Abstract
This study presents the thermoeconomic optimization of LiBr-H2O, LiCl-H2O, (CaCl2-LiBr-LiNO3)-H2O with R290, R123, R1234yf, and R1234ze in the compression-absorption cascade refrigeration system. The detailed thermodynamics and economic analysis have been presented. Nonlinear objective function formulated based on the concept of energy, exergy, economic, and environmental performance is minimized by using five recent computational intelligence techniques. Optimum cascade, evaporator, absorber, generator, condenser, and overlap temperatures and the effectiveness of the solution heat exchanger have been reported for the cascade refrigeration system. The effect of decision variables on the coefficient of performance, total exergy destruction, total heat exchanger area, and the total annual cost of cascade refrigeration system has also been reported within their upper and lower bounds for all absorbent-refrigerant-refrigerant combinations. Among all the studied algorithms, the coyote optimization algorithm reports the minimum total annual cost for all considered absorbent-refrigerant-refrigerant combinations. The minimum and maximum total annual costs are reported as 13,164.76 $ year−1 and 15,406.65 $ year−1 for (CaCl2-LiBr-LiNO3)-H2O-R290 and LiBr-H2O-R1234yf, respectively.








Similar content being viewed by others
Abbreviations
- \(a^{c}\) :
-
Capital recovery factor
- \(A\) :
-
Heat transfer area/m2
- \(b\) :
-
Specific exergy/kJ kg−1
- \(\dot{B}\) :
-
Exergy flow/kW
- \(\dot{B}_{{\text{D}}}\) :
-
Exergy destruction/kW
- \(\dot{B}_{{{\text{in}}}}\) :
-
Input exergy/kW
- \(\dot{B}_{{{\text{out}}}}\) :
-
Output exergy/kW
- \({\text{CCU}}\) :
-
Cost of cold utility/$ year−1
- \({\text{COP}}\) :
-
Coefficient of performance
- \({\text{C}}_{{{\text{CO}}_{2} }}\) :
-
Cost charged for CO2/$ ton−1
- \(C_{{{\text{el}}}}\) :
-
Electricity cost/$ kW−1 h−1
- \(C_{{{\text{env}}}}\) :
-
Penalty cost/Carbon tax/$ year−1
- \(C_{{{\text{exer}}}}\) :
-
Cost of exergy input/$ kW−1 h−1
- \(C_{P}\) :
-
Specific heat capacity/kJ kg−1 K−1
- \(C_{T}\) :
-
Annual cost/$ year−1
- \(\dot{E}\) :
-
Energy/kW
- H :
-
Specific enthalpy/kJ kg−1
- \(i_{{\text{r}}}\) :
-
Interest rate/%
- \(\dot{m}\) :
-
Mass flow rate/kg s−1
- \(m_{{\text{f}}}\) :
-
Maintenance factor
- \(m_{{{\text{CO}}_{2} }}\) :
-
Mass of CO2 emitted/kg
- \(N_{{\text{y}}}\) :
-
Period of repayment/years
- \(P\) :
-
Pressure/kPa, bar
- \(\dot{Q}\) :
-
Heat load/kW
- \(s\) :
-
Specific entropy/kJ kg−1 K−1
- \({\text{top}}\) :
-
Period of operation/hours
- T :
-
Temperature/°C, K
- T m :
-
Logarithmic mean temperature difference
- \(T_{{\text{C}}}\) :
-
Crystallization temperature/°C
- \(U\) :
-
Overall heat transfer coefficient /kW m−2 K−1
- \(\dot{W}\) :
-
Work/kW
- \(X,w\) :
-
Concentration of absorbent in solution /%
- \(Z\) :
-
Total system capital cost/$
- \(Z_{{{\text{comp}}}}\) :
-
Compressor cost/$
- \(\varepsilon\) :
-
Effectiveness of solution heat exchanger
- \(\eta\) :
-
Isentropic efficiency of compressor/pump
- \(\lambda\) :
-
Emission factor/kg kW–1 h–1
- \(\rho\) :
-
Density/kg m−3
- \(0\) :
-
Reference value
- \({\text{abs}}\) :
-
Absorber
- \(c\) :
-
Compressor
- \({\text{cascade}}\,\,{\text{con}}\) :
-
Cascade condenser
- \({\text{cond}}\) :
-
Condenser
- \({\text{env}}\) :
-
Environment
- \({\text{evp}}\) :
-
Evaporator
- \({\text{gen}}\) :
-
Generator
- \({\text{hx}}\) :
-
Solution heat exchanger
- \({\text{isen}}\) :
-
Isentropic
- \({\text{mix}}\) :
-
Mixture
- \(P\) :
-
Pump
- \(r\) :
-
Real
- \({\text{ref}}_{1}\) :
-
Refrigerant in VCRC
- \({\text{ref}}_{2}\) :
-
Refrigerant in VARC
- salt:
-
Absorbent
- \(T\) :
-
Total
- CRS:
-
Cascaded refrigeration system
- VARC:
-
Vapour absorption refrigeration cycle
- VCRC:
-
Vapour compression refrigeration cycle
- 1, 2, 3:
-
State points
- yr:
-
Year
References
Sheikholeslami M, Farshad SA, Ebrahimpour Z, Said Z. Recent progress on flat plate solar collectors and photovoltaic systems in the presence of nanofluid: a review. J Clean Prod. 2021;293:126119. https://doi.org/10.1016/J.JCLEPRO.2021.126119.
