Abstract
In this study, Cu-13.5Al-4Ni shape memory alloy (mass %) was tested. The alloy samples, at different pressures, were aged for an equal period of 5 min. The physical properties of Cu-13.5Al-4Ni alloy samples aged for an equal period were investigated. Differential scanning calorimetry (DSC) was used to investigate the impact on thermodynamic parameters. The differential thermal analysis (TG/DTA) measurements were made to obtain the ordered-disordered phase transformations from room temperature to 1200 °C. X-ray diffraction (XRD) and optic microscope observation were used to examine the microstructure. Microhardness measurements were performed on alloy samples aged for an equal period. The physical properties of the samples aged at different pressures for an equal period were compared to the measurement results of the homogenous sample.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The shape memory alloys (SMAs), which are based on the shape memory effect, superelastic, and pseudoelastic phenomena, are related to the first-order displacive and diffusionless martensitic transformation between a high-temperature austenite phase and a low-temperature martensite phase. These alloys have undergone significant technological advancement over the past two decades for their applications as functional materials [1,2,3,4,5].
NiTi alloys are the most popular among SMAs due to their excellent shape memory properties. Due to the high cost of NiTi SMAs, Cu-based SMAs have been developed as an alternative.
The Cu-based SMAs have thermal stability and are attractive for high-temperature applications. Among Cu-based SMAs CuZnAl alloys, exhibit good mechanical properties but have poor thermal stability. Although CuAlNi SMAs exhibit better thermal stability, they have found limited application due to their more brittleness. It is well known that different types of martensitic phases such as 2H, 18R, 6R occur in Cu–Al–Ni SMAs depending on both the chemical composition of the alloys, the applied load, test temperature and crystal orientation. Because these alloys are susceptible to aging after quenching under high-temperature heat treatment conditions, their transformation temperature, martensitic phase, and mechanical properties may change with operating time.
Since the shape memory performance of the alloys is closely related to the stabilities of both the parent and martensite phases, substantial work has been devoted to the study of ageing in Cu-based SMAs in recent years. Low-temperature ageing effects on Cu-based SMAs can affect their transformation behavior, limiting their reliability in temperature-sensitive component applications. The aging process, which creates changes in the characteristics of martensite and is both time-dependent and pressure-dependent, is undesirable because it limits the technological applications of SMAs. As a result, better management and development of SMAs for engineering applications are possible thanks to a better understanding of the aging mechanism.
Aging is a time-dependent process associated with the redistribution of atoms and defects, and pressure accelerates this process. The most important result of aging in SMAs is the stability of either the parent or martensite phase.
The homogeneous distribution of small second phase particles in the matrix phase after proper heat treatments can boost the hardness and strength of various metal alloys. Precipitates are small phase particles scattered throughout the structure. The alloy ages in a sense during this time because its strength develops over time. As a result, the process is also known as precipitation hardening or age hardening [6].
There are two types of aging: natural and artificial. Natural aging occurs over a lengthy period, and aging at typical ambient temperatures increases the material's strength. The artificial aging process is carried out at temperatures higher than room temperature. Artificial aging is aided by externally imposed stress. With an increase in the aging temperature and the stress applied, the hardening process accelerated [6].
Cu-based SMAs are subject to low aging, and achieving consistent martensitic transformation behavior necessitates careful control of the post-quench thermal history. These alloys' transition temperatures, martensitic phases, and mechanical qualities can change over time due to post-quench aging in high-temperature service conditions. Artificial aging can also be utilized to alter the SMA properties [7, 8]. When a material is mechanically loaded and deformed at temperatures over a certain threshold, it changes into super-elastic. As the material is unloaded under isothermal temperatures, the significant deformation is recovered [9]. There has been a lot of study on the effects of aging in ternary CuAlNi and CuZnAl SMAs [10]. Cu–Al–Ni SMAs work well in high-temperature situations [11].
In this study, (mass %) Cu-13.5Al-4Ni SMA was used. Different pressures were applied to the alloy samples at equal times at room temperature. In the article, a study on the martensite stabilization phenomenon in martensite phase-aged Cu-Al-Ni crystals exposed to mechanical pressures is included. The study was planned as measurements of homogeneous sample and samples aged at different pressures with equal periods.
