indicating that a lower local recurrence and increased survival rates (Thomas and Primeaux 2012; Cai et al. 2014). Increased Cyclooxygenase-2 (COX-2) expression has been correlated to the development of many cancers, including HNSCC (Lee et al. 2002). COX-2 is one of the enzymes that participates in the synthesis of prostaglandins (PGs). COX-2 expression is generally undetectable in normal cells (Zhang et al. 2020). However, its expression can be induced by inflammatory cytokines and growth factors. Higher expression of COX-2 is correlated with the development of HNSCC invasion, metastasis, and recurrence (Eun-Young et al. 2015; Celenk et al. 2013; Morita et al. 2012). Increased expression of COX-2 promotes the synthesis of prostaglandins, enhances cancer cell proliferation, and reinforces angiogenesis (Zhu et al. 2020). Consequently, COX-2 inhibition may be a novel strategy to treat HNSCC.
The Immune Response to HNSCC
The host immune system is closely correlated with the progression, growth, and metastasis of HNSCC (Ferris 2015). Although there have been recent advances in the development of novel cancer therapies, including anti-tumour vaccines, the results have not yet been developed into treatments that increase the survival rate of HNSCC patients. As a result, in order to establish effective immunotherapies, a better understanding of interactions between the immune system and HNSCC is required (Freiser et al. 2013). HNSCC patients have impaired DC and natural killer (NK) cell functions, higher immune-suppressive molecules, and regulatory T cells. There is also a loss of co-stimulatory molecules and MHC class Ι molecules, lower number of lymphocyte cell subsets and a poor response to antigen-presenting cells, APCs (Sittichai 2013; Kuss et al. 2004; Dasgupta et al. 2005; Bandoh et al. 2010; Strauss et al. 2007; Whiteside 2005). Immune evasion plays a key role in the progression of HNSCC, giving rise to the loss of anti-tumour immunity (Whiteside 2005).
Dendritic Cells in HNSCC
The most robust and effective APCs are DCs, which are crucial in anti-tumour immunity (Wang et al. 2020). DCs serve as a modulator between innate and adaptive immunity, triggering T cells activation and differentiation. Through the pattern recognition receptors (PRR), immature DCs can recognise a variety of antigens which induce DCs maturation and proliferation (Wculek et al. 2020). Moreover, antigens can be captured and processed by DCs and present them to naïve T cells (as peptides bound on the MHC), which stimulates T cell response (Wang et al. 2020; Wculek et al. 2020).
Developmental defects in systemic DCs result in decreased numbers of mature DC and an increase of immature DC (Almand et al. 2000). Mature DCs present antigens to T cells, which initiates the adaptive immune response. In the peripheral circulation, the majority of DCs are immature. Immature DCs cannot express normal amounts of MHC complex and co-stimulatory molecules, such as CD86, on their surface. Consequently, they have poor capability to uptake antigen and trigger T cells activation, giving rise to the inhibition of antigen-specific immune responses (Groux et al. 2004). Immature DCs may establish immune tolerance through the up-regulation of regulatory T cells (Zhou et al. 2013). Moreover, immature DCs cannot generate some inflammatory cytokines, such as IL-6 and IL-12 (Yoo and Ha 2016). Furthermore, CD4+ T cells from patients with HNSCC have low expression of co-stimulatory signals OX40 and 4-1BB. CD8+ T cells have higher production of the co-inhibitory receptor PD-1, which may significantly contribute to immune evasion (Baruah et al. 2012). This is because OX40 and 4-1BB are positive factors that prime T cell activation and PD-1 could limit T cell response (Baruah et al. 2012).
Compared with normal healthy cells, HNSCC cells do not constitutively express the human leukocyte antigen (HLA) class Ι molecules and lack antigen-processing machinery (APM) (Ferris 2015). HLA is one of the highly polymorphic genes located on human genome 6, encoding proteins on cell surface, which present processed antigen peptides to CD8+ T lymphocytes. APM cooperates with HLA-I in processing and presenting antigenic peptides, and plays a central role in regulate immune response (Ferris et al. 2006). Normal function of HLA and APM is to mediate cellular and humoral immunity. The lack of expression of both HLA and APM result in the host immune system failing to recognise and eliminate the tumour cells (Ferris 2015; Ferris et al. 2006; Hickey et al. 2016).
Lymphocytes in HNSCC
Another important strategy for the immune evasion of cancer cells is functional defects and apoptosis of T lymphocytes. Compared with healthy people, the level of mature CD4+ and CD8+ T cell subsets in circulation is greatly lower in the peripheral blood of HNSCC patients, which can lead to cancer recurrence or a secondary tumours (Kuss et al. 2004). Furthermore, T cell apoptosis may further reduce the number of T lymphocytes in HNSCC patients (Turksma et al. 2013). Immune dysfunctions of T cells have been reported to be associated with decreased expression of ζ chain in tumour patients, a signalling molecule in T lymphocytes that correlates with the T cell receptor (TCR)-CD3 complex (Whiteside 2004).
Tumour immune evasion can also be achieved through the actions of Tregs. Tregs are CD4+CD25+FoxP3+, accounting for approximately 4% of all peripheral blood CD4+ T cells in healthy people, playing a significant role in regulating immune system (Gasparoto, et al. xxxx). The participation of Tregs in immune responses is directed against cancer cells, pathogens and self-antigens. This may down-regulate NK cytotoxicity functions, DCs functions and cytotoxic T-lymphocyte (CTL) activity, leading to antitumour immune response (Ferris 2015). The percentage of Tregs in the peripheral circulation and cancer microenvironment is significantly higher in HNSCC patients compared with healthy humans (Schaefer et al. 2005). Nevertheless, it is still unclear that whether Tregs arise from tumour microenvironment or if they play a systemic immunity role (Elkord et al. 2010). Recent research has also shown that cancer-associated fibroblasts (CAFs) play a role in evasion from immune surveillance by blocking T cell proliferation, and inducing apoptosis of T cells (Takahashi et al. 2015). CAFs are one of the most abundant cell types in the cancer stroma, and are involved in cancer progression and invasion (Wang 2015b). As a result, the cancer cells can evade the host immune system and grow progressively (Schreiber et al. 2011).
Cytokines in HNSCC
HNSCC cells secrete cytokines and small molecular weight signalling molecules such as transforming growth factor-β (TGF-β), prostaglandin E2 (PGE2), COX-2, VEGF, interleukin (IL)-6 and interleukin (IL)-10, all of which are closely correlated with immunosuppression (Ferris 2015).
Overexpression of immunosuppressive cytokines such as TGF-β and IL-10 by tumour-associated macrophages (TAMs) systemically, which are commonly found in OSCC patients, significantly contributes to local immunosuppression and tumour development (Costa et al. 2013). TGF-β may inhibit the function of the CTLs and T-helper type 1 (Th1) cells, as well as blocking the migration of DCs to lymph nodes (Weber et al. 2005; Zamarron and Chen 2011; Moutsopoulos et al. 2008). In addition, TGF-β may affect the differentiation and maturation of B cells (Lebman and Edmiston 1999). TGF-β can increase the production of IL-10, which has been suggested to inhibit DCs development and MHC class I expression, affecting T cell activation (Lippitz 2013). VEGF inhibit the maturation of DCs in the tumour microenvironment, leading to immune dysfunction (Johnson et al. 2007). High levels of PGE2 can be found in many epithelial cancers, which is induced by COX-2 (Whiteside 2014). PGE2 plays a critical role in tumour evasion, inhibiting the proliferation of T cells and B cells, and the cytotoxic function of NK cells (Camacho et al. 2008). IL-6 is a pleiotropic cytokine and generated by several cells such as macrophages, B and T cells and cancer cells. High expression of IL-6 has been closely related to cancer metastasis and worse prognosis (**no et al. 2015). The differentiation and maturation of DCs are blocked by IL-6 via STAT3 activation, which can result in immunosuppression (Park, et al. 1950).