Said Z, Sundar LS, Tiwari AK, Ali HM, Sheikholeslami M, Bellos E, et al. Recent advances on the fundamental physical phenomena behind stability, dynamic motion, thermophysical properties, heat transport, applications, and challenges of nanofluids. Phys Rep. 2021. https://doi.org/10.1016/J.PHYSREP.2021.07.002.
Bhatti MM, Michaelides EE. Study of Arrhenius activation energy on the thermo-bioconvection nanofluid flow over a Riga plate. J Therm Anal Calorim. 2021;143:2029–38. https://doi.org/10.1007/S10973-020-09492-3.
Zhang L, Bhatti MM, Michaelides EE. Thermally developed coupled stress particle-fluid motion with mass transfer and peristalsis. J Therm Anal Calorim. 2021;143:2515–24. https://doi.org/10.1007/s10973-020-09871-w.
Turkyilmazoglu M. Nanoliquid film flow due to a moving substrate and heat transfer. Eur Phys J Plus. 2020;135:781. https://doi.org/10.1140/epjp/s13360-020-00812-y.
Turkyilmazoglu M. Cooling of particulate solids and fluid in a moving bed heat exchanger. J Heat Transf. 2019;141:114501. https://doi.org/10.1115/1.4044590.
Conor G, Fisher DH, Haider SA, Eve L, Devika S, Sara C, et al. The cooling imperative forecasting the size and source of future cooling demand. Econ Intell Unit. 2019;1–69. http://www.eiu.com/graphics/marketing/pdf/TheCoolingImperative2019.pdf
Sachar S, Campbell I, Kalanki A. Solving the global cooling challenge how to counter the climate threat from room air conditioners. Rocky Mt Inst. 2018;1–50. www.rmi.org/insight/solving_the_global_cooling_challenge
UNEP. Handbook for the montreal protocol on substances that deplete the ozone layer. 14th ed. Nairobi: Ozone Secr; 2020.
Polonara F, Kuijpers LJM, Peixoto RA. Potential impacts of the montreal protocol kigali amendment to the choice of refrigerant alternatives. Int J Heat Technol. 2017;35:S1-8. https://doi.org/10.18280/ijht.35Sp0101.
Salmi W, Vanttola J, Elg M, Kuosa M, Lahdelma R. Using waste heat of ship as energy source for an absorption refrigeration system. Appl Therm Eng. 2017;115:501–16. https://doi.org/10.1016/J.APPLTHERMALENG.2016.12.131.
Nami H, Arabkoohsar A, Anvari-Moghaddam A. Thermodynamic and sustainability analysis of a municipal waste-driven combined cooling, heating and power (CCHP) plant. Energy Convers Manag. 2019;201:112158. https://doi.org/10.1016/J.ENCONMAN.2019.112158.
Bellos E, Tzivanidis C, Antonopoulos KA. Exergetic and energetic comparison of LiCl-H2O and LiBr-H2O working pairs in a solar absorption cooling system. Energy Convers Manag. 2016;123:453–61. https://doi.org/10.1016/J.ENCONMAN.2016.06.068.
Li N, Luo C, Su Q. A working pair of CaCl2–LiBr–LiNO3/H2O and its application in a single-stage solar-driven absorption refrigeration cycle. Int J Refrig. 2018;86:1–13. https://doi.org/10.1016/J.IJREFRIG.2017.11.004.
Tripp JT. The UNEP montreal protocol: industrialized and develo** countries sharing the responsibility for protecting the stratospheric ozone layer. NYU J Int Law Polit. 1988;20:733.
Liang Y, Shu G, Tian H, Liang X, Wei H, Liu L. Analysis of an electricity–cooling cogeneration system based on RC–ARS combined cycle aboard ship. Energy Convers Manag. 2013;76:1053–60. https://doi.org/10.1016/J.ENCONMAN.2013.08.056.