Experimental
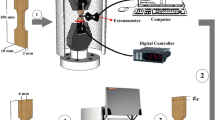
The CuAlNi SMA used in this study was provided from TREFIMETAUX Center Recherche in France. The nominal composition of the alloy is Cu-13.5Al-4Ni (mass %). Samples cut from this alloy were annealed in the β-phase field (1203 K for 30 min.) and then promptly quenched in iced brine to keep the phase at room temperature. The aging process of samples was carried out at different pressures 186 MPa, 372 MPa, 558 MPa, 745 MPa, 930 MPa in an equal period (5 min.). The transformation temperatures and thermodynamic characteristics of homogenous and aged samples were determined using differential scanning calorimetry (DSC) techniques. These parameters were determined by Perkin Elmer 8000 differential scanning calorimetry (DSC) with 10 °C min–1 heating/cooling rate. An Alumina sample crucible was used in DSC measurements. DSC measurements were carried ranging from 30 °C to 200 °C. The TG/DTA (HITACHI TG/DTA 7300) measurements of homogenous CuAlNi SMA sample and CuAlNi SMA samples aged were taken from room temperature to 1200 at a heating rate of 15 °C min–1 to identify order–disorder phase transitions at high temperatures. A Platinum sample crucible was used in DTA measurements.
For structure analysis of homogeneous and aged samples were used X-ray diffraction. X-ray analyses of alloy samples were taken with Bruker D8 Advance diffractometer. X-ray diffraction analysis of samples was made with CuKα radiation at room temperature in the 30°–90° range. The wavelength of the beam used is 1.54056 Ȧ. In addition, optical microscope observations were made for the structural changes of the samples. For optical microscope observations, first polishing and then chemical etching (5gr Fe3Cl-95 mL Ethanol-2 mL HCl) was applied to the samples. Metallographic observations were made using the NIKON ECLIPSE MA200 optical microscope. Then, Vickers hardness (VH) measurements of these samples were made. The VH of the samples was measured using a 100 g load. VH measurements were made on an EMCOTEST Dura Scan microhardness device.
Results and discussion
Differential scanning calorimetry measurements
Figure 1 depicts the DSC curve of homogeneous CuAlNi alloy sample. The tangent approach was used to determine alloy samples As, Af, Ms, and Mf transformation temperatures. The pressure versus transformation temperature curves of CuAlNi SMA samples aged for different pressures in equal period is given in Fig. 2 and transformation temperature values in Table 1. As seen in Table 1 and Fig. 2, the transformation temperatures (As, Af, Ms, Mf) of the alloy sample decreased at 186 MPa pressure. For pressures above 186 MPa, the changes in transformation temperatures are erratic. Ms, As, and Af temperature values of CuAlNi SMA aged under 373 MPa pressure increased and Mf temperature value decreased. The transformation temperatures of the CuAlNi alloy sample aged under 558 MPa pressure all showed an increasing trend. Af and Ms temperatures of the alloy sample aged under 745 MPa pressure continued to increase. The Ms temperature reached the Ms temperature of the homogeneous sample and the Af temperature approached the Af temperature of the homogeneous sample. A sharp decrease was observed in As and Mf temperatures, and they became lower than the As and Mf temperatures of the homogeneous sample. Forward and reverse transformation curves were not observed in DSC measurement of CuAlNi SMA sample aged under 930 Mpa pressure. The alloy sample aged under 930 MPa pressure lost its shape memory feature. The standard deviation in the transformation temperatures As, Af, Ms, and Mf of CuAlNi SMA aged for equal times under different pressures was calculated ± 4.47, ± 3.93, ± 3.93 and ± 6.43, respectively. Gastien et al. attributed the increase in the transition temperatures of the alloy to the ordering process that occurs during the aging process of the irregularly structured Cu–Al–Ni alloy in the quenched state [12]. Benke et al. argue that the change in transformation temperature during aging processes is a result of bainitic transformation [13]. Suresh and Ramamurty also obtained similar results and attributed the increase in transition temperature to the formation of the γ2 precipitate phase [7]. Rodriguez and Guenin observed that precipitation occurs gradually in the Cu–Al–Ni alloy when aged between 573 and 623 K, and the increase in transition temperatures is the most likely cause of this continuous [14].
Similar results were observed in the CuAlNi SMA alloy aged for equal periods of time under different pressures. The results are in agreement with the literature.
The thermodynamic parameters were also impacted by the variations in transformation temperatures (Table 2). T0 equilibrium temperature is defined as the temperature at which Gibbs free energy of the austenite and martensite phases is equal. The following equation is used to calculate it [15,16,17].
The enthalpy values, ΔHM→A and ΔHA→M have been calculated from the region thereunder endothermic and exothermic peaks in the DSC curves. The following equation is used to determine the entropy [18, 19].
The hysteresis in the transition, which is defined by the driving force for the nucleation of martensite, ΔGA→M, is represented by the supercooling T0–Ms [18,19,20].
