Abstract
This article presents and discusses the results of a compilation of experimental results of thermogravimetry with simultaneous differential scanning calorimetry (TG-DSC), obtained in the same apparatus and under similar experimental conditions, for a selection of nanoporous materials with interesting properties as adsorbents and catalysts, namely clays and clay-based materials (such as pillared-clays and porous clays heterostructures), zeolites and related materials (such as titanosilicates), mesostructured silicas and MOFs. Materials functionalized with a relatively common silane, the (3-aminopropyl)triethoxysilane (APTES) were also analyzed and discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The use of thermogravimetry with simultaneous differential scanning calorimetry (TG-DSC) for the characterization of solids has been often reviewed, particularly although not exclusively, for organic materials (such as polymers) and glasses [1,2,3,4,5,6]. TG-DSC can be highly informative since the two types of data, the mass loss or gain (TG) and the associated differential heat flux (DSC), are obtained at the same time in the same sample, with the possibility of detection of endo and exothermic effects that might not imply mass changes [7]. The principles and experimental description of TG-DSC were already provided in various publications and time spanning [1, 4, 7]. Basically, a microbalance is coupled with a Tian-Calvet [8] calorimeter, which allows the measurement of the mass loss or gain, and the heat/energy absorbed or released by the sample, respectively, when the sample is subject to a temperature path.
The purpose of the present work is to focus on the TG-DSC studies in nanoporous materials that are interesting as adsorbents and/or as catalysts such as zeolites, metal–organic frameworks (MOFs), clay based and mesostructured solids. Therefore, the studies herein discussed present illustrative TG-DSC results for examples of those materials, showing the importance that these data have in the characterization of different types of nanoporous materials. The data was always obtained in the same apparatus, in the same laboratory, and under similar experimental conditions. This allows a clear comparison of results amongst the materials, which is not always straightforward employing data from bibliographic sources and different apparatus/methodologies.
This text does not intend to make a complete detailed interpretation of the TG-DSC results but to enlighten some main features for each type of material that, as mentioned above, have relevant properties as adsorbents and/or catalysts. Additional bibliography for the interpretation of more specific aspects is also provided.
2 The Equipment and the Materials Studied
A TG-DSC model 111 (Setaram, France) was used in all the reported experiments. The measures, were made in air flow, the few cases where helium or nitrogen were used are indicated below. More details are given in Supporting Information—Section S1. The TG-DSC apparatus used has a safety limit temperature of 800 °C, but most of the studies described in this work were made at lower upper temperatures. Therefore, and to present the data in a uniform and comparable way, the results are presented from 25 to 600 °C. The few cases where a more restricted range of temperatures was used are justified below. A similar situation applies to the heating rate, which was 5 °C/min. The heat flow (DSC signal) is expressed in Watt per gram of solid material.
The studied materials were (i) zeolites: 3A, 4A, 5A, NaY, 13X, NaZSM-20, Chabazite, Ferrierite, Mordenite, ZSM-5, Na-ETS-4, Na and Ce-ETS-10; (ii) clay based materials: raw clays from three different origins, K10, clays pillared with aluminium oxide pillars (PILCs), and porous clays heterostructured (PCHs) of various compositions; (iii) mesostructured materials: MCM-41, SBA-15, SBA-16, HMS, and (iv) MOFs: Cu-BTC, Fe-BTC, IRMOF-8, UiO-66, MIL-53(Al). Detailed information on the origin and/or the preparation of the materials are given in Supporting Information—S.2. Schemes of the structures of the materials are given in Supporting Information—S.3.
3 Zeolites
Zeolite and zeolite-like materials are known for a long time in adsorption and catalysis, besides other fields of application. Various books and reviews have been published about these materials, their structures and applications [9,10,11,12]. The three-dimensional framework structures of zeolites resulting from the linkage of TO4 tetrahedra, where T is mainly but not exclusively, Si or Al, as well as their pore topologies, have been very detailed described by the Structure Commission of the International Zeolite Association in their publications and website [13, 14]. Schematic representations of the structures of the zeolites discussed in this section are given in Supporting Information—S.3
Figure 1 presents the results for zeolites of A type and Chabazite. Zeolites 3A, 4A and 5A have the same structure [14], and similar Si/Al ratios of 1.1 [15] but differ on the extra-framework cations: Na+–K+, Na+ and Ca2+–Na+, respectively. The pore openings of A zeolites are small [16] and the cations have a slight effect on the pore openings in relation to the sodic form (4.2 Å for the 4A sample) being smaller in 3A and larger in 5A. The TG-DSC curves in Fig. 1 are relatively similar to each other, they correspond to the water loss, presenting a broad endothermic DSC signal from ambient to near 250 °C. This signal is signal is broader for the 5A sample, as a probable consequence of the presence of the Ca2+ cations.
The highest temperature for the minimum (the peak) in the DSC curve (189 °C) was noticed for the material with the smallest pore openings (3A zeolite), while 4A and 5A zeolites presented similar values between them (166 °C). Chabazite (Fig. 1d) is also a zeolite with small pores that has Ca2+ as extra-framework cations, the maximum diameter of a hypothetical sphere that can diffuse along the structure of this zeolite has 3.7 Å [13], but has a higher Si/Al than the series of A zeolites. This Si/Al ratio is 2 thus having a lower number of extra-framework cations (less aluminum implies less charge compensating cations). The lowest peak in the DSC curve (132 °C) is also lower than for the type A zeolites.
