Abstract
In this work the distribution coefficients (D) of polonium between 0.1 mol dm−3 TOPO/toluene; TOPO/cyclohexane; TBP/toluene and TBP/cyclohexane and aqueous solutions of inorganic acids (HCl, HNO3, H2SO4 and H3PO4) were determinated. The molarities of inorganic acids were between 0.5 and 12.0 mol dm−3. The activity of extracted Po-209 was measured by means of liquid scintillation counting. The best result was obtained for TOPO/toluene and 2 mol dm−3 H2SO4 system (D = 62 ± 9). The most stable conditions of extraction were found for TOPO/toluene-HCl system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polonium-210 is a common element present in Earth crust (rocks and soils), but also in the atmosphere and hydrosphere. The occurrence of this element in the environment is an effect of widespread occurrence of its progenies (namely Ra-226 and Rn-222) [1]. The natural source of polonium can also be attributed to volcanic activity, forest fires, sea salts migration and resuspension of dust [2,3,4]. Natural polonium is often classified as NORM (Naturally Occurring Radioactive Material) and as such is rarely a concern from the point of radiation protection [5, 6]. Despite the fact that Po-210 is an alpha emitter and therefore poses a risk only after ingestion or inhalation it contributes significantly to the annual effective dose from natural radiation, introducing about 7% of the dose [7, 8]. Beside natural sources, Po-210 can also originate from the anthropogenic activity. It is often present in waste materials from heavy industry, liquid and solid waste from mining and fossil fuels processing (especially when mined material is described by relatively high uranium concentration), radium bearing waste, processing of phosphogypsium, metal ores processing and phosphate fertilizers production [1, 6, 9,10,11].
What is more, along with the development of new materials for nuclear industry a new problems considering polonium have been indicated. One of the examples can be found in so-called led-bismuth eutectic (LBE), a eutectic alloy composed of lead (Pb) and bismuth (Bi). Known for its low melting point and excellent heat transfer properties, making it suitable for various applications in the nuclear industry, particularly in advanced nuclear reactors and accelerator-driven systems. It has been described by Obara et al. that neutron activation of LBE lead to production of Po-210 [12], therefore demand on new methods for removal of this radionuclide is growing.
Among many known methods alpha spectrometry and mass spectrometry are most often used for polonium determination [13]. Although efficient, these methods put high requirements in terms of sample purity, which (despite the need of application of complicated radiochemical separation procedures) are not easily obtained for samples with complex matrix like industrial or environmental samples [14, 15]. Moreover, the activity concentrations of polonium in industrial samples are often very high, which makes the use of alpha spectrometry technically and economically unreasonable [16].
This problem can be solved by using liquid scintillation counting (LSC) for activity determination. This method is described by high counting efficiency for alpha particles, reaching up to 100%. Moreover LSC is less sensitive to high count rates than detectors used in alpha spectrometry. Using the alpha/beta discrimination betters the selectivity of the measurement allowing to reduce beta interferences [6, 17, 18]. Disadvantage of this method it is a relatively high sensitivity to chemical composition of the sample. Radioactive impurities (e.g. Ra-226) can affect the measurement of polonium by a liquid scintillation counter due to their potential interference with the detection process. These impurities can emit radiation that may overlap with the energy range of polonium, leading to inaccurate readings. What is more, presence of non-radioactive elements in the sample results in sample quenching, which lowers the energetic resolution and counting efficiency. For those reason the preferred purification procedure should reject all of radioactive and most of non-radioactive interference.
The promising way to achieve this requirement is to incorporate liquid–liquid extraction to radiochemical procedure. There are many reports in the literature on the extraction of polonium from various solutions of inorganic acids (HCl, HNO3, H2SO4, HF) using organic extractants such as: triisooctylamine (TIOA) [4, 19, 20], isopropyl ether, diisopropyl ketone, isobutyl phosphate (MIBK), tributyl phosphate (TBP), diethylammonium diethyldithiocarbamate (DDTC), methyl isobutyl ketone (MIBK), HTTA (thenoyltrifluoroacetone), diethylammonium dithiocarbamate (DDTC) [21], tri-n-octylphosphine oxide (TOPO) [4, 15, 22, 23], di(2-ethhexyl) phosphoric acid (HDEHP) [4, 24]. TOPO, as a promising extractant have been previously studied for the extraction of various metals [25], however, there are no reports about the distribution coefficient for polonium.