Wang L, Ma A, Tan Y, Cui X, Cui H. Study on solar-assisted cascade refrigeration system. Energy Procedia. 2012;16:1503–9. https://doi.org/10.1016/j.egypro.2012.01.236.
Garimella S, Brown AM, Nagavarapu AK. Waste heat driven absorption/vapor-compression cascade refrigeration system for megawatt scale, high-flux, low-temperature cooling. Int J Refrig. 2011;34:1776–85. https://doi.org/10.1016/J.IJREFRIG.2011.05.017.
Kairouani L, Nehdi E. Cooling performance and energy saving of a compression–absorption refrigeration system assisted by geothermal energy. Appl Therm Eng. 2006;26:288–94. https://doi.org/10.1016/J.APPLTHERMALENG.2005.05.001.
Colorado D, Velázquez VM. Exergy analysis of a compression–absorption cascade system for refrigeration. Int J Energy Res. 2013;37:1851–65. https://doi.org/10.1002/ER.3012.
Cimsit C, Ozturk IT. Analysis of compression–absorption cascade refrigeration cycles. Appl Therm Eng. 2012;40:311–7. https://doi.org/10.1016/J.APPLTHERMALENG.2012.02.035.
Jain V, Kachhwaha SS, Sachdeva G. Thermodynamic performance analysis of a vapor compression-absorption cascaded refrigeration system. Energy Convers Manag. 2013;75:685–700. https://doi.org/10.1016/j.enconman.2013.08.024.
Lijuan H, Wang S, Suxia L, Xuan W. Numerical and experimental evaluation of the performance of a coupled vapour absorption-compression refrigeration configuration. Int J Refrig. 2019;99:429–39. https://doi.org/10.1016/J.IJREFRIG.2018.11.023.
Xu Y, Jiang N, Pan F, Wang Q, Gao Z, Chen G. Comparative study on two low-grade heat driven absorption-compression refrigeration cycles based on energy, exergy, economic and environmental (4E) analyses. Energy Convers Manag. 2017;133:535–47. https://doi.org/10.1016/j.enconman.2016.10.073.
Wang J, Wang B, Wu W, Li X, Shi W. Performance analysis of an absorption-compression hybrid refrigeration system recovering condensation heat for generation. Appl Therm Eng. 2016;108:54–65. https://doi.org/10.1016/J.APPLTHERMALENG.2016.07.100.
Patel B, Kachhwaha SS, Modi B. Thermodynamic modelling and parametric study of a two stage compression-absorption refrigeration system for ice cream hardening plant. Energy Procedia. 2017;109:190–202. https://doi.org/10.1016/J.EGYPRO.2017.03.091.
He H, Wang L, Yuan J, Wang Z, Fu W, Liang K. Performance evaluation of solar absorption-compression cascade refrigeration system with an integrated air-cooled compression cycle. Energy Convers Manag. 2019;201:112153. https://doi.org/10.1016/j.enconman.2019.112153.
Jianbo L, Kai L, **aolong H, Chen Z, Fulin C, **angqiang K. A novel absorption–compression combined refrigeration cycle activated by engine waste heat. Energy Convers Manag. 2020;205:112420. https://doi.org/10.1016/j.enconman.2019.112420.
Agarwal S, Arora A, Arora BB. Energy and exergy analysis of vapor compression–triple effect absorption cascade refrigeration system. Eng Sci Technol an Int J. 2020;23:625–41. https://doi.org/10.1016/J.JESTCH.2019.08.001.
Chen W, Li Z, Sun Q, Zhang B. Energy and exergy analysis of proposed compression-absorption refrigeration assisted by a heat-driven turbine at low evaporating temperature. Energy Convers Manag. 2019;191:55–70. https://doi.org/10.1016/J.ENCONMAN.2019.04.024.
Longo GA, Mancin S, Righetti G, Zilio C. R1234yf and R1234ze(E) as environmentally friendly replacements of R134a: Assessing flow boiling on an experimental basis. Int J Refrig. 2019;108:336–46. https://doi.org/10.1016/j.ijrefrig.2019.09.008.
Goldberg DE. Genetic algorithms in search, optimization, and machine learning. Choice Rev Online. 1989;27:27-0936-27–0936. https://doi.org/10.5860/choice.27-0936.
Eberhart R, Kennedy J. A new optimizer using particle swarm theory. Int Symp Micro Mach Hum Sci. 1999. https://doi.org/10.1109/MHS.1995.494215.