High levels of programmed death 1 (PD-1): PD ligand 1(PD-L1) expression can be found in HPV-infected HNSCC patients, which plays a role in immune resistance (Zandberg and Strome 2014). PD-1 is an immune checkpoint receptor and a significant co-inhibitory receptor of the CD28 family. It is expressed on activated T and B cells, DCs, NK cells and monocytes, has a negative effect on activation of T lymphocytes (Lyford-Pike et al. 2013). PD-L1 can be found on the cell surface of cancer cells. The cooperation of PD-1 and its ligand PD-L1 is likely to suppress T cell activation and proliferation, leading to T cells dysfunction and an immunosuppressive microenvironment (Baruah et al. 2012; Zandberg and Strome 2014; Lyford-Pike et al. 2013; Keir et al. 2008). Bu et al. showed that in HNSCC patients, STAT3 signalling induces immunosuppression through upregulating the expression of PD-1/PD-L1 (Bu et al. 2017). Recent studies have found that targeting PD-1/PD-L1 pathway may be an effective method in cancer immunotherapy (Constantinidou et al. 2019; Ju et al. 2020), especially when combined with other therapies (Qiao, et al. 2020). However, the efficacy of PD-1/PD-L1 monotherapy varies substantially depending on the tumour type and PD-L1 expression (Zhao et al. 2020).
The Traditional Treatment of HNSCC
At present the standard treatment modalities for HNSCC patients include surgery, chemotherapy, and radiotherapy. Surgery and radiotherapy are considered as most common treatment methods for HNSCC patients (Argiris et al. 2008). In general, the treatment decision is not only determined by TNM stage, but also by the patient’s health condition, tumour size, nutritional status, the availability of resources and prognosis following treatment. The goal of HNSCC treatment is to cure the cancer, acquire organ preservation, and improve patient quality of life (Rivera 2015; Yao et al. 2007).
Surgery
For HNSCC patients, surgery is the primary and standard treatment (Swiecicki et al. 2016; Bessell, et al. xxxx). Nearly 30% of HNSCC patients are diagnosed at stages I or II, will be treated with surgery and/or radiotherapy, resulting in a 85% probability of survival of two years or longer (Gilbert et al. 2013). These treatments are, however, not effective for locally advanced cancer. Treatment for stages III and IV HNSCC usually use a combination of surgery, radiotherapy, and chemotherapy. Unfortunately, only 50% of stage III and IV HNSCC patients will survive for 2 years following treatment (Güneri and Epstein 2014). At present, open surgery and transoral resections are common surgical approaches used by doctors. Transoral resection patients recover quickly and the surgery is economical (Kwong et al. 2015). More recently, there is significant interest in transoral robotic surgery (TORS), which could improve visualization in the oropharyngeal and laryngeal cancer, can better retain speech and swallowing functions of patients. Nevertheless the long term effects is unclear and need to be determined (Kwong et al. 2015; Meulemans et al. 2015).
Radiotherapy
In order to acquire organ preservation and reinforce anti-tumour effects radiotherapy is an important primary or adjuvant approach for HNSCC patients, and is often combined with chemotherapeutic and /or targeted agents (Gilbert et al. 2013; Takahashi et al. 2014). For early stage of HNSCC, radiotherapy is often used as adjuvant treatment, with the intention to reduce side effects such as mucosal and bone necrosis (Kademani 2007). There have been some technical advances in radiotherapy during the last few decades, which may prolong the survival rate of patients and improve their quality of life (Gregoire et al. 2015). Intensity-modulated radiation therapy could substantially decrease the occurrence of grade 2–4 xerostomia, indicating this technique could be used as a standard treatment for HNSCC patients (Marta et al. 2014). Moreover, this therapy can also reduce the risk of swallowing dysfunction (Laan et al. 2013). However, this treatment has significant patient side effects such as acute and late toxicities and radio-resistance which still need to be addressed (Clement-Colmou et al. 2015). Recently, through preventing DNA repair, OBP-301 (a telomerase-specific oncolytic adenovirus) when combined with radiotherapy seems to prolong survival in HNSCC patients and overcome radio-resistance, providing a new strategy for these patients (Takahashi et al. 2014).
Chemotherapy
Chemotherapy is classified as an adjuvant treatment, and is frequently used for patients with tumour distant metastases and local recurrence with a low efficacy (Pignon et al. 2009). Chemotherapy can be divided into induction, concomitant, or adjuvant chemotherapy, and the effect of concomitant chemotherapy was notably greater than induction and adjuvant chemotherapy. The benefit of chemotherapy decreases with age, as patients of 71 years and older did not receive any treatment benefit (Pignon et al. 2009; Pignon et al. 2007). Chemo-radiotherapy combined with induction chemotherapy can be used to support organ preservation and decrease the incidence of distant metastasis (Haddad et al. 2013). Research found neoadjuvant chemotherapy can reduce tumour size in HNSCC patients with locally unresectable cancer. Further research, including clinical trials, are required to fully investigate these treatment modalities (Vishak et al. 2015).
Despite improvements in these treatments the prognosis for HNSCC patients is still poor, and the available treatments often have serious side effects. Surgery removal of cancer from patients fails to achieve organ preservation, preventing cancer spread and recurrence, and can be disfiguring. The treatment of both surgery and radiotherapy might impair the patient’s ability to speak, eat, and other regional functions (Furness, et al. xxxx). Significant swallowing dysfunction is common in patients after intensive radiotherapy and chemotherapy (Eisbruch et al. 2002). The poor prognosis of HNSCC patients is due to limited treatment modalities. In recent years the knowledge concerning HNSCC biology and the role of immune escape has expand significantly (Huang et al. 2021; Tinhofer et al. 2020). As a result, the development of novel and promising therapeutic immune approaches is crucial for HNSCC patients.
Therapeutic Cancer Peptide Vaccines
The development of vaccines is one of the most promising and cost-effective way to protect people from infectious diseases and cancers, leading to eradication of diseases such as smallpox (Purcell et al. 2007). There are two kinds of cancer vaccines: prophylactic (disease preventative) and therapeutic (disease treatment). The former could induce humoral immune response and protect people from tumour development, while the latter (Fig. 2) attempts to treat an existing tumour by eliciting a potent cell-mediated immune response against tumour associated-antigens (TAA), and consequently reversing immune evasion (Wierzbicka et al. 2014).
Therapeutic cancer peptide vaccines. The aim of cancer vaccines is to stimulate the body’s immune system to cure cancer and prevent them from spreading. After vaccine uptake, antigens will be captured and processed by antigen presenting cells. Then, antigen will be delivered through TCR/MHC complex to CD8+ T cells. CD8+ T cells could differentiate into cytotoxic T cells, which are able to directly kill cancer cells. TCR T cell receptor, MHC major histocompatibility complex
A therapeutic cancer vaccine has been suggested as a promising strategy to improve outcome by eliciting a potent adaptive immune response, giving rise to the eradication of cancer cells (Wierzbicka et al. 2014). The aim of therapeutic vaccine development is to use the patient’s own immune system to recognize and kill tumour cells (Wierzbicka et al. 2014). Compared with conventional treatment strategy, these approaches have several advantages. They can specifically target cancer cells, with minimal harm to normal cells. Additionally, they could stimulate systemic anti-tumour immunity. A long-lasting memory immune response could be induced, which provides continuing protection from tumour recurrences (Krishnamachari et al. 2011).