The Y and X zeolites have the same type of structure, in cages,—FAU [14], but the Si/Al ratios are different namely 2.5 and 1.2 for Y and 13X, respectively. In Fig. 2 the TG-DSC results for the sodic form of Y zeolite and X zeolites (13X) and for ZSM-20 (also in the sodic form) are presented. While the mass losses for Y and 13X are similar, the DSC curve presents a minimum for a higher value for 13X (190 °C) than for Y zeolite (110 °C) and, additionally, the curve is much broader for 13X. These results are compatible with the fact that 13X has a higher number of extra-framework cations that can populate the main cation sites in these materials differently [17]. The ZSM-20 zeolite (Fig. 2c) is a mixed cubic and hexagonal stackings of FAU sheets that results in an intergrowth of the FAU and EMT structures with and Si/Al ratio of 4.2 [18]. Nevertheless, the intergrowth of the structures does not translate into clear differences in the TG-DS results for instance in comparison with the Y zeolite (FAU structure).
TG-DSC results for zeolites type Y (a) and 13X (b); ZSM-20 [88] (c) and mordenite (d)
As the zeolites with the FAU and EMT structures, mordenite (Fig. 2d) is also considered a large pore zeolite [16], with a structure in channels with side pockets [14]. The Si/Al ratios of, for instance ZSM-20 (4.2) and mordenite (5) is not much different, meaning that the total number of (sodic) charge compensating cations might not be much different also but the types of cation sites and their distribution for those sites, accordingly to what has been extensively discussed in the literature [17, 19, 20] are necessarily diverse and the DSC curve, much broader for the mordenite zeolite is an experimental consequence of this different site locations in the two structures.
Within the temperature range studied, that is below 600 °C, the main transformations regarding both, mass loss and heat released or absorbed, are mostly related to water loss. Nevertheless, some zeolites can present changes in bond angles and structural or textural stability as reviewed elsewhere [21,22,23]. The effect of the temperature on crystal lattice of zeolites is studied to some extension, as reviewed for instance in Ref. [21] but still, trends are not always clear due to the large variation of structures and compensating cations. Nevertheless, in most of the cases as temperature rises a contraction in the cell volume (between less than 1 to 17%) can be noticed [22].
Salt occlusion in zeolites was studied for purposes such as the immobilization of radioactive species, electrorefining [24] or the slow release of nitrates as fertilizers [25,26,27]. Figure 3a presents an example for the occlusion of KNO3 in the Y zeolite, prepared as described elsewhere [25]. The date in this figure was obtained just until 370 °C C because all the transformations ascribed KNO3 occur below this temperature. The TG curve (for Y + KNO3 zeolite) shows a lower mass loss than in the case of the sample in Fig. 2a as a consequence of the process of occlusion of the salt that implied loss of porous volume that could be used to adsorb water from moisture. In Fig. 3a the DSC curve for pure KNO3 shows the phase transition and melting [28] endothermic signals at 133 and 335 °C respectively. After occlusion these endothermic signals, particularly the one related to the melting of KNO3, are shifted to lower temperatures. With the support of 23Na MAS NMR and 23Na triple-quantum (3Q) MAS NMR, this fact was interpreted by the authors as being due to the variation in the local environments of the exchangeable sodium cations upon the occlusion of potassium nitrate [25].
a TG-DSC data for Y zeolite with occluded KNO3 and DSC curve for pure KNO3 [25]. b 4A zeolite surface grafted with chitosan; c and d Y and 4A zeolites surface grafted with silanated poly(ethylene glycol)—PEG, respectively.
In the context of adsorption and subsequent delivery of gases with therapeutical effects, such as nitric oxide and hydrogen sulfide [29], 4A zeolite was grafted with chitosan and silanated poly(ethylene glycol), the latter was also used with Y zeolite, upon procedures described in the literature [30, 31], with the objective of produce a slow release of the adsorbed gases. The TG-DSC curves for the chitosan grafted in 4A zeolite (Fig. 3b) below 225 °C reveal a strong contribution for the mass decrease due to the water loss, corresponding to two endothermic signals in the DSC curve. This pattern is different from what was shown in the initial 4A zeolite (Fig. 1b), in which only one signal was recorded and can be ascribed to water released from the zeolite and also form the chitosan itself. After about 225 °C, the DSC signal is endothermic and can be ascribed to the chitosan polymer decomposition which occurs between this temperature and 525 °C in an oxidant atmosphere [32]. The amount of chitosan can be estimated subtracting the TG curves for the initial zeolite and the zeolite with chitosan between these temperature (225 and 525 °C) which, for the material in Fig. 3d) gives 10.5% [31]. In the case of the surface grafting with silanated poly(ethylene glycol) in Y and 4A zeolites, Fig. 3c and d, respectively, the melting of the polymer [33] is registered at a temperature that is higher in the Y (66.5 °C) than in 4A (60.3 °C) zeolite. Also the decomposition of the grafted PEG, which is reported to begin to occur near 200 °C [34], starts at a higher temperature for the material with Y zeolite, in comparison to the one obtained with the 4A zeolite. Both facts, the higher melting and decomposition temperature in Y zeolite, are compatible with the possible formation of heavy species in this case, due to the more voluminous cages of the Y zeolite structure.