The goal of this research was to obtain information about distribution coefficient (D) od polonium between toluene and cyclohexane solutions of TOPO and TBP, and varying concentrations of several mineral acids.
The mineral phases used in this study were sets of aqueous solutions of HCl, HNO3, H2SO4 and H3PO4 in concentrations varing from 0.5 up to 12 mol dm−3. The organic extractants were tri-octylphospine oxide (TOPO) and tri-butylphosphate (TBP), each prepared in two solvents, namely toluene and cyclohexane. The chosen extractants differs significantly in extraction mechanism. TOPO acts as a neutral extractant that forms a complex with polonium through coordination bonds on the other hand TBP is an acidic extractant that forms an organic phase by extracting polonium as an anionic species, therefore it was expected that organic solvents with higher polarity (in this case toluene) may enhance the extraction efficiency of TOPO by increasing the solubility of metal complexes, on the other hand less polar solvents (like cyclohexane) are preferred for extraction by TBP, as they minimize interference from other species present in the aqueous phase [26, 27]. The results of this study can be used in elaborating radiochemical separation procedure involving liquid–liquid extraction as polonium purification and/or concentration step.
Experimental
All reagents used in this study were of analytical grade purchased from Chempur (Poland), except for Tri-octylphospine oxide (TOPO) and Tri-butylphosphate (TBP) which were obtained from Sigma-Aldrich (USA). Deionized water with conductivity less than 0.09 µS cm−1 was prepared by SolPure 78 system. The Po-209 reference solution was prepared by Radioisotope Centre POLATOM (Poland), the activity concentration of the solution was 0.951 ± 0.010 Bq g−1. Scintillation cocktail Ultima Gold AB (PerkinElmer, USA) was used. The samples were measured in PerkinElmer liquid scintillation counter Tri-Carb 3180 TR/SL.
Polonium extraction
The experimental procedure was similar for all samples. A 10 mL of acid solution was spiked with known activity of Po-209. Activity of the spike was about 0.5 Bq. Sample was then transferred into separation funnel and 10 mL of organic phase was added. The funnel was then placed on the laboratory shaker and shaken for 10 min at room temperature (22 ± 2 °C). After shaking the funnel was fixed to the laboratory holder and left for 10 min for phases separation. After this step a 9 mL aliquot of upper (organic) phase was transferred into 20 mL plastic scintillation vial and mixed with 11 mL of scintillation cocktail. The sample was stored in a dark chamber overnight and placed in a liquid scintillation counter.
Typical counting time for samples was 300 min. The concentration of polonium remaining in the aqueous phase was calculated as the difference between the activity spiked and the activity measured in the organic phase.
Organic phases used in this study were toluene solutions of TOPO and TBP along with cyclohexane solutions of TOPO and TBP. All solutions were of molarity equal to 0.1 mol dm−3. The mineral phases were aqueous solutions of HCl, HNO3, H2SO4 and H3PO4 of concentrations between 0.5 and 12 mol dm−3. In total 57 different configurations were tested, among which HCl was tested in 15 configurations, while HNO3, H2SO4 and H3PO4 in 14 configurations each. The distribution coefficient (D) was calculated as a quotient ratio of the concentration of Po-209 in organic and aqueous phases, according to Eq. 1. The data confidence level for the distribution coefficients was 95%.
where Ao and Aaq are the activities in the organic and aqueous phases respectively; Vo and Vaq are the volumes of organic and aqueous phases.
It should be noted, that the experiment consisted of two stages: in the initial stage deionized water and two concentrations of all inorganic acids 2 and 8 mol dm−3 with all extracting systems were tested.
In the second stage of the experiment, the most promising extractant that showed a high coefficient values in tested aqueous phases, namely 0.1 mol dm−3 TOPO/toluene, was tested in additional concentrations of inorganic acids, i.e.: HCl: 0.5 mol dm−3; 0.75 mol dm−3; 1.0 mol dm−3. HNO3: 4 mol dm−3; 6 mol dm−3; H2SO4: 10 mol dm−3; 12 mol dm−3; H3PO4: 0,5 mol dm−3; 1 mol dm−3.
