Abstract
The mammalian neck adopts a variety of postures during daily life and generates numerous head trajectories. Despite its functional diversity, the neck is constrained to seven cervical vertebrae in (almost) all mammals. Given this low number, an unexpectedly high degree of modularity of the mammalian neck has more recently been uncovered. This work aims to review neck modularity in mammals from a developmental, morpho-functional, and paleontological perspective and how high functional diversity evolved in the mammalian neck after the occurrence of meristic limitations. The fixed number of cervical vertebrae and the developmental modularity of the mammalian neck are closely linked to anterior Hox genes expression and strong developmental integration between the neck and other body regions. In addition, basic neck biomechanics promote morpho-functional modularity due to preferred motion axes in the cranio-cervical and cervico-thoracic junction. These developmental and biomechanical determinants result in the characteristic and highly conserved shape variation among the vertebrae that delimits morphological modules. The step-wise acquisition of these unique cervical traits can be traced in the fossil record. The increasing functional specialization of neck modules, however, did not evolve all at once but started much earlier in the upper than in the lower neck. Overall, the strongly conserved modularity in the mammalian neck represents an evolutionary trade-off between the meristic constraints and functional diversity. Although a morpho-functional partition of the neck is common among amniotes, the degree of modularity and the way neck disparity is realized is unique in mammals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction - from Meristic Limitations to Evolutionary Modularity
Irrespective of neck length and body size of different species – long-necked giraffes and short-necked whales, big elephants and tiny shrews - the number of cervical vertebrae in mammals is constant at seven (Galis 1999; Narita and Kuratani 2005). The conspicuous constancy in the number of its constituting vertebrae makes the mammalian neck an appropriate subject for evolutionary biologists interested in conserved body plans. Only the extant tree sloths (Bradypus and Choloepus, Pilosa) and the manatees (Trichechus, Sirenia) deviate from this pan-mammalian pattern (Owen 1866; Buchholtz and Stepien 2009; Hautier et al. 2010; Varela-Lasheras et al. 2011; Buchholtz et al. 2014). The exceedingly low level of interspecific variation in the number of cervical vertebrae of mammals has puzzled biologists for nearly two centuries (Cuvier 1835; Owen 1866; Remane 1936; Galis 1999; Narita and Kuratani 2005; Buchholtz et al. 2012). In birds and sauropods the number of cervical vertebrae is highly variable (e.g., Owen 1866; Boas 1929; Taylor and Wedel 2013) and evolutionary variation in number has proven to be adaptive regarding avian neck functionality (Van Der Leeuw 1991). However, functional and morphological diversity of the mammalian neck seems to be as high as in birds, with numerous head-neck postures adopted during foraging, drinking, grooming, exploration, social interaction, and locomotion. Moreover, an unexpectedly high degree of modularity of the mammalian neck has more recently been uncovered given the low number of only seven cervical vertebrae (discussed below). What role has this modularity played in the evolution of the mammalian neck and how is it related to the limited number of cervical vertebrae? In this review, neck modularity is examined from a developmental, morpho-functional, and paleontological perspective in order to reveal how high functional diversity evolved in the mammalian neck after the occurrence of meristic limitations. The concept of modularity corresponds to the recognition that the phenotype can be decomposed into parts. Modules are tightly integrated by numerous and usually strong internal interactions compared to the few and weak interactions among different modules (Klingenberg 2008). Modularity, however, manifests on different level of integration, like genetics (e.g., pleiotropy), development, anatomical organization, or function, and thus results in different types of modules (i.e., genetic, developmental, functional modules etc.; Eble 2005; Wagner et al. 2007; Klingenberg 2008; Esteve-Altava 2017). The interplay and relationship of the different types of modules and the structured associations between the evolutionary divergence in different traits finally result in evolutionary modularity (Goswami 2006; Klingenberg 2008; Goswami et al. 2014). As this review aims to combine findings from different fields (developmental biology, veterinary biomechanics, comparative morphology, paleontology), it is hard to find modularity metrics that cover all type of data. Starting from the broad definition of Klingenberg (2008), the review therefore collects evidence for potential partition of the neck that are eventually discussed together in order to check if they argue for evolutionary modularity.
The Developmental Perspective: Constraints and Patterning
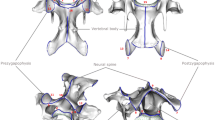
The developmental modularity of the neck is closely linked to the fixation of seven cervical vertebrae in mammals. The evolutionary origin of this meristic limitation is, however, not yet fully understood. Different hypotheses have been proposed but they agree in the fundamental role of Hox genes in determining neck patterning. Hox genes encode highly conserved homeodomain-containing transcription factors and are collinearly organized in clusters consecutively activated from cranial to caudal (Gaunt 1994; Burke et al. 1995; Wellik 2007). They are central players in patterning the vertebrate axial skeleton through region specific expression patterns (Kessel and Gruss 1991; Burke et al. 1995; Horan et al. 1995; Burke 2000; Deschamps and van Nes 2005; Mallo et al. 2010). In the mammalian neck, seven Hox genes of paralog groups 4 and 5 mediate the specification of the cervical vertebrae (Fig. 1) whereas three genes of paralog group 6 determine the development of the cervico-thoracic boundary (reviewed in Böhmer 2017). These genes show modular expression patterns that eventually also guide the specific shape of the neck vertebrae (Fig. 1). According the “Hox pleiotropy” hypothesis, changes in the number of cervical vertebrae in mammals are coupled with changes in the Hox gene expression patterns. These changes, however, are accompanied by a variety of congenital abnormalities and an increased susceptibility to cancer (Galis 1999; Galis et al. 2006; Galis and Metz 2007). For instance, cervical ribs on C7 in humans are interpreted as homeotic changes at the cervico-thoracic boundary and are frequently associated with anatomical abnormalities in stillborns (cardiovascular, nervous, or urogenital abnormalities, malformation of the neck-shoulder transition; Galis et al. 2006; Furtado et al. 2011; ten Broek et al. 2012). Thus, pleiotropic constraints are at the root of the evolutionary conservation of the number of cervical vertebrae in mammals (Fig. 1). It is proposed that the unavoidability of such pleiotropic effects is due to the strong interactions during the early developmental stage when the number of cervical vertebrae (i.e., the cervico-thoracic boundary) is determined in mammals (Galis et al. 2006, 2018). Altogether, these interactions result in strong prenatal selection against individuals with a changed number of cervical vertebrae (Galis et al. 2006, 2018; Varela-Lasheras et al. 2011).