Titanosilicates of ETS-4 and ETS-10 have been exploited as adsorbents and catalysts [35,36,37,38]. The structure of these crystalline microporous oxides is closely related to the zeolites [36]. At room temperature, ETS-4 and ETS-10 have micropores with pore openings of, approximately, 4 and 8 Å respectively [36, 37]. One interesting particularity of ETS-4 (Fig. 4a) is that the pore openings shrink between room temperature (4.27 Å) and 300 °C (3.90 Å) [36]. The two endothermic signals in the heat curve of ETS-4 were reported before [35] but they are not noticed for ETS-10 (Fig. 4b) for which the heat curve resembles those found for zeolites with similar pore openings, such as Y and ZSM-20 as discussed above. In the case of ion-exchange of Na-ETS-10 with Ce3+ cations (Fig. 4c) an additional transformation is noticed in the heat curve, near 400 °C that can tentatively be ascribed to the de-hydration of cerium hydroxide, that can be formed in aqueous solution at pH values near 7 [39], in parallel with the ion-exchange.
4 Clay Based Materials
Natural clays are a large group of materials [40, 41]. In this section the focus will be on a particular type of clays: the montmorillonites. The main reasons for this are because these lamellar clays are widely available, they possess important properties of ion-exchange and expandability [41] and, therefore, montmorillonites have being exploited for the preparation of materials that present permanent porosity and have important applications in adsorption and catalysis. Montmorillonites have, however, a much wider field of applications as materials as reviewed elsewhere [42]. By themselves, montmorillonites do not have permanent porosity as they swell on contract upon variations of humidity or polar solvents but, after experimental procedures leading to the introduction of some types of species between the clay layers, materials with permanent porosity can be achieved. These materials are essentially of two types: pillared interlayered clays (PILCs) and porous clays heterostructures (PCHs). These two types of materials (PILCs and PCHs) have been extensively reviewed concerning their preparation and also their properties of adsorption and catalysis [43,44,45,46,47]. In brief, while PILCs are prepared by ion exchange with of large oligomeric cations, for instance from aluminium, zirconium or other, that upon thermal treatment form rigid pillars; PCHs are prepared by first intercalating polar long chain organic molecules to expand the layers followed by the formation of pore walls, for instance by the polymerization of silica species followed by a thermal treatment to decompose the organic parts and provide free porosity [43,44,45,46,47]. The schematic representation of the structures of PILCs and PCHs are given in Supporting Information—Section S.3. Although the departure montmorillonite, being natural substances, are subjected to some degree of variability, some trends and common features can be obviously identified in the TG-DSC results, as well as for the PILCs and PCHs that are prepared from them.
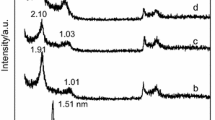
The two natural montmorillonites in Fig. 5a and c are from Wyoming (USA) and Porto Santo Island (Portugal)—WY and PS, respectively. Details on the characteristics of these samples can be found elsewhere [48]. In the range of temperatures up to 200 °C, the endothermic transformation corresponds to the loss of the water physically adsorbed between the clay sheets. The position of this transformation depends on the nature of the exchangeable cations and the nature of the surface chemistry [41, 49]. The second endothermic transformation, near 450 °C is attributed to the loss of OH groups, that is, dehydroxylations [41]. This transformation is usually more pronounced for the montmorillonites that have a higher degree of isomorphic substitutions, which is in fact the case for the PS sample when compared with the WY clay [48]. In the case of the PILCs (Fig. 5b, d), and after being calcined at 350 °C according to the procedure for their preparation [50], the endothermic transformation due to the water loss occurs in a wider range of temperatures, particularly for the Al-WY sample, since now the water is removed not only from the clay layers but from the pillars also.
Silylation of materials, often with (3-aminopropyl)triethoxysilane (APTES), can be used for the modification of oxide being applicable to the fields of catalysis, namely to attach active phases by cross-linkage, adsorption, and nanocomposites [51]. Figure 6 shows an example where K10 (a commercially available montmorillonite [52]) was studied by TG-DSC before and after functionalization with APTES [52]. The results for initial K10 clay are much similar to the PS clay discussed above, even though the results for the K10 sample were obtained in helium flux. After functionalization, with the results obtained also under helium flux (Fig. 6b), the initial decrease in the mass due to the water loss is decreased in relation to the initial K10, in line with the decrease in the hydrophobicity due to the presence of the organic moieties of the organo-silane. After 120 °C the DSC curve shows a continuous endothermic signal associated with a mass decrease due to the decomposition of APTES in the inert helium atmosphere.
TG-DSC results for the K10 montmorillonite before (a) and (b) after functionalization with (3-aminopropyl)triethoxysilane (APTES) [52]
The Porous Clays Heterostructures (PCHs) in Fig. 7a–c were prepared using as silica sources tetraethoxysilane (TEOS) and phenyltriethoxysilane (PhOS) (detailed preparation described in Ref. [53]) increasing the amount of PhOS from zero in PCH1) to 1:6 and 1:1 molar ratios TEOS:PhOS in PCH2 and PCH3, respectively. All materials show a mass loss for temperatures up to 150 ºC due to the physisorbed water and more evident for PCH1 and PCH2 (Fig. 7a, b), in line with the high adsorption capacity of these materials when compared with PCH3 [53] but the effect of the hydrophobic nature of the organic species, that is, the phenyl groups of the PhOS cannot be ruled out. Above 400 °C a significant mass decrease is observed for the samples where PhOS was used (PCH2 and PCH3) but not for PCH1. This mass loss above 400 °C is attributed to the decomposition of the organic moieties of the materials, due to the phenyl group of PhOS, indicating that these materials are stable until 400 °C, an important information if, for instance, these materials are considered for adsorption processes with thermal regeneration [53].