Results and discussion
Polonium extraction from HCl solutions
In hydrochloric acid environment polonium is present in the form of a stable Po (IV) ion, thus no losses by oxidation or adsorption on glass should be observed [21, 28]. According to literature data [29] good results in the extraction of polonium from hydrochloric acid by TBP are expected, which is explained by the high chemical stability of TBP and good properties for the extraction of heavy metals [27, 30].
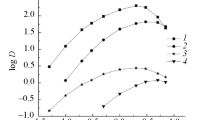
In our experiment, the highest values of the Po-209 distribution coefficient were observed for the hydrochloric acid solution extracted with the TOPO/toluene Fig. 1
The distribution coefficients ranged from 2.3 ± 0.4 (for 2 mol dm−3) to 6.5 ± 0.9 (for 0.5 mol dm−3). What is interesting, the results for 0.5 and 8 mol dm−3 were at the similar level (6.5 ± 0.9 and 6.2 ± 0.9), while for the intermediate concentrations the coefficient was slightly lover (about 4). For low concentrations of HCl comparable results were also obtained for TOPO/cyclohexane system. In case of this system the values of distribution coefficient are decreasing with the increasing acid concentration, giving coefficients of 0.37 ± 0.06 for 8 mol dm−3 solution.
Both systems based on TBP (namely TBP/toluene and TBP/cyclohexane) appeared to be much less effective than the extraction by TOPO. The highest value of 0.42 ± 0.06 was observed for 8 mol dm−3 HCl extracted with TBP/cyclohexane. In both cases, the lowest values (below 0.01) were observed for 2 mol dm−3 HNO3.
Polonium extraction from HNO 3 solutions
In case of extraction from HNO3 solutions, for all tested extracting systems the distribution coefficient decreased with the increasing acid molarity. The highest value (5.5 ± 0.8) was observed for TOPO/toluene and 2 mol dm−3 HNO3 solution, however any other system or acid concentration gave much lower results (Fig. 2). The lowest value was observed for TBP/toluene solutions, where no results exceeded 0.01. The sudden drop in the distribution coefficient values with the increase of the acid molarity for the HNO3–TOPO/toluene solutions were also described in the literature [15].
The obtained trends were partially observed by other researchers in HDEHP/cyclohexane system, where the decrease of distribution coefficient results from the increase of acid molarity.
Polonium extraction from H 2 SO 4
In case of H2SO4 solution the extraction of polonium was also most efficient in the TOPO/toluene system (Fig. 3).
The best result was obtained for an acid concentration of 2 mol dm−3, where D = 62 ± 9, turned out to be the best result of this series of measurements. The other acid concentrations also gave good results. The lowest observed result was 1.4 ± 0.2. It is worth mentioning, that in the case of the remaining systems, the distribution coefficient increases with the increase in sulfuric acid concentration, however the values obtained for high concentrations remain unsatisfactory for utilization of this acid in the final radiochemical procedure.
Extraction of polonium from aquatic solution H 3 PO 4
In case of extraction from phosphoric acid solutions, two extractants turned out to be almost equally good: TOPO/toluene and TOPO/cyclohexane (Fig. 4).
In comparison with other systems the extraction from phosphoric acid solution by TOPO/toluene was described by almost the best coefficient value for the acid concentration of 8 mol dm−3 (D = 51 ± 8) (Table 1). This result is comparable only to TOPO/toluene extraction from 2 mol dm−3 sulphuric acid extraction. What is more, phosphoric acid turned out to be the only aqueous phase, for which the result of polonium extraction with TBP/cyclohexane was higher than TBP/toluene.
Despite giving the best values of distribution coefficient, phosphoric acid turned out to be the most contaminated by natural Po-210 among all tested reagents. Nakanishi et al. [31] show that some commercially available high-purity phosphoric acids contain from 0.009 to 1.51 mBq dm−3 of Po-210. This phenomenon was also observed in this study. In order to obtain correct results each sample was followed by the blank sample in order to assess the level of contamination by natural polonium. This fact excluded H3PO4 from further studies as the intended use of studied liquid–liquid extraction process was to utilize this technique to sample purification. The introduction of additional polonium from reagents is in this case undesirable, as it may influence the results.