The temperature difference Mf–Ms is connected to the elastic energy GE contained in the self-accommodated martensitic variants by [15, 17],
Table 2 calculates and lists the values of T0, ΔHM→A, ΔSM→A, ΔGA→M (Ms), and GE of the samples aged for 5 min at various pressures. The equilibrium temperature of the homogeneous CuAlNi SMA sample is 347.78 K. The equilibrium temperature of CuAlNi alloy samples aged for different pressures at equal times decreased compared to the equilibrium temperature of the homogeneous sample. However, this decrease in equilibrium temperature does not show a regular variation for different pressures. Equilibrium temperature took the smallest value with 337.02 K under 186 MPa pressure. Then it showed a regular increase and the equilibrium temperature of the alloy sample aged under 745 Mpa pressure was 346,775 K. The alloy sample aged under 930 Mpa pressure lost its shape memory feature.
The ΔG change values and the GE change values of the alloy samples aged for equal times under different pressures are given in Table 2. As seen in Table 2, ΔG free energy value increased and disordered the elastic energy change of CuAlNi SMA samples aged under different pressure.
TG/DTA measurements
Differential thermal analysis TG/DTA measurements are used to determine the order–disorder phase transitions at high temperatures. The DTA results of the samples aged under different pressure for equal times as multiple curves can be seen in Fig. 3 Cu–Al–Ni SMA show a three-phase transition with the increase of the heating and these transitions are described as β (A2) → β2 (B2) → β1 (L21). In Cu-based SMAs β (A2) → β2 (B2) → β1 (L21) order–disorder phase transitions are observed at high temperatures [21,22,23].
As to the characteristic endothermic peaks on these curves it can firstly be said that by an increment of applied pressure the peak areas, i.e., the enthalpy change values of the martensite (β1') to austenite (β) transformations at around 300 K–350 K typically decreased like these transformations lost power by the effect of pressure. This happened may be because of some defects and lattice distortions that occurred by the effect of pressure. The second endothermic peaks (at about 760 K) are the typical β1(superlattice) → β2(metastable) transitions that occur in Cu–Al–based SMAs, and the enthalpy change of these peaks first decreases with a pressure of 186 MPa and then increase again at a pressure of 372 MPa. The enthalpy values again decreased with aging under pressures of 558 MPa and 745 MPa. In the DTA measurement of the sample aged under 930 MPa pressure, the (β1') → (β) and β1 → β2 transitions were lost. The third peaks are phase transitions from eutectoid solid to solid. The eutectoid reaction occurs between 850 K and 900 K. The enthalpy values of the samples aged under different pressures increased with increasing pressure. The enthalpy was highest with the aging of the sample under 930 MPa pressure.
X-ray diffraction (XRD) analysis results
Structural analysis of CuAlNi SMA samples aged under different pressures at equal times of 5 min was investigated using X-ray diffraction. X-ray diffraction patterns of alloy samples aged are given in Fig. 4a-f. In the crystal structure of homogenous CuAlNi SMA is seen peaks of β' and α martensite phases Fig. 4a. Changes in peak intensities may be seen in XRD patterns of alloy samples aged under various pressures. As can be seen from the XRD patterns of the samples aged under different pressures (186 Mpa, 372 Mpa, 558 Mpa, 745 Mpa, 930 Mpa) for equal periods, (122), (0018), (311) peaks remained. The applied aging process changed the intensities of these peaks. In addition, some peaks disappeared and new peaks were formed. Peaks in the XRD patterns are consistent with the literature [24,25,26,27].
The Debye–Scherrer equation given below was used to determine the crystallite size of the alloy samples [28,29,30].
where D indicates the crystal size, λ the X-ray wavelength, FWHM the full width at half the maximum peak, and θ Bragg angle. Table 3 lists the crystallite sizes for the alloy samples. As can be observed, the crystallite size of CuAlNi alloy reduced as it was aged under a constant period at various pressures.
Metallographic observations and micro-hardness results
Figure 5a-f shows the optical photographs of CuAlNi SMA samples aged under different pressures for an equal time. As seen in the photograph (Fig. 5a), a homogeneous CuAlNi SMA shows martensite structure and precipitate phase at room temperature. Precipitates, various martensite plates (needle-shaped, zigzag, and V-shaped martensites), and cracks (Fig. 5b-f) can be seen in the optical images of the samples aged at varied pressures for 5 min at room temperature [5, 31,32,33].
In the photographs, precipitates (P), grain boundaries (G-B), martensite (M), water spots (W-S), and cracks (C) are shown with symbols.