Current research includes virus-modified tumour vaccines, DC-based vaccines, DNA vaccines, protein vaccines, peptide-based vaccines and combined strategies (Shibata et al. 2021). All of these demonstrate encouraging outcomes for immunotherapies. Peptide-based vaccines are one of the most common strategies for cancer vaccination (Shibata et al. 2021; Bezu 2018). These vaccines generally deliver the MHC class I restricted peptide epitopes, which come from shared TAAs, with the intention to activate cancer specific CD8+ T cells (Calvo Tardón et al. 2019; Stephens et al. 2021). Compared with classic vaccines, there are several advantages of peptide vaccines (Skwarczynski and Toth 2016). Peptides don not have cytotoxicity to mammalian cells and are unlikely to induce allergic or autoimmune responses (Li et al. 2017; Li et al. 2017). With the development of solid phase peptide synthesis (SPPS) and SPOT synthesis, peptides are relatively simple to produce and are cost-effective, allowing production to be scaled up as required (Gaglione et al. 2019). The purity of peptide can be assessed by techniques such as mass spectrometry, and peptide stability can be demonstrated by standard physicochemical characterisation (Li et al. 2020; Li et al. 2021a). Additionally, peptides are usually water-soluble and could be freeze-dried, stable at room temperature, and easy for storage and distribution (Wanning et al. 2020). Peptides could be designed to target unique pathogen. They can also contain multiple epitopes to target the diverse phases of life cycle or different pathogens (Fries et al. 2021). Since whole proteins cannot be used in cancer vaccines as they are much the same endogenous human protein, which could lead to autoimmune diseases, peptides may be very useful to design cancer vaccines (Malonis et al. 2020). Further, compared with DNA vaccines, peptides vaccines do not have risk of genetic recombination. Lipids, carbohydrates and phosphate groups can be added to the peptide so as to improve stability and immunogenicity (Li et al. 2021b).
Peptide Vaccines for HNSCC Patients
Currently, several therapeutic peptide vaccines are under investigation for HNSCC (Shibata et al. 2021; Domingos-Pereira et al. 2019; Mora Roman et al. 2019; Miyazaki et al. 2011). Table 1 summarises recent peptide vaccines for HNSCC patients. For example, Trojan peptide-based vaccines were designed for advanced HNSCC patients. They consisted of HLA-I and HLA-II restricted peptide epitopes (melanoma antigen E, MAGE-A3 and HPV-16 TAA, respectively) (Voskens et al. 2012). MAGE, a cancer testis (CT) antigen, is highly expressed in HNSCC cells. HPV is strongly associated with OPSCC. MAGE and HPV were used to induce both HLA-I and HLA-II restricted immune responses. Results from clinical trials suggest that this vaccine could induce measurable systemic immune responses (Voskens et al. 2012). This vaccine was also evaluated in a phase 1 clinical trial in advanced HNSCC patients. It was found to induce an antibody response and an antigen-specific T cell response in most of patients (Zandberg et al. 2015).
More recently, a multiple peptide vaccine in a phase 2 clinical trial was demonstrated to induce a CTL response and improve the prognosis of HNSCC patients. These peptides are from three CT antigens LY6K, CDCA1, and IMP3, which are overexpressed in most of HNSCC cells rather than normal tissues. Following vaccination, the number of CD8+ T cells were increased in the peripheral circulation of HNSCC patients. This indicates that the frequency of CD8+ T cells was related to the overall increased survival of patients. One potential disadvantage of a peptide vaccine is that they can target one epitope of the TAA. However, this research used three peptides which together could induce stronger CTL response than one peptide (Yoshitake et al. 2015; Schlom 2012).
Targeting mutated proteins p53 has become a promising immunotherapy area in HNSCC patients (Albers et al. 2018). Recently, some researchers designed DCs loaded p53 peptides as novel anti-tumour vaccines and detected in a phase 1 clinical trial. These vaccines were shown to decrease regulatory T cells, a modest vaccine-specific immune response and inhibit cancer growth (Schuler et al. 2014). In addition to p53, p16 could also act as a therapy target for HNSCC. p16INK4a, the cyclin-dependent kinase inhibitor, is highly expressed in HNSCC cells. Recent research investigated P16INK4a as a vaccine target antigen for HNSCC. p16-based peptide vaccines are safe to these patients and may prime cellular and humoral immune responses (Reuschenbach 2016). A clinical study also showed that vaccination with p16INK4a could be an attractive immunotherapy for HNSCC patients. It would need more further investigations about the clinical efficacy of p16-based peptide vaccine (Reuschenbach 2015).
While it is now well established that the high-risk HPV is highly associated with oral cancer, research showed that HPV 16 is the major contributor for this cancer (Yete et al. 2018). The oncoproteins E6 and E7 are encoded by HPV 16, and thus immunotherapy targeting E6 and E7 may offer an effective way to prevent and treat HPV-related diseases (Yang et al. 2016; Aggarwal et al. 2019). Domingos-Pereira et al. used a synthesised HPV E7-long-peptide (E7LP) therapeutic vaccine with an encapsulated TLR9 agonist CpG, combined with carboplatin/paclitaxel (C + P) chemotherapy to target HPV-associated malignancies in a mouse model of disease. It was reported that compared to any of the dual treatments (C + P + E7LP, C + P + intravaginal CpG or E7LP + intravaginal CpG), the vaccine significantly increased survival. It was also shown that the addition of CpG greatly led to increased E7-specific CD8 T cells (Domingos-Pereira et al. 2019). Ye et al. reported that, in a OSCC model, a HPV-16mE6Delta/mE7/TBhsp70Delta fusion-protein vaccine not only suppressed tumour, but also elicited tumour death and provided protection against OSCC (Ye et al. 2013). In a recent study Yang and co-workers synthesized HPV E7 long peptide therapeutic vaccine, without using an adjuvant, aimed to against HPV-associated malignancies. Results indicated that this vaccine could generate both a local and systemic potent CD8+ T cell response and anti-tumour effects. In addition, results demonstrated that this vaccine is more effective in buccal mucosal cancers than subcutaneous tumours (Yang et al. 2016). The design of this vaccine has two advantages. First, this vaccine does not have adjuvant, simplifying the design of vaccine and reducing side effects that can come from adjuvant use. Another benefit is it uses long peptides rather than short peptides. The latter may be directly loaded onto any cells which have MHC I molecules, leading to interaction between MHC I complex and TCR without co-stimulatory signals, resulting in immune tolerance (Toes et al. 1996). HNSCC is usually less immunogenic than other cancer types. Tan and co-workers found that MOC2-E6/E7 C57BL/6-syngeneic vaccine greatly increases antigen uptake and effectively prevents HNSCC immune escape (Tan et al. 2018). In addition, peptide vaccine combined with other immune therapies could provide a novel treatment option for HNSCC patients. For example, a combination of intranasal HPV peptide vaccination plus 4-1BB and CTLA-4 antibodies showed curative effectiveness and a superior safety profile against orally implanted mEER tumours (Dorta-Estremera et al. 2018). Immunotherapy Targeting HPV16/18 combined PD-1/PD-L1 inhibition may improve therapeutic outcomes in HNSCC (Aggarwal et al. 2019).
Some of these vaccines have previously been used in clinical trials. A survivin-derived peptide vaccine in phase 1 clinical trial has demonstrated the induction of an effective CTL response, and consequently has therapeutic potential for advanced or recurrent oral cancer patients (Miyazaki et al. 2011). Survivin is an inhibitor of apoptosis that reinforces cell cycle progression and enhances angiogenesis. While it is rarely detectable in normal tissues, overexpression of survivin can be found in approximately 80% of OSCC, and consequently, it is often used as a tumour antigen for oral cancer (Lo Muzio et al. 2003; Altieri 2003). Recently, the Kinesin family member 11 (KIF11) has been identified as a potential therapeutic target for oral cancer. KIF11 is a motor protein that essential to establish a bipolar spindle in cell division, and high expression of KIF11 has been closely associated with poor prognosis in oral cancer patients (Daigo et al. 2018).
MAGED4B is a melanoma antigen that promotes cell proliferation and migration that overexpresses in OSCC and high expression. Research into MAGED4B has led to the identification of immunogenic MAGED4B peptides for potential oral cancer vaccine targets. The results demonstrated that the vaccine increased T-cell cytotoxic efficacy against OSCC patients (Lim et al. 2014). The same research group later investigated the effectiveness of a dual-antigenic peptide vaccination (PV1) made composed of MAGED4B and FJX1 peptides in HNSCC patients. They showed that after PV1 vaccination, patients’ T-cells were able to release cytotoxic cytokines. Additionally, patients with significant MAGED4B and FJX1 expression in their tumours were more sensitive to PV1 activation, indicating the PV1 peptide vaccine's specificity. PV1 might be a promising vaccination candidate for HNSCC patients and other malignancies that express these antigens (Chai et al. 2019).