Conclusions
In this study the initial steps to introduce liquid–liquid extraction, as a part of sample purification to polonium determination by means of liquid scintillation counting were investigated. The determined distribution coefficients between HCl, HNO3, H2SO4 and extractants: TOPO/toluene, TOPO/cyclohexane, TBP/toluene, TBP/cyclohexane allowed to notice that in the analysed concentration range the highest D value was recorded in the TOPO/toluene/inorganic acids extraction system (in particular HCl and H2SO4). The most best overall values were recorded for the HCl 0.5–8.0 mol dm−3. A slight loss of polonium activity was also observed in TOPO/toluene extraction and 2 mol dm−3 H2SO4. For H2SO4 concentration of 8.0 mol dm−3 comparable results for extractants: TOPO/toluene and TOPO/cyclohexane were obtained, which is the basis for us to start further research with environmental samples mineralized with a mixture of these two acids. Additional motivation to study the TOPO/cyclohexane system arises from the fact that cyclohexane is considered to be more environmentally friendly than cancerogenic toluene. Unfortunately, despite the satisfactory results of the distribution coefficients obtained for the extraction with the tested organic systems and H3PO4 solutions, this acid was rejected from further studies due to high level of contamination by polonium.
References
Blanchet-Chouinard G, Larivière D (2021) Determination of polonium in environmental samples using diglycolamide based cloud point extraction coupled to alpha spectrometry analysis. Appl Radiat Isot. https://doi.org/10.1016/j.apradiso.2020.109549
Poet SE, Moore HE, Martell EA (1972) Lead 210, bismuth 210, and polonium 210 in the atmosphere: accurate ratio measurement and application to aerosol residence time determination. J Geophys Res. https://doi.org/10.1029/JC077i033p06515
Jia G, Belli M, Blasi M, Marchetti A, Rosamilia S, Sansone U (2001) Determination of 210Pb and Po-210 in mineral and biological environmental samples. J Radioanal Nucl Chem. https://doi.org/10.1023/A:1010605804815
Thakur P, Ward AL (2020) Po-210 in the environment: insight into the naturally occurring polonium isotope. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-019-06939-2
Eitrheim ES, May D, Forbes TZ, Nelson AW (2016) Disequilibrium of naturally occurring radioactive materials (NORM) in drill cuttings from a horizontal drilling operation. Environ Sci Technol Lett. https://doi.org/10.1021/acs.estlett.6b00439
Szaciłowski G, Ośko J, Pliszczyński T (2019) Determination of Po-210 in air filters from metallurgic industry. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-019-06858-2
McDonald P, Fowler SW, Heyraud M, Baxter MS (1986) Polonium-210 in mussels and its implications for environmental alpha-autoradiography. J Environ Radioact. https://doi.org/10.1016/0265-931X(86)90004-4
UNSCEAR (2000) Sources and Effects of Ionizing Radiation. United Nations Scientific Committee on the Effects of Atomic Radiation. https://doi.org/10.18356/49c437f9-en
Ram R, Vaughan J, Etschmann B, Brugger J (2019) The aqueous chemistry of polonium (Po) in environmental and anthropogenic processes. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2019.06.002
Boryło A, Skwarzec B, Wieczorek J (2022) Sources of polonium Po-210 and radio-lead 210Pb in human body in Poland. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph19041984
Muggli ME, Ebbert JO, Robertson C, Hurt RD (2008) Waking a slee** giant: the tobacco industry’s response to the polonium-210. Issue Am J Public Health 98:1643–1650
Obara T, Miura T, Fujita Y, Ando Y, Sekimoto H (2003) Preliminary study of the removal of polonium contamination by neutron-irradiated lead-bismuth eutectic. Ann Nucl Energy. https://doi.org/10.1016/S0306-4549(02)00089-0