Developmental constraints and patterning of the mammalian neck. Left: Pleiotropic effects of Hox genes prevent changes in cervical number. Innervation by brachial plexus, phrenic nerve, and ansa cervicalis reveal cervical origin of muscles in the forelimb, diaphragm, and tongue, respectively. Underlying mesodermal cell streams are patterned according the modular Hox gene expression. Right: Detailed gene expression pattern of Hox paralogues group 4 and 5 in the mammalian neck. Anterior expression patterns correlate with morphological modularity of the cervical vertebrae. Hox gene pattern after Kessel and Gruss (1991) and Böhmer (2017). Murine vertebral outlines after Johnson and O’Higgins (1996)
According to the “mesoderm patterning” hypothesis, the fixation of the number of vertebrae is based on the patterning of mesodermal cell streams that leave the embryonic neck (Buchholtz et al. 2012; Buchholtz 2014). In tetrapods, migratory muscle precursor cells from the upper and lower neck contribute to the muscle of the tongue and the forelimb, respectively. In mammals in addition, a novel cell stream originates from the mid-cervical somites and muscularizes the diaphragm. In adults, phrenic nerve innervation is a testimony of this cervical origin of the diaphragm muscles (Greer et al. 1999; Fig. 1). Buchholtz and colleagues (Buchholtz et al. 2012) proposed a specialized mid-cervical module that provides the somitic origin of diaphragm muscles and the phrenic nerve. In contrast, the more cranial parts of the neck provide the somitic origin for parts of the tongue muscles (innervations by the ansa cervicalis) whereas forelimb muscles (innervates by the brachial plexus) originate from the caudal parts (Fig. 1). The patterning of the mesodermal cell streams is thereby guided by the modular expression of Hox genes in the neck (Buchholtz et al. 2012). The strong developmental integration between the cervical mesoderm and other structures (head, forelimb, and diaphragm) constrains variation in cervical organization by preventing meristic variability. This hypothesis is further supported by recent findings that evolutionary modifications at the head-trunk interface associated with migrating mesoderm cells are crucial for the general structuring of the trunk (Hirasawa and Kuratani 2013; Hirasawa et al. 2016).
Both hypotheses have their limits and cannot explain all aspects of mammalian neck development. On the one hand, incidence for abnormalities is not higher in embryos than that for adults in non-human mammals, contradicting assumption of the “Hox pleiotropy” hypothesis (Asher et al. 2011). On the other hand, the close relationship between the number of cervical vertebrae and the diaphragm (as proposed by the “mesoderm patterning” hypothesis) could not yet be verified by Hox mutant experiments (e.g., Kostic and Capecchi 1994). Additionally, the hypotheses are not mutually exclusive. No matter which of them will be proven as more reliable, they agree in the pivotal role of the Hox genes, their modular expression, and the strong developmental integration between the neck and other body regions (pleiotropic effects, emigrating mesodermal cell streams).
The Biomechanical Perspective: Anatomical and Postural Consequences
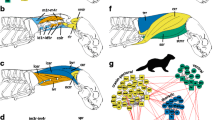
The cervical spine constitutes a simple beam that is supported at one end only (i.e., cantilever or loaded beam construction; Fig. 2; Slijper 1946, Kummer 1959). The gravitational effect of the weight of the head at the unsupported end results in permanent stress on the neck and the tendency of the head to collapse downward in an unbraced condition due to the bending moment. Head moment is increased in mammals due to the efficient yet heavy masticatory apparatus (e.g., extensive masticatory muscles). In accordance, head/neck support is maintained by dorsal passive (nuchal and spinal ligaments) and active (dorsal neck muscles) elements bridging the distance between the anterior trunk and the head/upper cervical vertebrae. Such a biomechanical construction compensates the bending moment of the head but limits variation in overall vertebral body shape or orientation of vertebral processes (Fig. 2; Kummer 1959; Arnold et al. 2017a). Additionally, the cervical spine is held as vertically as possible to reduce the distance between the weight of the head and the sustaining cervico-thoracic junction. This leads to the stereotypical vertical, s-shaped, and self-stabilizing resting posture of the mammalian cervical spine (Fig. 2; Vidal et al. 1986; Graf et al. 1995b). As head/neck movements start from this posture, orientation and gaze changes in the sagittal plane are restricted to the cranio-cervical (Occiput-C1-C2) and cervico-thoracic junction (C6-C7-Th1). The mid-cervical spine does not contribute to sagittal motion to any significant extent but is rather involved in axial rotation (Graf et al. 1995a, b). This functional modularity is supported by two prominent bony processes in mammals that provide major muscles attachments sites for head and neck motion: the enlarged spinous process on C2 and the ventral lamina on C6 (plate-like ventral tubercle of the transverse process, Chassaignac tubercle; Fig. 2).
Biomechanics of the mammalian neck. Left: Load of the head induces a permanent bending moment on the neck which is resisted by nuchal muscles and ligaments. The resulting axial load limits variation in vertebral shape. The lower neck is biomechanically linked with the forelimb via numerous muscle slips and the brachial plexus. This construction results in a tripartite functional modularity of the neck. Right: Stereotypical vertical, s-shaped, and self-stabilizing resting posture of the mammalian cervical spine, resulting in parallelogram-like movements of the head. The enlarged spinous process on C2 and the plate-like ventral lamina of C6 act as major muscles attachments sites and enable stereotypical neck posture and movement. Biomechanical model modified after Kummer (1959). Canine bone models from Arnold et al. (2016)
Moreover, several muscles span from the cervical spine to the pectoral girdle with repeated slips (Fig. 2). Through this connection, the posture and movements of the neck have crucial influence on the mechanics of the forelimb in terms of gait efficiency, balance, ground reaction forces, and kinematics (e.g., Runciman and Richmond 1997; Zsoldos and Licka 2015; Loscher et al. 2016). This interdependency further constrains variation in size and shape of vertebrae. Additionally, the position and arrangement of the brachial plexus poses a strong constraint in variation of the lower cervical spine (shape and size of intervertebral foramina). The limited amount of space between adjacent vertebrae suggests that their size and shape is generally adjusted to reduce the risk of nerve compression during neck motion or under variation of neck length (Breit and Künzel 2001; Sleutjens et al. 2010).