Rao RV, Savsani VJ, Vakharia DP. Teaching–learning-based optimization: a novel method for constrained mechanical design optimization problems. Comput Des. 2011;43:303–15. https://doi.org/10.1016/J.CAD.2010.12.015.
Kommadath R, Prakash K (2016) Sanitized teaching-learning based optimization. Tech Report, IIT Guwahati. 2016.
Ahmadi MH, Ahmadi MA, Mehrpooya M, Hosseinzade H, Feidt M. Thermodynamic and thermo-economic analysis and optimization of performance of irreversible four-temperature-level absorption refrigeration. Energy Convers Manag. 2014;88:1051–9. https://doi.org/10.1016/J.ENCONMAN.2014.09.041.
Rubio-Maya C, Pacheco-Ibarra JJ, Belman-Flores JM, Galván-González SR, Mendoza-Covarrubias C. NLP model of a LiBr–H2O absorption refrigeration system for the minimization of the annual operating cost. Appl Therm Eng. 2012;37:10–8. https://doi.org/10.1016/J.APPLTHERMALENG.2011.12.035.
Rezayan O, Behbahaninia A. Thermoeconomic optimization and exergy analysis of CO2/NH3 cascade refrigeration systems. Energy. 2011;36:888–95. https://doi.org/10.1016/J.ENERGY.2010.12.022.
Grosu L, Benelmir R, Feidt M. Technico-economic simulation and optimization of a compression refrigerating machine. Energy Convers Manag. 1999;40:1651–60. https://doi.org/10.1016/S0196-8904(99)00058-8.
Cimsit C, Ozturk IT, Kincay O. Thermoeconomic optimization of LiBr/H2O-R134a compression-absorption cascade refrigeration cycle. Appl Therm Eng. 2015;76:105–15. https://doi.org/10.1016/J.APPLTHERMALENG.2014.10.094.
Jain V, Sachdeva G, Kachhwaha SS. NLP model based thermoeconomic optimization of vapor compression-absorption cascaded refrigeration system. Energy Convers Manag. 2015;93:49–62. https://doi.org/10.1016/j.enconman.2014.12.095.
Turgut MS, Turgut OE. Comparative investigation and multi objective design optimization of a cascaded vapor compression absorption refrigeration system operating with different refrigerants in the vapor compression cycle. Heat Mass Transf. 2019;55:467–88. https://doi.org/10.1007/s00231-018-2430-3.
Boyaghchi FA, Mahmoodnezhad M, Sabeti V. Exergoeconomic analysis and optimization of a solar driven dual-evaporator vapor compression-absorption cascade refrigeration system using water/CuO nanofluid. J Clean Prod. 2016;139:970–85. https://doi.org/10.1016/J.JCLEPRO.2016.08.125.
Jain V, Colorado D. Thermoeconomic and feasibility analysis of novel transcritical vapor compression-absorption integrated refrigeration system. Energy Convers Manag. 2020;224:113344. https://doi.org/10.1016/J.ENCONMAN.2020.113344.
Li Z, Liu L, **g Y. Exergoeconomic analysis of solar absorption-subcooled compression hybrid cooling system. Energy Convers Manag. 2017;144:205–16. https://doi.org/10.1016/j.enconman.2017.04.052.
**g Y, Li Z, Liu L, Lu S. Exergoeconomic assessment of solar absorption and absorption-compression hybrid refrigeration in building cooling. Entropy. 2018;20(2):130. https://doi.org/10.3390/e20020130.
Jain V, Sharma N, Sachdeva G, Kachhwaha SS. Performance analysis and multi-objective optimization of cooling tower assisted vapor compression-absorptioin cascaded and hybrid refrigeration systems. Int J Green Energy. 2019;16:1024–45.
Salhi K, Korichi M, Ramadan KM. Thermodynamic and thermo-economic analysis of compression–absorption cascade refrigeration system using low-GWP HFO refrigerant powered by geothermal energy. Int J Refrig. 2018;94:214–29. https://doi.org/10.1016/J.IJREFRIG.2018.03.017.
Dixit M, Arora A, Kaushik SC. Energy, exergy, environment and economic analyses and optimization of two-stage absorption-compression combined refrigeration system. Clean Technol Environ Policy. 2017;19:2215–29. https://doi.org/10.1007/s10098-017-1404-3.
Zhao W, Wang L, Zhang Z. Atom search optimization and its application to solve a hydrogeologic parameter estimation problem. Knowledge-Based Syst. 2019;163:283–304. https://doi.org/10.1016/j.knosys.2018.08.030.