TG-DSC results for porous clays heterostructures (PCHs) prepared using as silica sources tetraethoxysilane (TEOS) and phenyltriethoxysilane (PhOS): (a) only TEOS; (b) 10% PhOS and (c) 50% PhOS [53]
Considering the heat-flow curves, it can be seen that the temperature at the minima of the ascribed to the water loss, which is observed at 129 °C for PCH1, is shifted to lower temperatures for PCH2 (119 °C) and PCH3 (104 °C). This series of temperatures can be related to the surface chemistry of the materials, namely of its increased hydrophobicity due to the presence of the phenyl groups, making the interactions with the water molecules weaker. If the DSC curves for the PCHs (Fig. 7) are compared with the DSC curve for a highly hydrophilic material such as the 13X zeolite (Fig. 2b) it can be noticed that the minimum in the DSC signal for 13X zeolite occurs near 200 °C, a value considerably higher than those found for the PCHs. In this way, the PCHs in Fig. 7 have and hydrophobic nature which is relevant for instance for the adsorption processes that involve adsorption of organic molecules which can be promoted even in the presence of some degree of humidity [53].
5 Mesostructured Materials
According to the IUPAC definition, mesoporous materials have pore openings between 2 and 50 nm [54]. Although mesoporous materials were known for long time [55], it was only after 1990 that a group of materials, mainly silicas, with regular mesopores became well established [56]. These materials were obtained using surfactants that form templates where silicates can condensate and form the pore walls, originating the mesostructured materials [57]. Some of the more know and studied, that were also some of the first reported in the literature, are MCM-41 (Mobil Composition of Matter 41, or Mobil Crystalline Material 41), HMS (Hexagonal Mesoporous Silica), SBA-15 (Santa Barbara Amorphous 15) where the mesoporous are assembled in a hexagonal symmetry and SBA-16 that is cubic cage-structured [56, 57]. The synthesis [58] and applications in adsorption [59] and in catalysis [60] of mesostructured materials were reviewed in the literature. In this section TG-DSC results obtained with MCM-41, SBA-15, SBA-16, HMS and the carbon replica CMK-3 [61], are discussed. The silica materials have pore openings between 2.5 nm for HMS [62] and 9.6 nm for SBA-15 [62] and the carbon replica CMK-3 between 8.5 and 10 nm [62].
As can be seen in Fig. 8a–d, a common feature related with the initial mass loss in these materials due to the water desorption, is that the respective heat flow curves present a minimum that is always below 100 °C. Comparing this with the heat flow curves for the materials discussed above, including the PCH materials, the temperature for this minimum is the lowest for the mesostructured materials in Fig. 8. This is in line with the fact that, unlikely to what occurs for the materials discussed until this point, the samples in Fig. 8 have pores with silica walls that do not have, for instance, cations or other highly polar species at their surface [56]. In this way, the interactions with the water molecules are essentially made via the surface OH groups [56], hence less strong.
TG-DSC results for the mesostructured silicas (a) MCM-41, (b) SBA-15, (c) SBA-16 and (d) HMS [62]
In the case of the mesostrutured silicas the lack of strong acidity/basicity or redox properties, and although these functions can be to some extent introduced in steps subsequent to their synthesis [63], did not promote their wide use as catalysts. However, their regular structures and pore dimensions revealed to be adequate to their use as supports of large metallic complexes, particularly those that are active in enantioselective reactions [64]. In this way, the usually expensive enantioselective metal-complex catalysts could, in principle, be more easily recovered and re-used therefore improving their efficiency. There are several experimental routes to anchor metal-complexes at the surface of mesostructured silicas, namely the use of a linker between the silica surface and the active phase (the metal-complex) and one of the common linker is the 3-aminopropyl)triethoxysilane (APTES) [64] that was also mentioned above in this text.
In Fig 9a the TG-DSC results for the MCM-41 sample functionalized with APTES [52] were obtained in inert atmosphere (helium flux). When comparing with the results for the non-functionalized material (Fig. 8a), the minimum in the DSC curve for the first endothermic transformation, due to water loss [65], is shifted for higher temperatures by near 20 °C. The complexity of the Si–O–Si and Si–O–C bonds in siloxanes was recently reviewed in detail [66] and this temperature shift is most probably related to the difference of the interactions of water with the (non-functionalized) MCM-41 by one hand and with the siloxane moiety of the functionalized material by another hand. For temperature above 140 °C the transformations are due to the decomposition of the APTES moieties and the partial dehydroxylation that is known to occur in silicas above 170 °C [65]. Also for the SBA-15-APTES material (data obtained in air flow)—Fig. 9b) the heat flow curve for the initial temperatures (until 200 °C) is much broader than for the non-functionalized SBA-15 (Fig 8b) as a consequence of the water not being adsorbed only at the silica surface and hydroxyl groups [65] but also in the APTES groups. The maximum in the heat-flow curve, at 244 °C (higher than the boiling point of liquid APTES, which is 217 °C [67]) is accompanied with a step in the mass loss, revealing partial decomposition of APTES in air at this temperature. The decomposition of APTES continues until the highest temperature achieved in the experiment.