The Morphological Perspective: Bone Shape and Muscle Topology
The characteristic shape variation among the vertebrae within the cervical spine is the most common feature to delimit morphological modules of the neck. There are distinct shape changes between C2 and C3 as well as C5 and C6 (Johnson et al. 1999; Buchholtz et al. 2012; Arnold et al. 2016; Böhmer 2017; Randau and Goswami 2017; Villamil 2018). C1 and C2 (atlas and axis), but also C6 and C7, each have unique morphologies, whereas C3 to C5 are more uniform. Although the cervical vertebrae show secondary adaptations to ecology and locomotor mode (Osburn 1903; Shimer 1903; Lull 1904) or body size (Arnold et al. 2016; Randau et al. 2016), this basic morphological pattern among them is conserved across species. The morphological peculiarities of specific vertebrae (e.g., C2, C6) and the sequence of position specific morphologies are retained across mammals with quite different necks like rat, fruit-bat, tapir, baboon, ecologically diverse felid species, and dog breeds of different body size and skull shape (Johnson et al. 1999; Buchholtz et al. 2012; Arnold et al. 2016; Randau and Goswami 2017; Fig. 3). Even in very small mammals in which vertebral processes are generally small as muscles have to move/sustain only small masses, spinous process and ventral laminae are most distinct at C2 and C6, respectively (Fig. 3). The developmental basis for the morphological tripartite modularity (upper, middle, lower cervical spine) has been shown by the close relationship of vertebral shape and the underlying Hox gene anterior-posterior pattern (see above; reviewed in Böhmer 2017). The global validity of this modularity was proven for extant tree sloths too, which represent two of the rare deviations from the mammalian pattern of seven cervical vertebrae. Although the number of cervical vertebrae in Choloepus and Bradypus is decreased or increased, respectively, the underlying Hox gene modularity likely remained unchanged (Böhmer et al. 2018). The typical sequence of vertebral shapes (including three morphological modules), however, is condensed to six (in Choloepus) or stretched to nine vertebrae (in Bradypus) (Böhmer et al. 2018). This suggests that meristic constraints may be broken in sloths, but developmental and morphological modularity is maintained.
Constancy of position specific morphologies of cervical vertebrae. Left: Axis/C2 (from top to bottom: mole Talpa, horse Equus, bandicoot Perameles). Right: C6 (from top to bottom: tapir Tapirus, gelada Theropithecus, mouse Mus). Redrawn from Lessertisseur and Saban (1967) and Alba et al. (2014). Schematic drawing of mouse C6. Black arrows indicate ventral laminae at C6
Though the patterns of morphological modularity are most obvious in the shape of the cervical vertebrae, it was also revealed in the topology of the neck muscles (Arnold et al. 2017b). When analyzed as an anatomical network, a tripartite axial modularity into an upper (occiput, C1), mid-cervical (C2-C4), and lower musculoskeletal module (C5-C7, upper thoracic spine) is highly conserved across mammals (Arnold et al. 2017b). These modules, defined as highly interconnect clusters in a topology-based network, are not completely consistent with those suggested by vertebral shape changes (see above). This is not surprising as musculoskeletal modules have to bridge the transitions between vertebral shape modules to allow for proper neck motion (i.e., the relative motion between vertebral shape modules).
The Allometric Perspective: The Impact of Body Size
The impact of body size on the morphology and modularity of the cervical spine is more complex compared to vertebral shape. As size and locomotor related changes in vertebral shape and proportions are usually attributed to the (thoraco)lumbar spine, allometric analyses of the neck are rare in the literature. Randau and colleagues (Randau et al. 2016) found common scaling properties in the shapes of C4 and C5 across felid species, whereas the more upper and lower vertebrae scale individually. An extended allometric analysis for a huge number of mammals revealed most prominent differences in the scaling properties between C1 and the rest of the cervical vertebrae (C2-C7) (Arnold et al. 2017a). C1 length increases with larger body size and accounts for increasing head load. In contrast, C2 to C7 decrease with larger body size and account for increasing distance between the head and trunk. Thus, allometric response of the vertebral lengths and proportions suggested a bipartite modularity only (Arnold et al. 2017a). Multivariate analysis uncovered additional subtle differences between outer (C2, C7) and inner vertebrae (C3 to C6) (Arnold et al. 2017a; Villamil 2018). Thus, neck modularity from an allometric perspective is quite different compared to vertebral shapes. Although vertebral proportions are relatively uniform across the majority of mammalian species, the general pattern is altered by increasing the proportion of one of the three modules (C1, C2 & C7, or C3 to C6) under specific loading conditions on the cervical spine (big heads or aquatic lifestyle, fossorial lifestyle, or elongated necks, respectively; Arnold et al. 2017a). For instance, increasingly longer necks are realized by increasing the proportions of the middle vertebrae (C3 to C6) in long-necked antelopes and camelids (Arnold et al. 2017a). Relatively shortened necks, in contrast, are achieved by shorting C2 to C7 but not C1 (thus increasing its proportion) to maintain head support at the cranio-cervical junction (Arnold et al. 2017a).