A 100 g-force (gf) load was given to the aged CuAlNi SMA samples to measure their microhardness. The average Vickers microhardness values of several alloy samples are shown in Table 3. With aging, the specimen's hardness increased.
Conclusions
This article examined the effect of CuAlNi SMA aged for equal periods under different pressures on transformation temperatures and shape memory functionality. Aging is a potential barrier to the use of SMAs. Phase stability and their tendency toward SME distortion limit the applications of SMA. The aging of the material was accelerated by using an equal period of 5 min for varying pressures applied at room temperature. With equal periods aging under different pressures, the transformation temperatures and equilibrium temperatures of CuAlNi SMA are reduced under 186 MPa pressure and then tend to increase under higher pressures. Externally applied pressure not only increases the starting temperature of martensite and Gibbs free energy but also increases the amount of converted volume, that is, the amount of martensite formed. The standard deviation of the T0 equilibrium temperature is ± 2.38. At Ms temperature, the T0–Ms temperature difference creates chemical free energy between the phases. This free energy creates the driving force necessary to initiate the transformation. The T0–Ms temperature difference of CuAlNi SMA samples aged for equal periods under different pressures decreased. This means that the driving force required to initiate the transformation decreases. That is, SME decreased with increasing pressure. The decrease and disappearance of SME can be associated with the decrease in the T0–Ms temperature difference as well as the increase in the γ precipitate phase. The thermal transformation temperatures disappeared after the alloy was aged for 5 min at 930 MPa pressure. This degradation can be associated with γ phase precipitates. Increases in the microhardness of the alloy are a result of the precipitates that developed in the structure. This conclusion is also supported by optical images and XRD measurements. The obtained results show that the CuAlNi SMA samples aged at an equal period under varied pressures modified their thermodynamic characteristics and microstructure.
References
Recarte V, Pérez-Landazábal J, Rodrı́guez P, Bocanegra E, Nó M, San-Juan J. Thermodynamics of thermally induced martensitic transformations in Cu–Al–Ni shape memory alloys. Acta Mater. 2004;52(13):3941–8.
Alaneme KK, Anaele JU, Okotete EA. Martensite aging phenomena in Cu-based alloys: effects on structural transformation, mechanicl and shape memory properties: a critical review. Sci Afr. 2021;12:e00760.
Bertrand N, Desgranges C, Gauvain D, Monceau D, Poquillon D. Low temperature oxidation of pure iron: growth kinetics and scale morphologies. In: Materials science forum. Stafa-Zurich: Trans Tech Publ Ltd; 2004.
Oliveira J, Zeng Z, Berveiller S, Bouscaud D, Fernandes FB, Miranda R, Zhou N. Laser welding of Cu–Al–Be shape memory alloys: microstructure and mechanical properties. Mater Des. 2018;148:145–52.
Dora TRK, Sampath V, Li Y, Hodgson P. In vitro cytotoxicity and corrosion studies of some copper base shape memory alloys. Mater Today Proc. 2017;4(10):10672–81.
Callister WD Jr, Rethwisch DG. Callister’s materials science and engineering. New York: Wiley; 2020.
Suresh N, Ramamurty U. Effect of aging on mechanical behavior of single crystal Cu–Al–Ni shape memory alloys. Mater Sci Eng A. 2007;454:492–9.
Shivaramu L, Shivasiddaramaiah A, Mallik U, Prashantha S. Effect of ageing on dam** characteristics of Cu–Al–Be–Mn quaternary shape memory alloys. Mater Today Proc. 2017;4(10):11314–7.
Shaw JA. Tips and tricks for characterizing shape memory alloy wire: part 1—differential scanning calorimetry and basic phenomena. Exp Tech. 2008;32:55–62. https://doi.org/10.1111/j.1747-1567.2008.00410.x.
Wei Z, Peng H, Zou W, Yang D. Aging effects in a Cu–12Al–5Ni–2Mn–1Ti shape memory alloy. Metall Mater Trans A. 1997;28(4):955–67.
Karagoz Z. Canbay CA Relationship between transformation temperatures and alloying elements in Cu–Al–Ni shape memory alloys. J Therm Anal Calorim. 2013;114(3):1069–74.
Gastien R, Corbellani CE, Bozzano PB, Sade ML, Lovey FC. Low temperature isothermal ageing in shape memory CuAlNi single crystals. J Alloys Compd. 2010;495(2):428–31.
Benke M, Mertinger V, Barkoczy P. Investigation of the kinetic of a bainitic reaction upon heating in a CuAlNiMn and a CuAlNiMnFe shape memory alloy. In: Materials science forum, vol. 752. Stafa-Zurich: Trans Tech Publ Ltd; 2013. p. 3–9.