Combination treatment provides a number of advantages, including the ability to use the synergy of various medicines to produce a satisfactory therapeutic outcome with lower medication dosages and fewer adverse effects (Karavasili et al. 2019). A clinical trial was performed with Wilms' tumour 1 peptide, in combination with DC vaccination and conventional chemotherapy, on metastatic or relapsed HNSCC patients. The researchers observed that none of the patients experienced any serious side effects. After DC immunisation, five patients had long-term disease stability, while six others experienced disease progression. The median progression-free survival was 6.4 months, and the overall survival was 12.1 months, respectively. Therefore, this vaccine DC-based immunotherapy in combination with conventional chemotherapy appears to be safe and viable for individuals with advanced HNSCC (Ogasawara et al. 2019). In addition, patients with HPV + HNSCC may benefit from E7 immunisation coupled with surgery. Recently, a nanoparticle conjugated with E7 long-peptide and CpG in an orthotopic immunocompetent mouse model was tested. In the absence of surgery, immunisation given before or after tumour-cell injection only minimally reduced tumour development and extended survival. However, when NP-E7LP vaccination was administered before primary tumour excision, no postsurgical recurrence was detected. This indicates that the removal of the primary tumour altered the tumour microenvironment, allowing the vaccine-induced anti-tumour response to have a therapeutic impact. Patients with HPV + HNSCC may benefit from E7 immunisation combined surgery therapy (Domingos-Pereira et al. 2021). Karavasili and co-workers demonstrated that for HNSCC patients, peptide hydrogel combined with drugs (doxorubicin and curcumin) dramatically changed apoptotic/anti-apoptotic gene expression (Karavasili et al. 2019). Another new immunologic strategy to treating HNSCC patients might be a combination of p53 peptides and chemotherapy. Ohara et al. found that p53 can induce effective T responses, and hemotherapeutic drugs increased the responses of these CD4 T cells by phosphorylating p53 (Ohara 2018).
Research is currently being conducted in order to identify immunotherapeutic targets for HNSCC. For example, placenta-specific 1 (PLAC1) is a cancer immunotherapy target that is expressed mainly in placental trophoblasts, but not in normal tissues. A recent study reported that PLAC1 is found in 74.5% of oropharyngeal tumours and 51.9% of oral cavity tumours, as well as many HNSCC cell lines. They also identified an HTL peptide epitope (PLAC131-50) that is able to induce potent T cell responses, suggesting PLAC1 might be a target antigen for HNSCC patients (Hayashi et al. 2021). Additionally, sperm protein (SP) 17, which is a CT antigen expressed in many types of cancers such as lung cancer and ovarian cancer. The SP17 protein was also found in HNSCC patients. This suggests that it is a promising immunotherapeutic target, as well as a possible disease biomarker for HNSCC (Schutt et al. 2017). These findings indicate that peptide-based vaccine is a promising treatment for advanced HNSCC.
Different peptide-based anti-cancer vaccines are now being tested in the clinical trials. Table 2 summarises clinical trials of peptide vaccines for HNSCC patients. On ClinicalTrials.gov, 14 studies were discovered using the keywords “head and neck cancer” and “peptide.” As shown in Table 1, there are six completed trials, some of which have already been mentioned. Although there have been recent advances in the development of novel therapies including anti-tumour vaccines, these results to date have not yet been transferred into clinical use and thus have not increased the survival rate of OSCC patients. As a result, further investigations need to be made to establish an effective immunotherapy.
Conclusions and Future Directions
In the past few years, there have been remarkable achievements in develo** the peptide-based vaccine for HNSCC patients. However, most peptide-based anticancer vaccines researched so far are less effective, and significant challenges still need to be solved. Most of the clinical research is still at an early stage, mainly concentrating on safety, availability, and immunogenicity (Whang et al. 2015). Although there have been recent advances in the development of novel therapies, including anti-tumour vaccines, these results to date have not yet been transferred into clinical use and thus have not increased the survival rate of HNSCC patients (Schneider et al. 2018). One of the main disadvantages of peptide vaccines is the lack of efficacy shown in clinical studies. For example, two phase 3 clinical trials (DERMA and MAGRIT) of MAGE-A3 immunotherapeutic with advanced melanoma (Dreno et al. 2018) and non-small-cell lung cancer, NSCLC (Vansteenkiste et al. 2016) patients failed. Vaccination with the MAGE-A3 immunotherapeutic elicited a strong immune response, resulting in substantial improvement in anti-MAGE-3 antibody concentrations. However, clinical effectiveness was not achieved. The reasons for the lack of clinical effectiveness might be connected to the antigen or immunostimulant used, or the failure to induce an antitumor immune response, especially cytotoxic T-lymphocyte responses (Dreno et al. 2018; Rosenberg and Restifo 2015). Another reason might be immune evasion and suppression. Many immunosuppressive mechanisms and immunosuppressive cytokines may inhibit antitumour T-cell responses (Dreno et al. 2018). To solve these problems, the most promising way is combination therapy. Due to the complex tumour evasion routes, a combination of different strategies will be important for cancer immunotherapy. More clinical trials combined with other immune therapies will be needed to guide the next generation of HNSCC vaccine and will be valuable for future research (Aggarwal et al. 2019; Dorta-Estremera et al. 2018; Karavasili et al. 2019).
Peptide-based vaccines have poor immunogenicity, and they require a delivery system or/and adjuvant such as TCR ligands, co-stimulatory molecules, and cytokines to induce the desired immunity. The challenges of develo** peptide vaccines are the selection of appropriate epitopes and adjuvants. Strong adjuvants are also critical to improving the efficacy of vaccines (Gouttefangeas and Rammensee 2018). Despite the side effects, adjuvants are needed to enhance the immune response (Leroux-Roels 2010). Another limitation of peptide vaccines is that they are MHC restricted. They must match the HLA in patients. In individuals with diverse MHC class I molecules, a specific peptide may not elicit robust cell-mediated immunity. It is difficult to design a peptide vaccine for all human population due to HLA polymorphisms. The development of long peptides that contain multiple antigenic epitopes could solve this problem (Chen et al. 2020; Slingluff 2011). However, many challenges still exist in the production of long peptides such as synthesis complexity, low yields and high costs (Isidro-Llobet et al. 2019). The future tendency in vaccination is the use of a minimal component from pathogens to induce potent and long-lasting immune responses (Skwarczynski and Toth xxxx; Skwarczynski and Toth 2014; Liu et al. 2012). Additionally, personalized peptide-based vaccines are highly promising ways to achieve clinical success (Shibata et al. 2021). Future studies focusing on personalized peptide-based vaccines will be needed more. When these hurdles are addressed, peptide cancer vaccines are expected to become a strong tool for stimulating an immune response against cancer and a standard treatment in cancer immunotherapy.