The Paleontological Perspective: Temporal Offset in Functional Specialization
The gradual transformation of the cervical spine in the evolutionary history of extant mammals from their non-mammalian synapsid ancestors can be traced in the fossil record. Shortly after the origin of amniotes, non-synapsid lineages and synapsids evolved very different degrees of plasticity in their vertebral numbers. Basal synapsids shared a more conserved (less variable) axial configuration with crown mammals but basal reptiles already demonstrated the plasticity of extant taxa (Müller et al. 2010). Accordingly, there was a clear early divergence in axial developmental plasticity. The actual fixation to only seven cervical vertebrae dates back to the Permian-Triassic boundary (Crompton and Jenkins 1973). Although they still had the full set of cervical ribs, advanced cynodonts like Thrinaxodon, Galesaurus, Cynognathus, and Kayentatherium (i.e., epicynodonts) had seven cervical vertebrae as defined by the shape of the spinous processes, the orientation of the articular facets and the presence of intercentra (Fig. 4; Jenkins 1971; Sues and Jenkins 2006). In non-cynodont therapsids like Patranomodon, Tapinocaninus, or Regisaurus and basal cynodonts like Procynosuchus, in contrast, cervical count still showed some variation (ranging from five to nine; Kemp and Parrington 1980; Rubidge and Hopson 1996; Govender et al. 2002; Fourie and Rubidge 2007). In addition to the fixation of cervical count, three further trends are reported in the literature that characterizes mammalian neck evolution: the consolidation of the specialized cranio-cervical junction (atlas-axis complex), the reduction and fusion of cervical ribs, the modification of the cervico-thoracic junction (Lessertisseur and Saban 1967; Buchholtz et al. 2012).
Trait evolution of the cervical spine across the synapsid-mammalian transition. Adopted and modified after Buchholtz et al. (2012). See references therein for character states of the taxa. Extended by characters c and i and character states of Asioryctes (Kielan-Jaworowska 1977), zalambdalestids (Kielan-Jaworowska 1979), and Nemegtbaatar (Kielan-Jaworowska and Gambaryan 1994). Position of multituberculates after Bi et al. (2014)
The fixation to seven cervical vertebrae was followed by an early onset of the consolidation of the specialized head junction in the Triassic (early mammaliaform Megazostrodon) that proceeded into the early Jurassic when eutriconodonts and multituberculates emerged (Fig. 4; Jenkins and Parrington 1976; Ji et al. 1999). The ‘reptilian’ multi-element occipito-cervical complex was replaced by a two-element atlas-dens-axis-joint due to the stepwise loss of the proatlas and axis’ anterior articular facets as well as the fusion of the different ossification centers of the atlas and axis, respectively (characters a-g in Fig. 4; Lessertisseur and Saban 1967; Jenkins 1969; Jenkins and Parrington 1976; Li and Luo 2006; Buchholtz et al. 2012). Some derived cervical traits (e.g., atlantal foramen, no suture between atlantal and axial part of the axis) nevertheless did not evolve until the rise of crown placentals and marsupials, as even early eutherian mammals (e.g., zalambdalestids, asioryctitherians) retained several of the plesiomorphic features of monotremes and triconodonts (Kielan-Jaworowska 1977; Kielan-Jaworowska 1979). In contrast to the early onset of atlas-axis modification, the onset of cervical rib modification was historically much more recent (Fig. 4), as movable postatlantal cervical ribs were still present in Cretaceous symmetrodonts such as Maotherium or Zhangheotherium (Li and Luo 2006; Luo et al. 2007; Buchholtz et al. 2012). This suggests a Late-Jurassic origin of fused cervical ribs (as seen in stem-cladotherians; Chimento et al. 2012). Rib rudiments still occur in the early development of extant mammals but completely fuse to the vertebral bodies (forming the cervical pleurapophysis dorsal, lateral, and ventral to the transverse foramen; Cave 1975). In monotremes, the pleurapophyses are still longer and are even separated by sutures from the vertebral body in the platypus (Ornithorhynchus; Cave 1975). A modification of the cervico-thoracic junction due to the morphological specialization of the sixth cervical vertebrae (complete transformation of the fused rib rudiment into the ventral lamina) occurred with the rise of therians (Kielan-Jaworowska 1977; Kielan-Jaworowska 1979; Krause and Jenkins 1983). Thus, modifications of the lower neck (characters h-i in Fig. 4) originated not until the Jurassic-Cretaceous boundary and thus long after the fixation of cervical number and the consolidation of the derived atlas-dens-axis joint (Fig. 4). These fossil evidences altogether reveal that the functional specialization of neck modules did not evolve all at once but started much earlier in the upper than in the lower one.
Interestingly, monotremes are homoplastic in several traits of extant therians (fused atlas ossifications centers, absence of axis prezygapophyses, fusion of dens and axis, absence of free postaxial cervical ribs; Fig. 4) but their origin and temporal sequence cannot be traced as postcranial material of early australosphenidians (i.e., stem-monotremes) is completely lacking.
The Evolutionary Perspective: Meristic Constraints Meets Functional Diversity
Previous studies reviewed above proposed different modes of modularity of the mammalian neck. Interpreted conjunctly, they act on different levels of morphological integration (i.e., structural, developmental, functional; Table 1). Most of the proposed modes of modularity are very similar in dividing the neck into an upper (C1, C2), middle (C3 to C5), and lower (C6, C7) compartment, although alternative structural partitions of the neck occur (Table 1). The interplay of the different levels of integration leading to evolutionary modularity of the neck is two-fold: On the one hand, conserved modular suites of Hox genes give rise to morphological modularity (Johnson and O’Higgins 1996; Buchholtz et al. 2012; Woltering and Duboule 2015; Böhmer 2017). Pleiotropic effects and the developmental integration of the neck with other body parts constrain alterations in the modular Hox gene expression pattern (see above). This conservation, however, also maintains morphological modules and their integration, which becomes hard to override by (directional) selection (evolutionary cannalization; Wagner 1989; Kuratani 2009). On the other hand, the developmental and morphological modularity allows for specialization through the evolutionary (semi-)autonomy of the modules. The highly derived atlas-axis complex might be the best example here. The three neck modules differ in many cervical traits (summarized in Table 1 and the references therein) but the differences are generally similar across species (Fig. 3). Body size, locomotion mode, and prey capture behavior seem to affect these modules to a minor extent only (Arnold et al. 2016, 2017a; Randau et al. 2016; Villamil 2018). Functional demands for different modules of the neck have led to morphological specialization of different modules (evolutionary plasticity; Kuratani 2009). The neck functions as the manipulator of the head and has a leading biological role during many activities like exploration, orientation, foraging, drinking, gaze stabilization during locomotion, grooming, social display, etc. It has to meet a wide variety of functional demands in terms of different head trajectories during many different behaviors. Thus, the strongly conserved modularity in the mammalian neck represents an evolutionary trade-off between the meristic constraints and functional diversity (see Böhmer 2017). In this, the neck closely resembles mammalian axial regionalization in general as basic (Hox gene mediated) modularity was followed by morphological and functional heterogeneity of the modules (Jones et al. 2018). However, modularity has never been tested across levels of integration (developmental, structural, functional) or type of data (developmental, morphological, functional, paleontological) as adequate approaches are missing.