Using the mesostructured silicas as a template, and upon a process of polymerization inside the pores, calcination and dissolution of the silica, carbon replicas can be obtained [61]. This is the case of CMK-3, the carbon replica of SBA-15 [61, 68] for which the TG-DSC data, obtained in inert atmosphere, is presented in Fig. 9c. A curious aspect when comparing the curves for SBA15 (Fig. 8b) and CMK-3 is that, although the nature of the surface of the materials is completely different, the minimum in the heat-flow curve only differs by 7°: 78 and 85 °C for CMK-3 and SBA-15, respectively. The remaining of the TG and DSC curves are also relatively similar for both materials, being the total mass loss higher for the SBA-15 sample, of course, properties in adsorption and catalysis are rather different [68, 69].
6 Metal–Organic Frameworks (MOFs)
Metal–organic frameworks (MOFs) have been one of the focuses of research in porous materials for the last three decades, due to the potential of combinations in composition, structures (more than 20,000 by 2015 [70]) and potential applications namely as adsorbents and catalysts, as extensively reviewed in the literature [70,71,72,73,74].
A MOF, can be considered as a porous crystalline polymer constructed by metal sites and organic building blocks that should display strong bonding providing robustness, and a geometrically well-defined structure [71, 75]. The number of MOF structures is so high that selecting some of them to illustrate a particular feature can be consider somehow arbitrary. Nevertheless, Fig. 10 displays the TG-DSC results for a copper and an iron MOFs where the linker is benzene-1,3,5-tricarboxylate (BTC). The Cu-BTC material was one of the MOFs first reported to have a high surface area value [76] (1661 m2 g−1 BET specific surface area for the sample in Fig. 10) and is presently commercially available and is one of the most widely studied MOFs. Its iron analogue received less attention in the literature, most probably due to the lower crystalline order of its structure as discussed elsewhere [77]. The pore network of Cu-BTC is composed by channels of about 9 Å in diameter, which intersects forming large cavities of 16 Å [77, 78]—Structure in Supporting Information—S.3. In Fig. 10, after the initial mass loss below 130 °C, due to the removal of water and solvent molecules [77] a marked exothermal transformation (and mass loss) is detected in the heat-flow curve due to the oxidative degradation of the organic BTC linkers (that, when pure, sublime at 300 °C [79]). The maximum in the heat curves is 304 and 313 °C for Cu and Fe-BTC respectively, denoting that the latter structure is somehow ore stable, as noticed previously by other authors [77].
The cavities in the metal-organic framework IRMOF-8 [80] are smaller than those of Cu-BTC. This may imply that IRMOF-8 can have a lower adsorption capacity for some gases, in comparison to Cu-BTC but, for selective adsorption, that is, for the separation of gas mixtures, smaller pores are not necessarily a disadvantage. In fact, since interactions between the adsorbed molecules and the pore walls are then promoted, the possibilities for a selective differentiation (selective adsorption) of molecules in a mixture increase [81]. The structure of IRMOF-8 is made of Zn4O tetranuclear clusters connected by naphthalene-2,6-dicarboxylate linkers creating a cubic framework [82]—Structure in Supporting Information—S.3. The TG-DSC results for IRMOF-8 are reported in Fig. 11 both, in an oxidative (air) and in inert (nitrogen) atmospheres.
TG-DSC results for IRMOF-8 in air (a) and in nitrogen (b); UiO-66 (c) [85] and MIL-53 (Al) (d)
The oxidative decomposition of IRMOF-8 is exothermal, starts near 387 °C and has its maximum value in the heat-flow curve at 458 °C. In the inert atmosphere the decomposition is endothermic, and the structure is more stable since it starts to decompose at 415 °C and presents its maximum value in the heat-flow curve at 513 °C. In the sense of MOFs with small pores, UiO-66 [83] is one material with small pore openings. Its structure is a three-dimensional arrangement of cages of 8 and 11 Å connected through narrow triangular windows of 7 Å [84]. It is a thermally stable MOF that, after a first endothermal signal in the heat-flow, associated to an initial mass loss that will include removal of solvent molecules [84, 85], shows an exothermic signal in the heat-flow, ascribed to the degradation of the structure that shows a maximum for 502 °C (Fig. 11c).
The MOF MIL-53(Al) it is now a commercial aluminum MOF, where the linker is the 1,4-benzenedicarboxylate (BDC) and has the most interesting property of structure breathing [86]. In this way, after synthesis, the rhombic one dimensional channels have the dimensions of 7.3 × 7.7 Å and are occupied by BDC species that, after heating, are removed and the channels have then the dimensions of 8.5 × 8.5 Å [86]. After the MOF is left at room temperature, moisture adsorption will occur and the channels have then the dimensions of 2.6 × 13.6 Å. This breathing nature has been explored, for instance, for adsorption purposes [87]. The TG-DSC curves (Fig. 11d) show a first endothermal transformation near 100 °C, due to water loss [86] and an exothermal peak that starts at 479 °C and has a maximum at 561 °C, due to the structure collapse with the departure of the linker [86] illustrating, nevertheless, the relatively elevated thermal stability of MIL-53 (Al).