References
Acin S et al (2011) Gain-of-function mutant p53 but not p53 deletion promotes head and neck cancer progression in response to oncogenic K-ras. J Pathol 225(4):479–489
Aggarwal C et al (2019) Immunotherapy targeting HPV16/18 generates potent immune responses in HPV-associated head and neck cancer. Clin Cancer Res 25(1):110–124
Albers AE et al (2018) Phenotype of p53 wild-type epitope-specific T cells in the circulation of patients with head and neck cancer. Sci Rep 8(1):10716
Almand B et al (2000) Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res 6(5):1755–1766
Almand B et al (2001) Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol 166(1):678–689
Alnuaimi AD et al (2015) Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: a matched case–control study. Oral Oncol 51(2):139–145
Alsahafi E et al (2019) Clinical update on head and neck cancer: molecular biology and ongoing challenges. Cell Death Dis 10(8):540
Alshadwi A et al (2013) Nutritional considerations for head and neck cancer patients: a review of the literature. J Oral Maxillofac Surg 71(11):1853–1860
Altieri DC (2003) Validating survivin as a cancer therapeutic target. Nat Rev Cancer 3(1):46–54
Amit M et al (2020) Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 578(7795):449–454
Amtha R et al (2014) Tobacco (kretek) smoking, betel quid chewing and risk of oral cancer in a selected Jakarta population. Asian Pac J Cancer Prev 15(20):8673–8678
Argiris A et al (2008) Head and neck cancer. Lancet (lond Engl) 371(9625):1695–1709
Bandoh N et al (2010) HLA class I antigen and transporter associated with antigen processing downregulation in metastatic lesions of head and neck squamous cell carcinoma as a marker of poor prognosis. Oncol Rep 23(4):933–939
Baruah P et al (2012) Decreased levels of alternative co-stimulatory receptors OX40 and 4–1BB characterise T cells from head and neck cancer patients. Immunobiology 217(7):669–675
Bessell A et al (2011) Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment. Cochrane Database Syst Rev 9:CD006205
Bezu L et al (2018) Trial watch: peptide-based vaccines in anticancer therapy. OncoImmunology 7(12):e1511506
Bonner JA et al (2010) Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 11(1):21–28
Bu LL et al (2017) STAT3 induces immunosuppression by upregulating PD-1/PD-L1 in HNSCC. J Dent Res 96(9):1027–1034
Cai C et al (2014) Keratinizing-type squamous cell carcinoma of the oropharynx: p16 overexpression is associated with positive high-risk HPV status and improved survival. Am J Surg Pathol 38(6):809–815
Calixto G et al (2014) Nanotechnology-based drug delivery systems for treatment of oral cancer: a review. Int J Nanomed 9:3719–3735
Calvo Tardón M et al (2019) Peptides as cancer vaccines. Curr Opin Pharmacol 47:20–26
Camacho M et al (2008) Prostaglandin E(2) pathway in head and neck squamous cell carcinoma. Head Neck 30(9):1175–1181
Canning M et al (2019) Heterogeneity of the head and neck squamous cell carcinoma immune landscape and its impact on immunotherapy. Front Cell Dev Biol 7:52
Celenk F et al (2013) Expression of cyclooxygenase-2, 12-lipoxygenase, and inducible nitric oxide synthase in head and neck squamous cell carcinoma. J Craniofac Surg 24(4):1114–1117
Chai SJ et al (2019) In vitro evaluation of dual-antigenic PV1 peptide vaccine in head and neck cancer patients. Hum Vaccines Immunother 15(1):167–178
Chang JS et al (2013) Investigating the association between oral hygiene and head and neck cancer. Oral Oncol 49(10):1010–1017
Chen X et al (2020) Personalized neoantigen vaccination with synthetic long peptides: recent advances and future perspectives. Theranostics 10(13):6011–6023
Chen CH, Chen RJ (2011) Prevalence of telomerase activity in human cancer. J Formos Med Assoc 110(5):275–289
Clement-Colmou K et al (2015) Clinical and paraclinical follow-up after radiotherapy for head and neck cancer. Cancer Radiother 19(6–7):597–602
Constantinidou A, Alifieris C, Trafalis DT (2019) Targeting Programmed Cell Death-1 (PD-1) and Ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther 194:84–106
Costa NL et al (2013) Tumor-associated macrophages and the profile of inflammatory cytokines in oral squamous cell carcinoma. Oral Oncol 49(3):216–223
Costache MI et al (2015) VEGF expression in pancreatic cancer and other malignancies: a review of the literature. Rom J Intern Med 53(3):199–208
D’Souza G et al (2007) Case–control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 356(19):1944–1956
Daigo K et al (2018) Characterization of KIF11 as a novel prognostic biomarker and therapeutic target for oral cancer. Int J Oncol 52(1):155–165
Dasgupta S et al (2005) Inhibition of NK cell activity through TGF-beta 1 by down-regulation of NKG2D in a murine model of head and neck cancer. J Immunol 175(8):5541–5550
Domingos-Pereira S et al (2019) Carboplatin/paclitaxel, E7-vaccination and intravaginal CpG as tri-therapy towards efficient regression of genital HPV16 tumors. J Immunother Cancer 7(1):122
Domingos-Pereira S et al (2021) Vaccination with a nanoparticle E7 vaccine can prevent tumor recurrence following surgery in a human papillomavirus head and neck cancer model. OncoImmunology 10(1):1912473
Dorta-Estremera S et al (2018) Mucosal HPV E6/E7 peptide vaccination in combination with immune checkpoint modulation induces regression of HPV+ oral cancers. Cancer Res 78(18):5327–5339
Dreno B et al (2018) MAGE-A3 immunotherapeutic as adjuvant therapy for patients with resected, MAGE-A3-positive, stage III melanoma (DERMA): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 19(7):916–929
Eisbruch A et al (2002) Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys 53(1):23–28
Elango KJ et al (2011) Role of human papilloma virus in oral tongue squamous cell carcinoma. Asian Pac J Cancer Prev 12(4):889–896
Elkord E et al (2010) T regulatory cells in cancer: recent advances and therapeutic potential. Expert Opin Biol Ther 10(11):1573–1586
Estêvão D et al (2019) Hallmarks of HPV carcinogenesis: the role of E6, E7 and E5 oncoproteins in cellular malignancy. Biochim Biophys Acta Gene Regul Mech 1862(2):153–162
Eun-Young LEE, Ji-Yeon K, Kyoung-Won KIM (2015) Expression of cyclooxygenase-2, peroxiredoxin I, peroxiredoxin 6 and nuclear factor-κB in oral squamous cell carcinoma. Oncol Lett 10(5):3129–3136
Fakhry C, D’Souza G (2013) Discussing the diagnosis of HPV-OSCC: common questions and answers. Oral Oncol 49(9):863–871
Ferlay J et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 5:359
Ferris RL (2015) Immunology and immunotherapy of head and neck cancer. J Clin Oncol 33(29):3293–3304
Ferris RL, Whiteside TL, Ferrone S (2006) Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res 12(13):3890–3895
Freiser ME, Serafini P, Weed DT (2013) The immune system and head and neck squamous cell carcinoma: from carcinogenesis to new therapeutic opportunities. Immunol Res 57(1–3):52–69
Fries CN et al (2021) Advances in nanomaterial vaccine strategies to address infectious diseases impacting global health. Nat Nanotechnol 16(4):1–14
Furness S et al (2011) Interventions for the treatment of oral cavity and oropharyngeal cancer: chemotherapy. Cochrane Database Syst Rev 4:CD006386
Gabrilovich D et al (1998) Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92(11):4150–4166
Gaglione R et al (2019) Cost-effective production of recombinant peptides in Escherichia coli. N Biotechnol 51:39–48
Gasparoto TH et al (2010) Patients with oral squamous cell carcinoma are characterized by increased frequency of suppressive regulatory T cells in the blood and tumor microenvironment. Cancer Immunol Immunother 59(6):819–828
Gilbert MR, Lim C-M, Kim S (2013) Head and neck cancer. In: Experimental metastasis: modeling and analysis. Springer, Berlin, p 7–26
Gouttefangeas C, Rammensee H-G (2018) Personalized cancer vaccines: adjuvants are important, too. Cancer Immunol Immunother 67(12):1911–1918
Gregoire V, Langendijk JA, Nuyts S (2015) Advances in radiotherapy for head and neck cancer. J Clin Oncol 33(29):3277–3284