Unique Patterns of Neck Evolution in Mammals
Fossils like Thrinaxodon indicate that the reduction/fusion of lumbar ribs and thus a thoraco-lumbar differentiation date back to Triassic cynodonts (but with multiple homoplastic events; Crompton and Jenkins 1973; Luo et al. 2007; Buchholtz et al. 2012). Such bony markers of a thoraco-lumbal differentiation have been suggested as being also indicative for the muscularization of the diaphragm, in consistence with an increase in locomotor and breathing efficiency during the synapsid-mammalian transition (Perry et al. 2010; Buchholtz et al. 2012). Thus, the onset of the fixed cervical count was contemporaneous with the differentiation of the dorsal series into thoracic and lumbar spine and the inferred origin of the muscularized diaphragm. The developmental fixation to seven cervical vertebrae is therefore likely an evolutionary by-product of key innovations (increased metabolism, muscularized diaphragm, thoraco-lumbal differentiation) in mammalian metabolic and locomotor performance (Buchholtz et al. 2012, 2014; Galis et al. 2014), no matter if the fixation is directly linked with the muscularized diapragm or not (see developmental hypotheses above). Accordingly, the meristic constraints are independent from the actual function of the neck as the main actuator of the head in space. Post-Triassic modifications and modularity of the cervical spine, however, increasingly allowed to cope with the developmental constraints and biomechanical determinants in the neck. These modifications can be related to an increase in neck mobility in the sagittal plane, an elevated head/neck posture, and an increased neuromuscular control of the forelimb (Jenkins and Parrington 1976; Kielan-Jaworowska 1977).
It is not surprising to then find a tripartite modularity in a kinematic chain as uppermost and lowermost elements will tend to be modified according to their transitional function (in terms of the neck, the cranio-cervical and cervico-thoracic transition). Mid-cervical vertebrae do not have such a close functional connection to other body parts and therefore do not show functional specializations. Given the low number of vertebrae in the neck and the functional demands to the transitional vertebrae, it seems unavoidable to find a morphological tripartition of the neck, no matter of the underlying development. Compared to other amniotes’ necks, however, mammals differ in having sharp distinction between the vertebrae (C2/3 and C5/6, i.e., no smooth transition from an upper to a lower neck). Neck modularity is a trend observable in other amniotes, too, but they have mostly more modules with smoother transition (morphologically) between adjacent modules (Boas 1929; Böhmer et al. 2015; Böhmer and Werneburg 2017). Even in non-mammalian amniotes with a comparatively low number of cervical vertebrae (turtles: 8; broadbill: 11; cockatoo: 11; hummingbird: 11; woodpecker: 11; penguins: 12–13), four or more modules are identified (Guinard and Marchand 2010; Böhmer and Werneburg 2017; Terray et al. 2020). In contrast, the number of modules does not increase in three-toed sloths (Bradypus) with nine cervical vertebrae and their penultimate eighth vertebra resembles the sixth of other mammals, although they have more cervical vertebrae than turtles (Varela-Lasheras et al. 2011; Böhmer et al. 2018). Thus, mammal necks seem to be more limited in the number of modules, likely based on the fixation of the cervico-thoracic boundary and anterior Hox gene expression patterns in early development (see developmental perspective above). Due to the stronger biomechanical demands on the neck in mammals (massive heads, muscular coupling to the forelimb; see biomechanical perspective above), however, the need for a morpho-functional partition is as high (or even higher) as in other amniotes and therefore expressed in a higher degree of distinctiveness among modules (sharp shape changes, muscular topology, scaling patterns; see Table 1).
Do Conserved Meristic and Modular Patterns Limit Neck Disparity in Mammals?
The constraints and the trade-off, however, do not minimize or prevent disparity in the neck across mammalian lineages. Disparity is nonetheless achieved in cervical traits not directly related to modularity. For instance, variation in cervical spine length as a whole is a major source of disparity in the neck, whereas intracervical proportions are uniform across the majority of species (Arnold et al. 2017a). Additionally, shifts in growth rate or the morphology of the cervico-thoracic transition allow for extreme long necks such as in the giraffe (Van Sittert et al. 2010; Gunji and Endo 2016). This highlights that the way mammals achieve very long or short necks is fundamentally different from other amniotes, as neck disparity in most other amniote taxa is achieved by variation in cervical number (Müller et al. 2010; Taylor and Wedel 2013; Ward and Mehta 2014; Soul and Benson 2017). Furthermore, pectoral muscles (having expanded onto the trunk and neck in mammalian evolution) are quite variable in their number, attachments and function in head/neck motion across species whereas axial, intervertebral muscles show a similar modularity as the vertebrae (Arnold et al. 2017b). The fusion of two or more vertebrae increases cervical stability in aquatic or fossorial mammals (VanBuren and Evans 2017). However, even in cases in which C2 and C6 are incorporated in the fusion, their morphological peculiarities (and thus the underlying modularity) are still observable (e.g., Buchholtz 2011; VanBuren and Evans 2017). Finally, even the meristic constraints were broken and the number of cervical vertebrae was changed in sloths and manatees (Buchholtz and Stepien 2009; Varela-Lasheras et al. 2011; Buchholtz et al. 2014), but the underlying modularity is still present (Böhmer et al. 2018). Mammalian neck disparity is not constrained just because the number of cervical vertebrae is fixed to seven. Instead, the neck’s morphological evolution was channeled into variation in morphology of specific vertebrae or modules while vertebral count stayed fixed. Accordingly, the mammalian neck provides insights into the evolvability and adaptability of conserved body plans.