Rodriguez P, Guenin G. Thermal aging behaviour and origin of a Cu–Al–Ni shape memory alloy. Mater Sci Eng A. 1990;129(2):273–7.
Wayman C, Tong H. On the equilibrium temperature in thermoelastic martensitic transformations. Scr Metall. 1977;11(5):341–3.
Tong H, Wayman C. Characteristic temperatures and other properties of thermoelastic martensites. Acta Metall. 1974;22(7):887–96.
Huo Y, Zu X. On the three phase mixtures in martensitic transformations of shape memory alloys: thermodynamical modeling and characteristic temperatures. Contin Mech Thermodyn. 1998;10(3):179–88.
Balo ŞN, Sel N. Effects of thermal aging on transformation temperatures and some physical parameters of Cu–13.5 wt.% Al–4 wt.% Ni shape memory alloy. Thermochim Acta. 2012;536:1–5.
Romero R, Pelegrina J. Change of entropy in the martensitic transformation and its dependence in Cu-based shape memory alloys. Mater Sci Eng A. 2003;354(1–2):243–50.
Zu X, Zhang C, Zhu S, Huo Y, Wang Z, Wang L. Electron irradiation-induced changes of martensitic transformation characteristics in a TiNiCu shape memory alloy. Mater Lett. 2003;57(13–14):2099–103.
Sutou Y, Kainuma R, Ishida K. Effect of alloying elements on the shape memory properties of ductile Cu–Al–Mn alloys. Mater Sci Eng A. 1999;273–275:375–9.
Fernandez J, Benedetti AV, Guilemany JM. Zhang XM Thermal stability of the martensitic transformation of Cu–Al–Ni–Mn–Ti. Mater Sci Eng A. 2006;438–440:723–5.
Canbay CA, Genc ZK, Sekerci M. Thermal and structural characterization of Cu–Al–Mn–X (Ti, Ni) shape memory alloys. Appl Phys A. 2014;115(2):371–7.
Straumal B, Kilmametov A, López G, López-Ferreño I, Nó M, San Juan J, Hahn H, Baretzky B. High-pressure torsion driven phase transformations in Cu–Al–Ni shape memory alloys. Acta Mater. 2017;125:274–85.
Yang GS, Lee JK, Jang WY, Jeong SJ, Inoue K. Effect of aging and microstructure on mechanical rolling CuAlNi shape memory alloy. In: Materials science forum. Stafa-Zurich: Trans Tech Publ Ltd; 2007.
Izadinia M, Dehghani K. Structure and properties of nanostructured Cu-13.2 Al-5.1 Ni shape memory alloy produced by melt spinning. Trans Nonferrous Met Soc China. 2011;21(9):2037–43.
Pelosin V, Riviére A. Structural and mechanical spectroscopy study of the β1′ martensite decomposition in Cu-12% Al-3% Ni (wt.%) alloy. J Alloys Compd. 1998;268(1–2):166–72.
Cullity B. Elements of proteins X-ray crystallography. Massachusetts: Addison-Wesley Publishing Company Inc; 1978.
Balo ŞN, Yakuphanoglu F. The effects of Cr on isothermal oxidation behavior of Fe–30Mn–6Si alloy. Thermochim Acta. 2013;560:43–6.
Suryanarayana C, Norton MG. X-ray diffraction: a practical approach. New York USA: Plenum Press; 1998. p. 207.
Mallik U, Sampath V. Effect of composition and ageing on dam** characteristics of Cu–Al–Mn shape memory alloys. Mater Sci Eng A. 2008;478(1–2):48–55.
Pereira EC, Mathlakova LA, da Silva Figueiredo ABH, Lima ES, dos Santos PA, Monteiro SN. Grain ejection associated with thermocycling of Cu–Al–Ni shape memory alloy. Mater Lett. 2018;210:54–7.
Pereira EC, Matlakhova LA, Matlakhov AN, de Araújo CJ, Shigue CY, Monteiro SN. Reversible martensite transformations in thermal cycled polycrystalline Cu-13.7% Al-4.0% Ni alloy. J Alloys Compd. 2016;688:436–46.
Acknowledgements
The study carried out was supported by Firat University Scientific Research Projects Unit (FÜBAP) under project number FF.21.16
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Balo, Ş.N., Manguri, H.S.A. Thermodynamic and structural change of Cu-13.5Al-4Ni shape memory alloy aged under different pressures for equal times. J Therm Anal Calorim 149, 2025–2032 (2024). https://doi.org/10.1007/s10973-023-12822-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12822-w