7 Conclusions
The present work presented a series of simultaneous TG-DSC experiments for temperatures between 25 and 600 °C, determined in the same apparatus, in a group of nanoporous materials that are interesting for adsorption and for catalysis. It was possible to illustrate a group of typical results that can be obtained in selected clays and clay based materials, zeolites, mesostrutured silicas and MOFs. In the context of the study of nanoporous adsorbents and catalysts, more than the absolute results obtained, it is the high complementary nature of TG-DSC, with other techniques and methodologies of analysis, that makes this technique highly valuable.
References
P.J. Haines, Thermal Methods of Analysis-Principles, Applications and Problems (Springer, Dordrecht, 2012)
A.E. Slobozeanu, S.E. Bejan, I.A. Tudor, A.-M. Mocioiu, A.M. Motoc, M.D. Romero-Sanchez, M. Botan, C.G. Catalin, C.L. Madalina, R.R. Piticescu, C. Predescu, Manuf. Rev. 8, 1 (2021)
R. Mansa, S. Zou, Environ. Adv. 5, 100117 (2021)
Q. Zheng, Y. Zhang, M. Montazerian, O. Gulbiten, J.C. Mauro, E.D. Zanotto, Y. Yue, Chem. Rev. 119, 7848 (2019)
I. Blanco, V. Siracusa, Materials (Basel). 14, 1686 (2021)
X. Byrn, S.R. Zografi, G. Chen, Solid-State Properties and Pharmaceutical Development (Wiley, Hoboken, 2017), pp.124–141
M.E. Brown, Handbook of Thermal Analysis and Calorimetry: Principles and Practice (Elsevier, Amsterdam, 1998)
R.W. Attree, R.L. Cushing, J.A. Ladd, J.J. Pieroni, Rev. Sci. Instrum. 29, 491 (1958)
K. Margeta and A. Farkaš, in Zeolites, edited by K. Margeta and A. Farkaš (IntechOpen, Rijeka, 2020).
Y. Li, J. Yu, Chem. Rev. 114, 7268 (2014)
S. Kulprathipanja, Zeolites in Industrial Separation and Catalysis (Wiley, Weinheim, 2010)
A. C. J. Cejka, in (WILEY-VCH, Weinheim, 2010)
Structure Commission of the International Zeolite Association (2021)
C. Baerlocher, L.B. McCusker, D.H. Olson, Atlas of Zeolite Framework Types (Elsevier, Amsterdam, 2007)
J. Kim, T. Jung, D.-W. Cho, C.-Y. Yoo, J. Ind. Eng. Chem. 110, 274 (2022)
E. M. Flanigen, R. W. Broach, and S. T. Wilson, in Zeolites Ind. Sep. Catal. (John Wiley & Sons, Ltd, 2010), pp. 1–26
M. Jeffroy, C. Nieto-Draghi, A. Boutin, Chem. Mater. 29, 513 (2017)
J.M. Newsam, M.M.J. Treacy, D.E.W. Vaughan, K.G. Strohmaier, W.J. Mortier, J. Chem. Soc. Chem. Commun. (1989). https://doi.org/10.1039/c39890000493
G. Maurin, R.G. Bell, S. Devautour, F. Henn, J.C. Giuntini, J. Phys. Chem. B 108, 3739 (2004)
G. Maurin, P. Senet, S. Devautour, P. Gaveau, F. Henn, V.E. Van Doren, J.C. Giuntini, J. Phys. Chem. B 105, 9157 (2001)
G. Cruciani, J. Phys. Chem. Solids 67, 1973 (2006)
G. Cametti, A.C. Scheinost, S.V. Churakov, ACS Omega 5, 31774 (2020)
O.O. Shichalin, E.K. Papynov, V.A. Nepomnyushchaya, A.I. Ivanets, A.A. Belov, A.N. Dran’kov, S.B. Yarusova, I.Y. Buravlev, A.E. Tarabanova, A.N. Fedorets, S.A. Azon, Z.E. Kornakova, S.Y. Budnitskiy, I.G. Tananaev, Y. Shi, Y. **ong, H. Wang, J. Eur. Ceram. Soc. 42, 3004 (2022)
A. Harward, L. Gardner, C.M.D. Oldham, K. Carlson, T.-S. Yoo, G. Fredrickson, M. Patterson, M.F. Simpson, J. Nucl. Fuel Cycle Waste Technol. 20, 259 (2022)
A. Carvalho, J. Pires, P. Veloso, M. Machado, M. Brotas de Carvalho, J. Rocha, Microporous Mesoporous Mater. 58, 163 (2003)
M. Park, J.S. Kim, C.L. Choi, J.-E. Kim, N.H. Heo, S. Komarneni, J. Choi, J. Control Release 106, 44 (2005)
N.C. Hyatt, J.A. Hriljac, A. Choudhry, L. Malpass, G.P. Sheppard, E.R. Maddrell, MRS Proc. 807, 359 (2003)
N. Kumar, R. Nath, Polym. Eng. Sci. 53, 1856 (2013)
K.M. Dillon, R.J. Carrazzone, J.B. Matson, K. Kashfi, Biochem. Pharmacol. 176, 113931 (2020)
S. Jo, K. Park, Biomaterials 21, 605 (2000)
M. Batista, M.L. Pinto, F. Antunes, J. Pires, S. Carvalho, Materials (Basel) 14, 6701 (2021)
P.-Z. Hong, S.-D. Li, C.-Y. Ou, C.-P. Li, L. Yang, C.-H. Zhang, J. Appl. Polym. Sci. 105, 547 (2007)
K. Pielichowski, K. Flejtuch, Polym. Adv. Technol. 13, 690 (2002)
L.U.A. Gerasimov, M. Ziganshin, V. Gorbatchuk, Int. J. Pharm. Pharm. Sci. 6, 372 (2014)
G. De Raffele, A. Aloise, P. De Luca, D. Vuono, A. Tagarelli, J.B. Nagy, J. Porous. Mater. 23, 389 (2016)
S.M. Kuznicki, V.A. Bell, S. Nair, H.W. Hillhouse, R.M. Jacubinas, C.M. Braunbarth, B.H. Toby, M. Tsapatsis, Nature 412, 720 (2001)
S.B. Waghmode, S.M. Sabne, S. Sivasanker, Green Chem. 3, 285 (2001)
Z. Gao, M. **ang, M. He, W. Zhou, J. Chen, J. Lu, Z. Wu, Y. Su, Molecules 28, 2272 (2023)
M. Pourbaix, Atlas of Electrochemical Equilibria in Aqueous Solutions (National Association of Corrosion Engineers, 1974).