References
Alba DM, Colombero S, Delfino M, Martínez-Navarro B, Pavia M, Rook L (2014) A thorny question: the taxonomic identity of the Pirro Nord cervical vertebrae revisited. J Hum Evol 76:92-106
Arnold P, Amson E, Fischer MS (2017a) Differential scaling patterns of vertebrae and the evolution of neck length in mammals. Evolution 71:1587-1599
Arnold P, Esteve-Altava B, Fischer MS (2017b) Musculoskeletal networks reveal topological disparity in mammalian neck evolution. BMC Evol Biol 17:251
Arnold P, Forterre F, Lang J, Fischer MS (2016) Morphological disparity, conservatism, and integration in the canine lower cervical spine: insights into mammalian neck function and regionalization. Mammal Biol 81:153-162
Asher RJ, Lin KH, Kardjilov N, Hautier L (2011) Variability and constraint in the mammalian vertebral column. J Evol Biol 24(5):1080-1090
Bi S, Wang Y, Guan J, Sheng X, Meng J (2014) Three new Jurassic euharamiyidan species reinforce early divergence of mammals. Nature 514(7524):579-584
Boas JEV (1929) Biologisch-anatomische Studien über den Hals der Vögel. D Kgl Danske Vidensk Selsk Skrifter, Naturvidensk Math Afd (Série 9) 1:105–222
Breit S, Künzel W (2001) Osteological features in pure-bred dogs predisposing to cervical spinal cord compression. J Anat 199(5):527-537
Buchholtz EA (2011) Vertebral and rib anatomy in Caperea marginata: implications for evolutionary patterning of the mammalian vertebral column. Mar Mammal Sci 27:382-397.
Buchholtz EA (2014) Crossing the frontier: a hypothesis for the origins of meristic constraint in mammalian axial patterning. Zoology 117:64-69
Buchholtz EA, Bailin HG, Laves SA, Yang JT, Chan MY, Drozd LE (2012) Fixed cervical count and the origin of the mammalian diaphragm. Evol Dev 14:399-411
Buchholtz EA, Stepien CC (2009) Anatomical transformation in mammals: developmental origin of aberrant cervical anatomy in tree sloths. Evol Dev 11:69-79
Buchholtz EA, Wayrynen KL, Lin IW (2014) Breaking constraint: axial patterning in Trichechus (Mammalia: Sirenia). Evol Dev 16:382-393
Burke AC (2000) Hox genes and the global patterning of the somitic mesoderm. Curr Top Dev Biol 47:155-183
Burke AC, Nelson CE, Morgan BA, Tabin C (1995) Hox genes and the evolution of vertebrate axial morphology. Development 121:333-346
Böhmer C (2017) Correlation between Hox code and vertebral morphology in the mouse: towards a universal model for Synapsida. Zool Lett 3:1-8
Böhmer C, Amson E, Arnold P, van Heteren AH, Nyakatura JA (2018) Homeotic transformations reflect departure from the mammalian ‘rule of seven’cervical vertebrae in sloths: inferences on the Hox code and morphological modularity of the mammalian neck. BMC Evol Biol 18:84
Böhmer C, Rauhut OWM, Wörheide G (2015) New insights into the vertebral Hox code of archosaurs. Evol Dev 17:258-269
Böhmer C, Werneburg I (2017) Deep time perspective on turtle neck evolution: chasing the Hox code by vertebral morphology. Sci Rep 7:8939
Cave AJE (1975) The morphology of the mammalian cervical pleurapophysis. J Zool 177:377-393
Chimento NR, Agnolin FL, Novas FE (2012) The Patagonian fossil mammal Necrolestes: a Neogene survivor of Dryolestoidea. Rev Mus Argent Cienc Nat 14:261-306
Crompton A, Jenkins FA Jr (1973) Mammals from reptiles: a review of mammalian origins. Annu Rev Earth Planet Sci 1(1):131-155
Cuvier G (1835) Lecons d'anatomie comparée, Tome premiere. Crochard, Paris
Deschamps J, van Nes J (2005) Developmental regulation of the Hox genes during axial morphogenesis in the mouse. Development 132:2931-2942
Eble GJ (2005) Morphological modularity and macroevolution: conceptual and empirical aspects. In: Callebaut W, Rasskin-Gutman D (eds) Modularity: Understanding the Development and Evolution of Natural Complex Systems. MIT Press, Cambridge, pp 221-238
Esteve-Altava B (2017) In search of morphological modules: a systematic review. Biol Rev 92:1332-1347
Fourie H, Rubidge BS (2007) The postcranial skeletal anatomy of the therocephalian Regisaurus (Therapsida: Regisauridae) and its utilization for biostratigraphic correlation. Palaeontol Afr 42:1-16
Furtado LV, Thaker HM, Erickson LK, Shirts BH, Opitz JM (2011) Cervical ribs are more prevalent in stillborn fetuses than in live-born infants and are strongly associated with fetal aneuploidy. Pediatr Devel Pathol 14:431-437
Galis F (1999) Why do almost all mammals have seven cervical vertebrae? Developmental constraints, Hox genes, and cancer. J Exp Zool 285:19-26
Galis F, Carrier DR, Van Alphen J, Van der Mije SD, Van Dooren TJM, Metz JAJ, Ten Broek CMA (2014) Fast running restricts evolutionary change of the vertebral column in mammals. Proc Natl Acad Sci USA 111:11401-11406
Galis F, Metz JAJ (2007) Evolutionary novelties: the making and breaking of pleiotropic constraints. Integr Comp Biol 47:409-419
Galis F, Metz JAJ, van Alphen JJM (2018) Development and evolutionary constraints in animals. Annu Rev Ecol Evol Syst 49:499-522
Galis F, Van Dooren TJM, Feuth JD, Metzt JAJ, Witkam A, Ruinard S, Steigenga MJ, Wijnaendts LCD (2006) Extreme selection in humans against homeotic transformations of cervical vertebrae. Evolution 60:2643-2654
Gaunt SJ (1994) Conservation in the Hox code during morphological evolution. Int J Dev Biol 38:549-552
Goswami A (2006) Cranial modularity shifts during mammalian evolution. Am Nat 168:270-280
Goswami A, Smaers J, Soligo C, Polly P (2014) The macroevolutionary consequences of phenotypic integration: from development to deep time. Philos Trans R Soc B 369:20130254