Groux H, Fournier N, Cottrez F (2004) Role of dendritic cells in the generation of regulatory T cells. Semin Immunol 16(2):99–106
Guerra A et al (2021) Simulation of the process of angiogenesis: quantification and assessment of vascular patterning in the chicken chorioallantoic membrane. Comput Biol Med 136:104647
Guha N et al (2014) Betel quid chewing and the risk of oral and oropharyngeal cancers: a meta-analysis with implications for cancer control. Int J Cancer 135(6):1433–1443
Güneri P, Epstein JB (2014) Late stage diagnosis of oral cancer: components and possible solutions. Oral Oncol 50(12):1131–1136
Haddad R et al (2013) Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol 14(3):257–264
Hanken H et al (2014) CCND1 amplification and cyclin D1 immunohistochemical expression in head and neck squamous cell carcinomas. Clin Oral Investig 18(1):269–276
Hayashi R et al (2021) Expression of placenta-specific 1 and its potential for eliciting anti-tumor helper T-cell responses in head and neck squamous cell carcinoma. OncoImmunology 10(1):1856545
Hettmann A et al (2015) Infectious agents associated with head and neck carcinomas. Adv Exp Med Biol 897:63–80
Hickey MJ, Valenzuela NM, Reed EF (2016) Alloantibody generation and effector function following sensitization to human leukocyte antigen. Front Immunol 7:30
Hsu HW et al (2014) Combination antiangiogenic therapy and radiation in head and neck cancers. Oral Oncol 50(1):19–26
Hu J, Ge W, Xu J (2016) HPV 16 E7 inhibits OSCC cell proliferation, invasion, and metastasis by upregulating the expression of miR-20a. Tumor Biol 37(7):9433–9440
Huang B et al (2006) Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 66(2):1123–1131
Huang C et al (2021) Proteogenomic insights into the biology and treatment of HPV-negative head and neck squamous cell carcinoma. Cancer Cell 39(3):361-379.e16
Hubbers CU, Akgul B (2015) HPV and cancer of the oral cavity. Virulence 6(3):244–248
Hwang T-Z et al (2015) Incidence trends of human papillomavirus-related head and neck cancer in Taiwan, 1995–2009. Int J Cancer 137(2):395–408
Isidro-Llobet A et al (2019) Sustainability challenges in peptide synthesis and purification: from R&D to production. J Org Chem 84(8):4615–4628
**no T et al (2015) Increased expression of interleukin-6 predicts poor response to chemoradiotherapy and unfavorable prognosis in oral squamous cell carcinoma. Oncol Rep 5:2161
Johnson BF et al (2007) Vascular endothelial growth factor and immunosuppression in cancer: current knowledge and potential for new therapy. Expert Opin Biol Ther 7(4):449–460
Johnson DE et al (2020) Head and neck squamous cell carcinoma. Nat Rev Dis Primers 6(1):92–92
Ju X et al (2020) Regulation of PD-L1 expression in cancer and clinical implications in immunotherapy. Am J Cancer Res 10(1):1–11
Kademani D (2007) Oral cancer. Mayo Clin Proc 82(7):878–887
Kaminagakura E et al (2012) High-risk human papillomavirus in oral squamous cell carcinoma of young patients. Int J Cancer 130(8):1726–1732
Kao SY, Lim E (2015) An overview of detection and screening of oral cancer in Taiwan. Chin J Dent Res 18(1):7–12
Karatzanis AD et al (2012) Molecular pathways of lymphangiogenesis and lymph node metastasis in head and neck cancer. Eur Arch Oto-Rhino-Laryngol off J Eur Fed Oto-Rhino-Laryngol Soc Affil Ger Soc Oto-Rhino-Laryngol Head Neck Surg 269(3):731–737
Karavasili C et al (2019) Synergistic antitumor potency of a self-assembling peptide hydrogel for the local co-delivery of doxorubicin and curcumin in the treatment of head and neck cancer. Mol Pharm 16(6):2326–2341
Keir ME et al (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704
Klein HL (2008) The consequences of Rad51 overexpression for normal and tumor cells. DNA Repair (amst) 7(5):686–693
Krishnamachari Y et al (2011) Nanoparticle delivery systems in cancer vaccines. Pharm Res 28(2):215–236
Kuss I et al (2004) Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clin Cancer Res 10(11):3755–3762
Kwong FN, Puvanendran M, Paleri V (2015) Transoral robotic surgery in head neck cancer management. B-ENT Suppl 24:7–13
van der Laan HP et al (2013) The potential of intensity-modulated proton radiotherapy to reduce swallowing dysfunction in the treatment of head and neck cancer: a planning comparative study. Acta Oncol 52(3):561–569
Lebman DA, Edmiston JS (1999) The role of TGF-β in growth, differentiation, and maturation of B lymphocytes. Microbes Infect 1(15):1297–1304
Lee DW et al (2002) Increased cyclooxygenase-2 expression in human squamous cell carcinomas of the head and neck and inhibition of proliferation by nonsteroidal anti-inflammatory drugs. Anticancer Res 22(4):2089–2096
Lee Y-CA et al (2019) Tobacco smoking, alcohol drinking, betel quid chewing, and the risk of head and neck cancer in an East Asian population. Head Neck 41(1):92–102
Leroux-Roels G (2010) Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine 28:C25–C36
Li W et al (2017) C-terminal modification and multimerization increase the efficacy of a proline-rich antimicrobial peptide. Chem Eur J 23(2):390–396
Li W et al (2017) The effect of selective D- or Nα-methyl arginine substitution on the activity of the proline-rich antimicrobial peptide, Chex1-Arg20. Front Chem. https://doi.org/10.3389/fchem.2017.00001
Li W et al (2020) Chemical modification of cellulose membranes for SPOT synthesis. Aust J Chem 73(3):78–84
Li M et al (2021a) Structurally related peptide impurity identification and accurate quantification for synthetic oxytocin by liquid chromatography–high-resolution mass spectrometry. Anal Bioanal Chem 413(7):1861–1870
Li W et al (2021b) Chemically modified and conjugated antimicrobial peptides against superbugs. Chem Soc Rev 50(8):4932–4973
Lim KP et al (2014) Identification of immunogenic MAGED4B peptides for vaccine development in oral cancer immunotherapy. Hum Vaccine Immunother 10(11):3214–3223
Lippitz BE (2013) Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol 14(6):e218–e228
Liu TY et al (2012) Advances in peptide-based human papillomavirus therapeutic vaccines. Curr Top Med Chem 12(14):1581–1592
Liu D et al (2012) Association between polymorphisms in the promoter regions of matrix metalloproteinases (MMPs) and risk of cancer metastasis: a meta-analysis. PLoS ONE 7(2):e31251
Lo Muzio L et al (2003) Survivin expression in oral squamous cell carcinoma. Br J Cancer 89(12):2244–2248
Lyford-Pike S et al (2013) Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res 73(6):1733–1741
Machiels JP, Schmitz S (2015) Epidermal growth factor receptor inhibition in squamous cell carcinoma of the head and neck. Hematol Oncol Clin N Am 29(6):1011–1032
Mali SB (2015) Review of STAT3 (Signal Transducers and Activators of Transcription) in head and neck cancer. Oral Oncol 51(6):565–569
Malonis RJ, Lai JR, Vergnolle O (2020) Peptide-based vaccines: current progress and future challenges. Chem Rev 120(6):3210–3229
Mao L, Hong WK, Papadimitrakopoulou VA (2004) Focus on head and neck cancer. Cancer Cell 5(4):311–316
Marta GN et al (2014) Intensity-modulated radiation therapy for head and neck cancer: systematic review and meta-analysis. Radiother Oncol 110(1):9–15
Marur S et al (2010) HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 11(8):781–789
Masuda M et al (2002) Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res 62(12):3351–3355
McQueen N et al (2016) Smoking cessation and electronic cigarette use among head and neck cancer patients. Otolaryngol Head Neck Surg 154(1):73–79
Mehanna H et al (2010) Head and neck cancer—Part 1: epidemiology, presentation, and prevention. BMJ 341:c4684
Mehanna H et al (2013) Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer—systematic review and meta-analysis of trends by time and region. Head Neck 35(5):747–755
Meulemans J, Delaere P, Vander Poorten V (2015) Early experience in transoral robotic surgery (TORS) for non-oropharyngeal head and neck malignancies: a review of functional and oncologic outcomes. B-ENT Suppl 24:21–31