B. Velde, Introduction to Clay Minerals: Chemistry, Origins, Uses and Environmental Signicance (Chapman and Hall, London, 1992)
R.E. Grim, Clay Mineralogy, 2nd edn. (McGraw Hill, New York, 1968)
F. Bergaya, G. Lagaly, Dev. Clay Sci. 1, 1–18 (2006)
Y. Cardona, S.A. Korili, A. Gil, Appl. Clay Sci. 203, 105996 (2021)
A. Gil, M.A. Vicente, Curr. Opin. Green Sustain. Chem. 21, 56 (2020)
J.A. Cecilia, C. García-Sancho, E. Vilarrasa-García, J. Jiménez-Jiménez, E. Rodriguez-Castellón, Chem. Rec. 18, 1085 (2018)
N. Chouikhi, J.A. Cecilia, E. Vilarrasa-García, S. Besghaier, M. Chlendi, F.I. Franco Duro, E. Rodriguez Castellon, M. Bagane, Minerals 9, 514 (2019)
C.H. Zhou, Appl. Clay Sci. 53, 87 (2011)
J. Pires, M. Brotas De Carvalho, A.P. Carvalho, J.M. Guil, J.A. Perdigón-Melón, Clays Clay Miner. 48, 385 (2000)
F. Rouquerol, J. Rouquerol, and P. Llewellyn, in Handb. Clay Sci., edited by F. Bergaya and G. Lagaly (Elsevier, 2013), pp. 361–379.
R. Ferreira, C. Freire, B. De Castro, A.P. Carvalho, J. Pires, M.B. De Carvalho, Eur. J. Inorg. Chem. 2002, 3032 (2002)
P. Yuan, P.D. Southon, Z. Liu, M.E.R. Green, J.M. Hook, S.J. Antill, C.J. Kepert, J. Phys. Chem. C 112, 15742 (2008)
T. Borrego, M. Andrade, M.L. Pinto, A. RosaSilva, A.P. Carvalho, J. Rocha, C. Freire, J. Pires, J. Colloid Interface Sci. 344, 603 (2010)
J. Pires, M. Bestilleiro, M. Pinto, A. Gil, Sep. Purif. Technol. 61, 161 (2008)
M. Thommes, K. Kaneko, A.V. Neimark, J.P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol, K.S.W. Sing, Pure Appl. Chem. 87, 1051 (2015)
S.J. Gregg, K.S. Sing, H.W. Salzberg, Adsorption, Surface Area and Porosity (Academic Press, Boca Raton, 1967)
K. Ariga, A. Vinu, Y. Yamauchi, Q. Ji, J.P. Hill, Bull. Chem. Soc. Jpn. 85, 1 (2012)
S. Soltani, N. Khanian, U. Rashid, T.S. Yaw Choong, RSC Adv. 10, 16431 (2020)
V. Meynen, P. Cool, E.F. Vansant, Microporous Mesoporous Mater. 125, 170 (2009)
Z. Wu, D. Zhao, Chem. Commun. 47, 3332 (2011)
J.A. Melero, R. van Grieken, G. Morales, Chem. Rev. 106, 3790 (2006)
S. Jun, S.H. Joo, R. Ryoo, M. Kruk, M. Jaroniec, Z. Liu, T. Ohsuna, O. Terasaki, J. Am. Chem. Soc. 122, 10712 (2000)
A.R. Silva, L. Carneiro, A.P. Carvalho, J. Pires, Catal. Sci. Technol. 3, 2415 (2013)
A. Taguchi, F. Schüth, Microporous Mesoporous Mater. 77, 1 (2005)
J.M. Fraile, J.I. García, C.I. Herrerías, J.A. Mayoral, E. Pires, Chem. Soc. Rev. 38, 695 (2009)
L. Peng, W. Qisui, L. **, Z. Chaocan, Colloids Surfaces A 334, 112 (2009)
F. Dankert, C. von Hänisch, Eur. J. Inorg. Chem. 2021, 2907 (2021)
J. Buckingham, S.M. Donaghy, Sep. Purif. Technol. 75, 366–376 (2010)
V.K. Saini, M. Andrade, M.L. Pinto, A.P. Carvalho, J. Pires, Sep. Purif. Technol. 75, 366 (2010)
A. Węgrzyniak, S. Jarczewski, P. Kuśtrowski, P. Michorczyk, J. Porous Mater. 25, 687 (2018)
M. Peplow, Nature 520, 148 (2015)
Inamuddin, R. Boddula, M.I. Ahamed, A.M. Asiri (eds.), Applications of Metal–Organic Frameworks and Their Derived Materials (Wiley, Hoboken, 2020)
R.-B. Lin, S. **ang, W. Zhou, B. Chen, Chem 6, 337 (2020)
M. Rubio-Martinez, C. Avci-Camur, A.W. Thornton, I. Imaz, D. Maspoch, M.R. Hill, Chem. Soc. Rev. 46, 3453 (2017)