Govender R, Rubidge BS, Renaut AJ (2002) The first complete vertebral column of a basal tapinocephalid dinocephalian (Synapsida: Therapsida). S Afr J Sci 98:391-392
Graf W, de Waele C, Vidal P (1995a) Functional anatomy of the head-neck movement system of quadrupedal and bipedal mammals. J Anat 186:55-74
Graf W, de Waele C, Vidal P, Wang D, Evinger C (1995b) The orientation of the cervical vertebral column in unrestrained awake animals. II. Movement strategies. Brain Behav Evol 45:209-231
Greer JJ, Allan DW, Martin-Caraballo M, Lemke RP (1999) An overview of phrenic nerve and diaphragm muscle development in the perinatal rat. J Appl Physiol 86:779-786
Guinard G, Marchand D (2010) Modularity and complete natural homeoses in cervical vertebrae of extant and extinct penguins (Aves: Sphenisciformes). Evol Biol 37:210-226
Gunji M, Endo H (2016) Functional cervicothoracic boundary modified by anatomical shifts in the neck of giraffes. R Soc Open Sci 3:150604
Hautier L, Weisbecker V, Sánchez-Villagra MR, Goswami A, Asher RJ (2010) Skeletal development in sloths and the evolution of mammalian vertebral patterning. Proc Natl Acad Sci USA 107:18903-18908
Hirasawa T, Fujimoto S, Kuratani S (2016) Expansion of the neck reconstituted the shoulder–diaphragm in amniote evolution. Dev Growth Differ 58:143-153
Hirasawa T, Kuratani S (2013) A new scenario of the evolutionary derivation of the mammalian diaphragm from shoulder muscles. J Anat 222:504-517
Horan GSB, Kovàcs EN, Behringer RR, Featherstone MS (1995) Mutations in paralogous Hox genes result in overlap** homeotic transformations of the axial skeleton: evidence for unique and redundant function. Dev Biol 169:359-372
Jenkins FA Jr (1969) The evolution and development of the dens of the mammalian axis. Anat Rec 164:173-184
Jenkins FA Jr (1971) The postcranial skeleton of African cynodonts: problems in the early evolution of the mammalian postcranial skeleton. Bull Peabody Mus Nat Hi 36:1–216
Jenkins FA Jr, Parrington FR (1976) The postcranial skeletons of the Triassic mammals Eozostrodon, Megazostrodon and Erythrotherium. Philos Trans R Soc B 273:387-431
Ji Q, Luo Z, Ji S (1999) A Chinese triconodont mammal and mosaic evolution of the mammalian skeleton. Nature 398(6725):326-330
Johnson D, McAndrew T, Oguz Ö (1999) Shape differences in the cervical and upper thoracic vertebrae in rats (Rattus norvegicus) and bats (Pteropus poiocephalus): can we see shape patterns derived from position in column and species membership? J Anat 194:249-253
Johnson D, O'Higgins P (1996) Is there a link between changes in the vertebral “hoxcode” and the shape of vertebrae? A quantitative study of shape change in the cervical vertebral column of mice. J Theor Biol 183:89-93
Jones K, Angielczyk K, Polly P, Head JJ, Fernandez V, Lungmus JK, Tulga S, Pierce SE (2018) Fossils reveal the complex evolutionary history of the mammalian regionalized spine. Science 361:1249-1252
Kemp TS, Parrington FR (1980) The primitive cynodont Procynosuchus: structure, function and evolution of the postcranial skeleton. Philos Trans R Soc B 288:217-258
Kessel M, Gruss P (1991) Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell 67:89-104
Kielan-Jaworowska Z (1977) Evolution of the therian mammals in the Late Cretaceous of Asia. Part II. Postcranial skeleton in Kennalestes and Asioryctes. Palaeontol Pol 37:65-84
Kielan-Jaworowska Z (1979) Evolution of the therian mammals in the Late Cretaceous of Asia. Part III. Postcranial skeleton in the Zalambdalestidae. Acta Palaeontol Pol 38:5-41
Kielan-Jaworowska Z, Gambaryan PP (1994) Postcranial anatomy and habits of Asian multituberculate mammals. Foss Strat 36:1-92
Klingenberg CP (2008) Morphological integration and developmental modularity. Annu Rev Ecol Evol Syst 39:115-132
Kostic D, Capecchi MR (1994) Targeted disruptions of the murine Hoxa-4 and Hoxa-6 genes result in homeotic transformations of components of the vertebral column. Mech Dev 46:231-247
Krause DW, Jenkins FA Jr (1983) The postcranial skeleton of North American multituberculates. Bull Mus Comp Zool Harvard Univ 150:199-246
Kummer B (1959) Biomechanik des Säugetierskeletts. In: Helmcke J-G, Legerken Hv, Starck D (eds) Handbuch der Zoologie, Band 8 (6). Walter de Gruyter & Co. Verlag, Berlin, pp 1-80
Kuratani S (2009) Modularity, comparative embryology and evo-devo: developmental dissection of evolving body plans. Dev Biol 332:61-69
Lessertisseur J, Saban R (1967) Squelette axial. In: Grassé P-P (ed) Traité de Zoologie, Mammifères: Tèguments et Squelettes. Masson et Cie, Paris, pp 584-708
Li G, Luo Z-X (2006) A Cretaceous symmetrodont therian with some monotreme-like postcranial features. Nature 439:195-200
Loscher DM, Meyer F, Kracht K, Nyakatura JA (2016) Timing of head movements is consistent with energy minimization in walking ungulates. Proc R Soc Lond B 283:20161908
Lull RS (1904) Adaptations to aquatic, arboreal, fossorial and cursorial habits in mammals. IV. Cursorial adaptations. Am Nat 38:1-11
Luo Z-X, Chen P, Li G, Chen M (2007) A new eutriconodont mammal and evolutionary development in early mammals. Nature 446:288-293
Mallo M, Wellik DM, Deschamps J (2010) Hox genes and regional patterning of the vertebrate body plan. Dev Biol 344:7-15