Miyamoto R et al (2003) Prognostic significance of cyclin D1 amplification and overexpression in oral squamous cell carcinomas. Oral Oncol 39:610–618
Miyazaki A et al (2011) Phase I clinical trial of survivin-derived peptide vaccine therapy for patients with advanced or recurrent oral cancer. Cancer Sci 102(2):324–329
van Monsjou HS et al (2010) Oropharyngeal squamous cell carcinoma: a unique disease on the rise? Oral Oncol 46(11):780–785
Mora Roman JJ et al (2019) Immunotherapeutic potential of mollusk hemocyanins in combination with human vaccine adjuvants in murine models of oral cancer. J Immunol Res 2019:7076942
Morita Y et al (2012) Cyclooxygenase-2 promotes tumor lymphangiogenesis and lymph node metastasis in oral squamous cell carcinoma. Int J Oncol 41(3):885–892
Moutsopoulos NM, Wen J, Wahl SM (2008) TGF-β and tumors—an ill-fated alliance. Curr Opin Immunol 20(2):234–240
Munshi T, Heckman CJ, Darlow S (2015) Association between tobacco waterpipe smoking and head and neck conditions: a systematic review. J Am Dent Assoc 146(10):760–766
Münger K, Howley PM (2002) Human papillomavirus immortalization and transformation functions. Virus Res 89(2):213–228
Nasman A et al (2015) Incidence of human papillomavirus positive tonsillar and base of tongue carcinoma: a stabilisation of an epidemic of viral induced carcinoma? Eur J Cancer 51(1):55–61
Noorlag R et al (2015) The diagnostic value of 11q13 amplification and protein expression in the detection of nodal metastasis from oral squamous cell carcinoma: a systematic review and meta-analysis. Virchows Arch 466(4):363–373
Ogasawara M et al (2019) Phase I/II pilot study of Wilms’ tumor 1 peptide-pulsed dendritic cell vaccination combined with conventional chemotherapy in patients with head and neck cancer. Ther Apher Dial 23(3):279–288
Ohara K et al (2018) Targeting phosphorylated p53 to elicit tumor-reactive T helper responses against head and neck squamous cell carcinoma. OncoImmunology 7(9):e1466771
Omar E (2015) Current concepts and future of noninvasive procedures for diagnosing oral squamous cell carcinoma—a systematic review. Head Face Med 11:6
Park S-J et al (2004) IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol (baltim MD 1950) 173(6):3844–3854
Park JW et al (2014) Human papillomavirus type 16 E7 oncoprotein causes a delay in repair of DNA damage. Radiother Oncol 113(3):337–344
Patel SB, McCormack C, Hodge JC (2020) Non-fusion mutations in endometrial stromal sarcomas: what is the potential impact on tumourigenesis through cell cycle dysregulation? J Clin Pathol 73(12):830–835
Patel SG, Shah JP (2005) TNM staging of cancers of the head and neck: striving for uniformity among diversity. CA Cancer J Clin 55(4):242–258
Pavia M et al (2006) Association between fruit and vegetable consumption and oral cancer: a meta-analysis of observational studies. Am J Clin Nutr 83(5):1126–1134
Perri F et al (2015a) Radioresistance in head and neck squamous cell carcinoma: biological bases and therapeutic implications. Head Neck 5:763
Perri F et al (2015b) Radioresistance in head and neck squamous cell carcinoma: biological bases and therapeutic implications. Head Neck 37(5):763–770
Pignon J-P et al (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 92(1):4–14
Pignon JP, le Maitre A, Bourhis J (2007) Meta-Analyses of Chemotherapy in Head and Neck Cancer (MACH-NC): an update. Int J Radiat Oncol Biol Phys 69(2 Suppl):S112–S114
Poomsawat S et al (2016) Expression of cdk6 in head and neck squamous cell carcinoma. Clin Oral Investig 20(1):57–63
Purcell AW, McCluskey J, Rossjohn J (2007) More than one reason to rethink the use of peptides in vaccine design. Nat Rev Drug Discov 6(5):404–414
Qiao X-W et al (2020) The evolving landscape of PD-1/PD-L1 pathway in head and neck cancer. Front Immunol 11:1721
Rabinowits G, Haddad RI (2012) Overcoming resistance to EGFR inhibitor in head and neck cancer: a review of the literature. Oral Oncol 48(11):1085–1089
Reuschenbach M et al (2013) Lack of evidence of human papillomavirus-induced squamous cell carcinomas of the oral cavity in southern Germany. Oral Oncol 49(9):937–942
Reuschenbach M (2015) Targeting p16(INK4a) by therapeutic vaccination: concept and status of clinical investigations in HPV-associated head and neck cancers. HNO 63(2):104–110
Reuschenbach M et al (2016) A phase 1/2a study to test the safety and immunogenicity of a p16 peptide vaccine in patients with advanced human papillomavirus-associated cancers. Cancer 122(9):1425–1433
Rietbergen MM et al (2013) Increasing prevalence rates of HPV attributable oropharyngeal squamous cell carcinomas in the Netherlands as assessed by a validated test algorithm. Int J Cancer 132(7):1565–1571
Rivera C (2015) Essentials of oral cancer. Int J Clin Exp Pathol 8(9):11884–11894
Rodrigo JP et al (2014) Time trends in the prevalence of HPV in oropharyngeal squamous cell carcinomas in northern Spain (1990–2009). Int J Cancer 134(2):487–492
Rosenberg SA, Restifo NP (2015) Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348(6230):62–68
Rushatamukayanunt P et al (2014) Lack of association between high-risk human papillomaviruses and oral squamous cell carcinoma in young Japanese patients. Asian Pac J Cancer Prev 15(10):4135–4141
Rutkowski T (2014) The role of tumor volume in radiotherapy of patients with head and neck cancer. Radiat Oncol 9(1):1–16
Ruttkay-Nedecky B et al (2013) Relevance of infection with human papillomavirus: the role of the p53 tumor suppressor protein and E6/E7 zinc finger proteins (Review). Int J Oncol 43(6):1754–1762
Schaefer C et al (2005) Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer 92(5):913–920
Schlom J (2012) Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst 104(8):599–613
Schneider K et al (2018) Therapeutic human papillomavirus vaccines in head and neck cancer: a systematic review of current clinical trials. Vaccine 36(45):6594–6605
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331(6024):1565–1570
van der Schroeff MP, Baatenburg de Jong RJ (2009) Staging and prognosis in head and neck cancer. Oral Oncol 45(4–5):356–360
Schuler PJ et al (2014) Phase I dendritic cell p53 peptide vaccine for head and neck cancer. Clin Cancer Res 20(9):2433–2444
Schutt CA et al (2017) The cancer-testis antigen, sperm protein 17, a new biomarker and immunological target in head and neck squamous cell carcinoma. Oncotarget 8(59):100280–100287
Shao Y et al (2013) miR-145 inhibits oral squamous cell carcinoma (OSCC) cell growth by targeting c-Myc and Cdk6. Cancer Cell Int 13(1):51
Shibata H et al (2021) Personalized cancer vaccination in head and neck cancer. Cancer Sci 112(3):978–988
Siegel RL et al (2021) Cancer statistics, 2021. CA Cancer J Clin 71(1):7–33
Simard EP, Torre LA, Jemal A (2014) International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol 50(5):387–403
Sittichai K (2013) The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J Cancer 1:66
Skwarczynski M, Toth I (2014) Recent advances in peptide-based subunit nanovaccines. Nanomedicine (LOnd) 9(17):2657–2669
Skwarczynski M, Toth I (2016) Peptide-based synthetic vaccines. Chem Sci 7(2):842–854
Skwarczynski M, Toth I (2016) ChemInform abstract: peptide-based synthetic vaccines. ChemInform. https://doi.org/10.1002/chin.201612281
Slingluff CL Jr (2011) The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination? Cancer J 17(5):343–350
Stephens AJ, Burgess-Brown NA, Jiang S (2021) Beyond just peptide antigens: the complex world of peptide-based cancer vaccines. Front Immunol 12:696791
Stransky N et al (2011) The mutational landscape of head and neck squamous cell carcinoma. Science 333(6046):1157–1160
Strauss L et al (2007) A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res 13(15 Pt 1):4345–4354
Sturgis EM, Cinciripini PM (2007) Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer 110(7):1429–1435