H.-C. Zhou, S. Kitagawab, Chem. Soc. Rev. 43, 5415 (2014)
J.L.C. Rowsell, O.M. Yaghi, Micropor. Mesopor. Mater. 73, 3 (2004)
S.S.-Y. Chui, S.M.-F. Lo, J.P.H. Charmant, A.G. Orpen, I.D. Williams, Science (80-) 283, 1148 (1999)
N. Torres, J. Galicia, Y. Plasencia, A. Cano, F. Echevarría, L.F. Desdin-Garcia, E. Reguera, Colloids Surf. A 549, 138 (2018)
M.G. Plaza, A.F.P. Ferreira, J.C. Santos, A.M. Ribeiro, U. Mueller, N. Trukhan, J.M. Loureiro, A.E. Rodrigues, Microporous Mesoporous Mater. 157, 101 (2012)
R. M. Stephenson and S. Malanowski, in Handb. Thermodyn. Org. Compd. (Springer, Dordrecht, 1987), pp. 1–471.
J.K. Mohamed Eddaoudi, Science (80-) 295, 469 (2002)
J. Pires, M.L. Pinto, V.K. Saini, A.C.S. Appl, Mater. Interfaces 6, 12093 (2014)
I. Gutiérrez, E. Díaz, A. Vega, S. Ordónez, J. Chromatogr. A 1274, 173 (2013)
J.H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga, K.P. Lillerud, J. Am. Chem. Soc. 130, 13850 (2008)
P.S. Bárcia, D. Guimarães, P.A.P. Mendes, J.A.C. Silva, V. Guillerm, H. Chevreau, C. Serre, A.E. Rodrigues, Micropor. Mesopor. Mater. 139, 67 (2011)
M.L. Pinto, S. Dias, J. Pires, A.C.S. Appl, Mater. Interfaces 5, 2360 (2013)
T. Loiseau, C. Serre, C. Huguenard, G. Fink, F. Taulelle, M. Henry, T. Bataille, G. Férey, Chemistry A 10, 1373 (2004)
V. Finsy, L. Ma, L. Alaerts, D.E. De Vos, G.V. Baron, J.F.M. Denayer, Micropor. Mesopor. Mat. 120, 221 (2009)
J. Pires, J. Fernandes, A.C. Fernandes, M. Pinto, Sep. Sci. Technol. Technol. 52, 51 (2017)
M.L. Pinto, J. Rocha, J.R.B. Gomes, J. Pires, J. Am. Chem. Soc. 133, 6396 (2011)
L. Mafra, T. Cendak, S. Schneider, P.V. Wiper, J. Pires, J.R.B. Gomes, M.L. Pinto, J. Am. Chem. Soc. 139, 389 (2017)
Acknowledgements
Centro de Química Estrutural is a Research Unit funded by Fundação para a Ciência e Tecnologia through Projects UIDB/00100/2020 and UIDP/00100/2020. Institute of Molecular Sciences is an Associate Laboratory funded by FCT through Project LA/P/0056/2020. J. P. thanks to Sílvia Carvalho for valuable discussions.
Funding
Open access funding provided by FCT|FCCN (b-on). Funding was provided by Fundação para a Ciência e a Tecnologia (Grant Nos. UIDB/00100/2020 - (https://doi.org/10.54499/UIDB/00100/2020) and UIDP/00100/2020 - (https://doi.org/10.54499/UIDP/00100/2020)). Institute of Molecular Sciences is an Associate Laboratory funded by FCT through project LA/P/0056/2020 - (https://doi.org/10.54499/LA/P/0056/2020).
Author information
Authors and Affiliations
Contributions
J. Pires made the conception and design, search, preparation and writing of this review.
Corresponding author
Ethics declarations
Competing Interests
The author has no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pires, J. Simultaneous Thermogravimetry-Differential Scanning Calorimetry (TG-DSC) in Nanoporous Materials: Examples of Data for Zeolites, Metal–Organic Frameworks (MOFs), Clay Based and Mesostructured Solids. J Inorg Organomet Polym (2024). https://doi.org/10.1007/s10904-024-03048-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10904-024-03048-w