Pierezan J, Dos Santos Coelho L (2018) Coyote optimization algorithm: a new metaheuristic for global optimization problems. In: 2018 IEEE Congr Evol Comput CEC 2018—Proc. https://doi.org/10.1109/CEC.2018.8477769
Punnathanam V, Kotecha P. Yin-Yang-pair optimization: a novel lightweight optimization algorithm. Eng Appl Artif Intell. 2016;54:62–79. https://doi.org/10.1016/j.engappai.2016.04.004.
Cheraghalipour A, Hajiaghaei-Keshteli M, Paydar MM. Tree growth algorithm (TGA): a novel approach for solving optimization problems. Eng Appl Artif Intell. 2018;72:393–414. https://doi.org/10.1016/j.engappai.2018.04.021.
Makkitaya S, Kotecha P, R A (2019) Thermo economic optimizaion of the cascaded system; A Tech report IIT Guwahati 2019
Jain V, Sachdeva G, Kachhwaha SS. Energy, exergy, economic and environmental (4E) analyses based comparative performance study and optimization of vapor compression-absorption integrated refrigeration system. Energy. 2015;91:816–32. https://doi.org/10.1016/J.ENERGY.2015.08.041.
Ahmad S, Linnhoff B, Smith R. Cost optimum heat exchanger networks-2. Targets and design for detailed capital cost models. Comput Chem Eng. 1990;14:751–67. https://doi.org/10.1016/0098-1354(90)87084-3.
Lansing FL (1976) Computer modeling of a single-stage lithium bromide/water absorption refrigeration unit. PL Deep Sp Netw Prog Rep, pp 247–57
Kaita Y. Thermodynamic properties of lithium bromide-water solutions at high temperatures. Int J Refrig. 2001;24:374–90. https://doi.org/10.1016/S0140-7007(00)00039-6.
Grover GS, Eisa MAR, Holland FA. Thermodynamic design data for absorption heat pump systems operating on water-lithium chloride—Part one. Cool Heat Recover Syst CHP. 1988;8:33–41. https://doi.org/10.1016/0890-4332(88)90039-7.
Conde MR. Properties of aqueous solutions of lithium and calcium chlorides: formulations for use in air conditioning equipment design. Int J Therm Sci. 2004;43:367–82. https://doi.org/10.1016/J.IJTHERMALSCI.2003.09.003.
Chaudhari SK, Patil KR. Thermodynamic properties of aqueous solutions of lithium chloride. Phys Chem Liq. 2002;40:317–25. https://doi.org/10.1080/0031910021000004883.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix
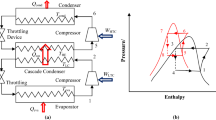
Thermo-physical properties of absorbent-refrigerant (VARC) working pairs (LiBr-H2O, LiCl-H2O, and (CaCl2-LiBr-LiNO3)-H2O) used in this study are calculated using the following equations.
LiBr-H2O
The concentration, enthalpy, and specific heat of LiBr solution are calculated as follows [57]:
where X6 and X9 (in %) are the concentrations of LiBr in weak solution and strong solution, respectively. Enthalpy and specific heat for the streams consisting of LiBr-H2O (streams: 6–11 in Fig. 1) are given as [58]:
Constant values used in Eqs. A.3–A.4 are given in Table 6.
LiCl-H2O
The concentration and specific heat capacity of LiCl-H2O are calculated as follows [59, 60]:
In Eq. (A.5), the temperature is in °C, concentration is in %, and the constant values are given in Table 6.
where T is in K; ζ is the mass fraction of the absorbent in the solution. The parameters used in Eqs. A.7–A.9 are listed in Table 7.
Enthalpy equation for LiCl-H2O is given by Chaudhari and Patil [61] for the concentration range of 0–50% of LiCl.
where T is in °C and X is in %. The coefficient values of Eqs. A.11 and A.12 are given in Table 6.
CaCl2-LiBr-LiNO3/H2O
Thermo-physical property expressions for specific heat capacity, vapour pressure, specific enthalpy are obtained from the literature [14]. The equation for vapour pressure is given as
The coefficient values of Eq. A.13 are given in Table 6. The specific heat capacity and enthalpy of the combination are given in Eqs. A.14 and A.15:
where P is in kPa, T is in °C, and w is in %. The coefficient values of Eqs. A.13–A.15 are mentioned in Table 6.
See Figs. 9
and 10
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nagraj, M.S., Maharana, D., Kotecha, P. et al. Thermoeconomic optimization of cascade refrigeration system using computational intelligence techniques. J Therm Anal Calorim 147, 13805–13827 (2022). https://doi.org/10.1007/s10973-022-11516-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11516-z