Semenishchev VS, Polyakov EV, Kulyashova EN, Rogozhnikov VA (2023) A new method for evaluation of diffusion coefficients of alpha emitters via mathematical treatment of alpha spectra. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-022-08689-0
Ham GJ, Ewers LW, Clayton RF (1997) Improvements on lead-210 and polonium-210 determination in environmental materials. J Radioanal Nucl Chem. https://doi.org/10.1007/BF02063625
Jia G, Torri G, Petruzzi M (2004) Distribution coefficients of polonium between 5% TOPO in toluene and aqueous hydrochloric and nitric acids. Appl Radiat Isot. https://doi.org/10.1016/j.apradiso.2004.03.021
Hou X (2018) Liquid scintillation counting for determination of radionuclides in environmental and nuclear application. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-018-6258-6
Case GN, McDonald WJ (1982) An improved sensitive assay for polonium-210 by use of a background-rejecting extractive liquid-scintillation method. Talanta. https://doi.org/10.1016/0039-9140(82)80253-1
Choudhury D, Lahiri S (2018) Estimation of polonium radionuclides in proton irradiated lead-bismuth eutectic (LBE) targets by LSC-TDCR technique and gamma spectrometry. Eur Phys J A. https://doi.org/10.1140/epja/i2018-12646-7
Aarkrog A (2001) A rapid method for the separation of 210 Po from 210 Pb by TIOA extraction. J Radioanal Nucl Chem. https://doi.org/10.1023/A:1013298215640
Naskar N, Lahiri S (2021) Separation of 206-Po from alpha particle irradiated lead bismuth eutectic target. Appl Radiat Isot. https://doi.org/10.1016/j.apradiso.2021.109717
Chen Q, Hou X, Dahlgaard H, Nielsen SP, Aarkrog A (2001) A rapid method for the separation of 210-Po from 210-Pb by TIOA extraction. J Radioanal Nucl Chem. https://doi.org/10.1023/A:1013298215640
Kim CK, Lee M, Martin P (2009) Method validation of a procedure for determination of 210-Po in water using DDTC solvent extraction and Sr resin. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-008-7337-x
Uesugi M, Noguchi M, Yokoyama A, Nakanishi T (2010) Improvements on the method for determining of 210Pb and Po-210 in lead. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-009-0440-9
Jia G, Torri G (2008) Distribution coefficients of polonium between 0.75 M HDEHP in cyclohexane and aqueous hydrochloric and nitric acids. Open J Inorg Chem. https://doi.org/10.2174/1874098700802010018
White JC, Ross WJ (1961) Separations by solvent extraction with tri-n-octyl-phosphine oxide. National Academies Press, Washington. https://doi.org/10.2172/4033780
Yuan L, Sun M, Liao X, Zhao Y, Chai Z, Shi W (2014) Solvent extraction of U(VI) by trioctylphosphine oxide using a room-temperature ionic liquid. Sci China Chem. https://doi.org/10.1007/s11426-014-5194-8
Younes A, Alliot C, Mokili B, Deniaud D, Montavon G, Champion J (2017) Solvent extraction of polonium(IV) with tributylphosphate (TBP). Solvent Extr Ion Exch. https://doi.org/10.1080/07366299.2017.1279917
Matsura N, Ouchi A, Kojima M (1961) Studies on extraction of polonium(IV) by hexone from acid solution. B Chem Soc Jpn. https://doi.org/10.1080/07366299.2017.1279917
Karraker DG, Templeton DH (1951) Polonium isotopes produced with high energy particles. Phys Rev. https://doi.org/10.1103/PhysRev.81.510
Lum H, Stevens GW, Kentish SE (2012) The modelling of water and hydrochloric acid extraction by tri-n-butylphosphate. Chem Eng Sci. https://doi.org/10.1016/j.ces.2012.07.036
Nakanishi T, Fukuda H, Hirose M (1999) Determination of polonium-210 in phosphoric acid. J Radioanal Nucl Chem. https://doi.org/10.1007/BF02349867
Funding
Financial support was not provided for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaczyńska, G., Szaciłowski, G. The study on distribution coefficient of polonium between tri-octylphosphine oxide (TOPO) and tri-butylphosphate (TBP) in toluene and cyclohexane and selected inorganic acids. J Radioanal Nucl Chem (2023). https://doi.org/10.1007/s10967-023-09274-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10967-023-09274-9