Sung H et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Swiecicki PL, Malloy KM, Worden FP (2016) Advanced oropharyngeal squamous cell carcinoma: pathogenesis, treatment, and novel therapeutic approaches. World J Clin Oncol 7(1):15–26
Syrjanen S (2005) Human papillomavirus (HPV) in head and neck cancer. J Clin Virol 32(Suppl 1):S59-66
Takahashi H et al (2014) Telomerase-specific oncolytic adenovirus: antitumor effects on radiation-resistant head and neck squamous cell carcinoma cells. Head Neck 36(3):411–418
Takahashi H et al (2015) Immunosuppressive activity of cancer-associated fibroblasts in head and neck squamous cell carcinoma. Cancer Immunol Immunother 64(11):1407–1417
Takenaka S, Sato S (2015) Telomerase as biomarker for oral cancer. In: Biomarkers in cancer. Springer, Dordrecht, p 753. ISBN 978-94-007-7680-7
Tan YS et al (2018) Mitigating SOX2-potentiated immune escape of head and neck squamous cell carcinoma with a STING-inducing nanosatellite vaccine. Clin Cancer Res 24(17):4242–4255
Thomas J, Primeaux T (2012) Is p16 immunohistochemistry a more cost-effective method for identification of human papilloma virus-associated head and neck squamous cell carcinoma? Ann Diagn Pathol 16(2):91–99
Tinhofer I, Braunholz D, Klinghammer K (2020) Preclinical models of head and neck squamous cell carcinoma for a basic understanding of cancer biology and its translation into efficient therapies. Cancers Head Neck 5(1):9
Toes RE et al (1996) Peptide vaccination can lead to enhanced tumor growth through specific T-cell tolerance induction. Proc Natl Acad Sci USA 93(15):7855–7860
Turksma AW et al (2013) Immunotherapy for head and neck cancer patients: shifting the balance. Immunotherapy 5(1):49–61
Tuttle TR et al (2016) The cyclic GMP/protein kinase G pathway as a therapeutic target in head and neck squamous cell carcinoma. Cancer Lett 2:279
Vangara BS, Grandis JR (2014) Jak/STAT signaling in HNC. In: Molecular determinants of head and neck cancer. p. 163. https://doi.org/10.1007/978-1-4614-8815-6_8
Vansteenkiste JF et al (2016) Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 17(6):822–835
Vassilakopoulou M, Psyrri A, Argiris A (2015) Targeting angiogenesis in head and neck cancer. Oral Oncol 51(5):409–415
Vigneswaran N, Williams MD (2014) Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Sur Clin N Am 26:123–141
Vilen ST et al (2013) Fluctuating roles of matrix metalloproteinase-9 in oral squamous cell carcinoma. Sci World J 2013:920595
Vishak S, Rangarajan B, Kekatpure VD (2015) Neoadjuvant chemotherapy in oral cancers: Selecting the right patients. Indian J Med Paediatr Oncol 36(3):148–153
Voskens CJ et al (2012) Induction of MAGE-A3 and HPV-16 immunity by Trojan vaccines in patients with head and neck carcinoma. Head Neck 34(12):1734–1746
Wang M et al (2015b) Cancer-associated fibroblasts in a human HEp-2 established laryngeal xenografted tumor are not derived from cancer cells through epithelial–mesenchymal transition, phenotypically activated but karyotypically normal. PLoS ONE 10(2):e0117405
Wang WM et al (2015a) Epidermal growth factor receptor inhibition reduces angiogenesis via hypoxia-inducible factor-1alpha and Notch1 in head neck squamous cell carcinoma. PLoS ONE 10(2):e0119723
Wang Y et al (2020) Dendritic cell biology and its role in tumor immunotherapy. J Hematol Oncol 13(1):107
Wang XJ, Feng CW, Li M (2013) ADAM17 mediates hypoxia-induced drug resistance in hepatocellular carcinoma cells through activation of EGFR/PI3K/Akt pathway. Mol Cell Biochem 380(1–2):57–66
Wanning S, Süverkrüp R, Lamprecht A (2020) Impact of excipient choice on the aerodynamic performance of inhalable spray-freeze-dried powders. Int J Pharm 586:119564
Warnakulasuriya S (2009) Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 45(4–5):309–316
Wculek SK et al (2020) Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 20(1):7–24
Weber F et al (2005) Transforming growth factor-beta1 immobilises dendritic cells within skin tumours and facilitates tumour escape from the immune system. Cancer Immunol Immunother 54(9):898–906
Whang SN, Filippova M, Duerksen-Hughes P (2015) Recent progress in therapeutic treatments and screening strategies for the prevention and treatment of HPV-associated head and neck cancer. Viruses 7(9):5040–5065
Whiteside TL (2004) Down-regulation of ζ-chain expression in T cells: a biomarker of prognosis in cancer? Cancer Immunol Immunother 53(10):865–878
Whiteside TL (2005) Immunobiology of head and neck cancer. Cancer Metastasis Rev 24(1):95–105
Whiteside TL (2014) Regulatory T cell subsets in human cancer: are they regulating for or against tumor progression? Cancer Immunol Immunother 63(1):67–72
Wierzbicka M et al (2014) HPV vaccination in head and neck HPV-related pathologies. Otolaryngol Pol 68(4):157–173
Yang MC et al (2016) Buccal injection of synthetic HPV long peptide vaccine induces local and systemic antigen-specific CD8+ T-cell immune responses and antitumor effects without adjuvant. Cell Biosci 6:17
Yao M et al (2007) Current surgical treatment of squamous cell carcinoma of the head and neck. Oral Oncol 43(3):213–223
Ye D et al (2013) Inhibitory effect of the HPV-16mE6Delta/mE7/TBhsp70Delta vaccine on oral squamous cell carcinoma. Am J Med Sci 345(5):380–384
Yete S, D’Souza W, Saranath D (2018) High-risk human papillomavirus in oral cancer: clinical implications. Oncology 94(3):133–141
Yoo S, Ha SJ (2016) Generation of tolerogenic dendritic cells and their therapeutic applications. Immune Netw 16(1):52–60
Yoshitake Y et al (2015) Phase II clinical trial of multiple peptide vaccination for advanced head and neck cancer patients revealed induction of immune responses and improved OS. Clin Cancer Res 21(2):312–321
Yuji M et al (2015) Telomerase activity in the occurrence and progression of oral squamous cell carcinoma. J Oral Sci 57(4):295
Zamarron BF, Chen W (2011) Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci 7(5):651–658
Zandberg DP et al (2015) A phase I dose escalation trial of MAGE-A3- and HPV16-specific peptide immunomodulatory vaccines in patients with recurrent/metastatic (RM) squamous cell carcinoma of the head and neck (SCCHN). Cancer Immunol Immunother 64(3):367–379
Zandberg DP, Strome SE (2014) The role of the PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol 50(7):627–632
Zhang Y, Tighe S, Zhu Y-T (2020) COX-2 signaling in the tumor microenvironment. In: Birbrair A (ed) Tumor microenvironment: molecular players—Part B. Springer, Cham, pp 87–104
Zhao B, Zhao H, Zhao J (2020) Efficacy of PD-1/PD-L1 blockade monotherapy in clinical trials. Ther Adv Med Oncol 12:1758835920937612
Zhou F et al (2013) Immune tolerance induced by intravenous transfer of immature dendritic cells via up-regulating numbers of suppressive IL-10(+) IFN-γ(+)-producing CD4(+) T cells. Immunol Res 56(1):1–8
Zhou J et al (2015) Correlation of human papilloma virus with oral squamous cell carcinoma in Chinese population. Int J Clin Exp Med 8(10):18172–18178
Zhu Y et al (2020) High COX-2 expression in cancer-associated fibroblasts contributes to poor survival and promotes migration and invasiveness in nasopharyngeal carcinoma. Mol Carcinog 59(3):265–280
Ziemann F et al (2015) Increased sensitivity of HPV-positive head and neck cancer cell lines to X-irradiation +/− cisplatin due to decreased expression of E6 and E7 oncoproteins and enhanced apoptosis. Am J Cancer Res 5(3):1017–1031
Acknowledgements
This work was supported by the funding from the Shandong Provincial Hospital Affiliated to Shandong First Medical University, Grant Number 2020FY027. The authors gratefully acknowledge the financial support provided by the Shandong Provincial Hospital Affiliated to Shandong First Medical University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, Z., Sun, X., Chen, Z. et al. Head and Neck Squamous Cell Carcinoma: Risk Factors, Molecular Alterations, Immunology and Peptide Vaccines. Int J Pept Res Ther 28, 19 (2022). https://doi.org/10.1007/s10989-021-10334-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s10989-021-10334-5