Müller J, Scheyer TM, Head JJ, Barrett PM, Werneburg I, Ericson PGP, Pol D, Sánchez-Villagra MR (2010) Homeotic effects, somitogenesis and the evolution of vertebral numbers in recent and fossil amniotes. Proc Natl Acad Sci USA 107:2118-2123
Narita Y, Kuratani S (2005) Evolution of the vertebral formulae in mammals: a perspective on developmental constraints. J Exp Zoll Part B 304:91-106
Osburn RC (1903) Adaptation to aquatic, arboreal, fossorial and cursorial habits in mammals. I. Aquatic adaptations. Am Nat 37-651-665
Owen R (1866) On the Anatomy of Vertebrates, Vol. II: Birds and Mammals. Longmans, Green and Co., London
Perry SF, Similowski T, Klein W, Codd JR (2010) The evolutionary origin of the mammalian diaphragm. Respir Physiol Neurobiol 171(1):1-16
Randau M, Goswami A (2017) Morphological modularity in the vertebral column of Felidae (Mammalia, Carnivora). BMC Evol Biol 17:1-12
Randau M, Goswami A, Hutchinson JR, Cuff AR, Pierce SE (2016) Cryptic complexity in felid vertebral evolution: shape differentiation and allometry of the axial skeleton. Zool J Linnean Soc 178:183–202
Remane A (1936) Wirbelsäule und ihre Abkömmlinge. In: Bolk L, Göpert E, Kallius E, Lubosch W (eds) Handbuch der vergleichenden Anatomie der Wirbeltiere. Urban & Schwarzenberg, Berlin, pp 1-206
Rubidge BS, Hopson JA (1996) A primitive anomodont therapsid from the base of the Beaufort Group (Upper Permian) of South Africa. Zool J Linnean Soc 117:115-139
Runciman RJ, Richmond FJ (1997) Shoulder and forelimb orientations and loading in sitting cats: implications for head and shoulder movement. J Biomech 30:911-919
Shimer H (1903) Adaptations to aquatic, arboreal, fossorial and cursorial habits in mammals. III. Fossorial adaptations. Am Nat 37:819-825
Sleutjens J, Voorhout G, Van Der Kolk JH, Wijnberg ID, Back W (2010) The effect of ex vivo flexion and extension on intervertebral foramina dimensions in the equine cervical spine. Equine Vet J 42:425-430.
Slijper EJ (1946) Comparative biologic–anatomical investigations on the vertebral column and spinal musculature of mammals. Verh K Ned Akad We Afd Natuurk Sect 2 42:1–128
Soul LC, Benson RB (2017) Developmental mechanisms of macroevolutionary change in the tetrapod axis: a case study of Sauropterygia. Evolution 71:1164-1177
Sues H-D, Jenkins FA Jr (2006) The postcranial skeleton of Kayentatherium wellesi from the Lower Jurassic Kayenta Formation of Arizona and the phylogenetic significance of postcranial features. In: Carrano MT, Blob RW, Wible JR (eds) Amniote Paleobiology: Perspectives on the Evolution of Mammals, Birds, and Reptiles. University of Chicago Press, Chicago, pp 114-152.
Taylor MP, Wedel MJ (2013) Why sauropods had long necks; and why giraffes have short necks. PeerJ 1:e36
Ten Broek CMA, Bakker AJ, Varela-Lasheras I, Bugiani M, Van Dongen S, Galis F (2012) Evo-devo of the human vertebral column: on homeotic transformations, pathologies and prenatal selection. Evol Biol 39:456-471
Terray L, Plateau O, Abourachid A, Böhmer C, Delapré A, de la Bernardie X, Cornette R (2020) Modularity of the neck in birds (Aves). Evol Biol. https://doi.org/10.1007/s11692-020-09495-w
Van Der Leeuw A (1991) Scaling effects on cervical kinematics in drinking Anatidae. Neth J Zool 42:23-59
Van Sittert SJ, Skinner JD, Mitchell G (2010) From fetus to adult—an allometric analysis of the giraffe vertebral column. J Exp Zool Part B 314:469-479
Van Buren CS, Evans DC (2017) Evolution and function of anterior cervical vertebral fusion in tetrapods. Biol Rev, 92:608-626
Varela-Lasheras I, Bakker AJ, van der Mije SD, Metz JA, van Alphen J, Galis F (2011) Breaking evolutionary and pleiotropic constraints in mammals: On sloths, manatees and homeotic mutations. EvoDevo 2:11
Vidal PP, Graf W, Berthoz A (1986) The orientation of the cervical vertebral column in unrestrained awake animals. Exp Brain Res 61:549-559
Villamil CI (2018) Phenotypic integration of the cervical vertebrae in the Hominoidea (Primates). Evolution 72:490-517
Wagner GP (1989) The origin of morphological characters and the biological basis of homology. Evolution 43:1157-1171
Wagner GP, Pavlicev M, Cheverud JM (2007) The road to modularity. Nat Rev Genet 8-921-931
Ward AB, Mehta RS (2014) Differential occupation of axial morphospace. Zoology 117:70-76
Wellik DM (2007) Hox patterning of the vertebrate axial skeleton. Dev Dyn 236:2454-2463
Woltering JM, Duboule D (2015) Tetrapod axial evolution and developmental constraints; Empirical underpinning by a mouse model. Mech Dev 138:64-72
Zsoldos RR, Licka TF (2015) The equine neck and its function during movement and locomotion. Zoology 118:364-376
Acknowledgements
I am grateful to Martin Fischer for his support during my work on this project and his comments and discussion on the manuscript. I thank two anonymous reviewers and the editor whose comments greatly enhanced the manuscript. This work is based on parts of my PhD thesis at the University of Jena, Germany. I thank Carolin Altmann for language improvement. The author was in parts funded by the Max Planck Society.
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declarations
The author declares that he has no conflict of interest. All data can be found within the publication.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arnold, P. Evolution of the Mammalian Neck from Developmental, Morpho-Functional, and Paleontological Perspectives. J Mammal Evol 28, 173–183 (2021). https://doi.org/10.1007/s10914-020-09506-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-020-09506-9