Abstract
The rapid surge in antibiotic resistance to pathogens has emerged as a grave threat to public health, globally. This multiple drug resistance (MDR) is directly linked to the high rates of morbidity and mortality worldwide due to untreated microbial infections. Therefore, it is inevitable to identify some novel, efficient, and comparatively safer antimicrobial agents to rescue the declining health index. In this regard, nanomaterials with modified structure, size, and infinity have risen as the sole source to tackle the MDR either through ameliorating the efficacy of existing drugs or by triggering entirely new bactericidal mechanisms. Out of all the nanomaterials, metals, and metal oxide nanoparticles with biopolymer-induced reduction have fetched the attention of global researchers due to their significant and promising pathogen-killing ability without any hint of resistance. The current review covers the updated molecular modes of resistance development in Gram-positive and Gram-negative bacteria, comprehensively. This review also highlighted the detailed mode of action of various metallic nanoparticles (silver, zinc, copper, titanium, and cobalt) against MDR pathogens. Moreover, this review article thoroughly discussed the correlation between the mechanisms of resistance and alternative NPs bactericidal modes for better understanding for the readers. Last but not least, toxicity analysis is also explained for safe further use.
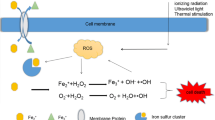
Graphical Abstract













Similar content being viewed by others
Data Availability
All data supporting the findings of this study are available within the review article.
References
S. Bassetti, S. Tschudin-Sutter, A. Egli, M. Osthoff, Optimizing antibiotic therapies to reduce the risk of bacterial resistance. European J. Intern. Med. (2022). https://doi.org/10.1016/j.ejim.2022.01.029
S. Das, R. Samantaray, A. Mallick, S.K. Sahu, S. Sharma, Types of organisms and in-vitro susceptibility of bacterial isolates from patients with microbial keratitis: a trend analysis of 8 years. Indian J. Ophthalmol. 67(1), 49 (2019)
A. Selvaraj, A. Valliammai, C. Sivasankar, M. Suba, G. Sakthivel, S.K. Pandian, Antibiofilm and antivirulence efficacy of myrtenol enhances the antibiotic susceptibility of Acinetobacter baumannii. Sci. Rep. 10(1), 21975 (2020)
A.M. Díez-Pascual, Antibacterial activity of nanomaterials. Nanomaterials 2018(8), 359 (2018)
X. Pang, X. Song, M. Chen, S. Tian, Z. Lu, J. Sun, H.G. Yuk, Combating biofilms of foodborne pathogens with bacteriocins by lactic acid bacteria in the food industry. Compr. Rev. Food Sci. Food Safety 21(2), 1657–1676 (2022)
Q. **n, H. Shah, A. Nawaz, W. **e, M.Z. Akram, A. Batool, J.R. Gong, Antibacterial carbon-based nanomaterials. Adv. Mater. 31(45), 1804838 (2019)
G. Dantas, M.O. Sommer, R.D. Oluwasegun, G.M. Church, Bacteria subsisting on antibiotics. Science 320(5872), 100–103 (2008)
M. Lobanovska, G. Pilla, Focus: drug development: Penicillin’s discovery and antibiotic resistance: lessons for the future? Yale J. Biol. Med. 90(1), 135 (2017)
Wuo, M. G., Dulberger, C. L., Brown, R. A., Sturm, A., Ultee, E., Bloom-Ackermann, Z., & Kiessling, L. L. (2022). Antibiotic action revealed by real-time imaging of the mycobacterial membrane. bioRxiv.
M.E. Enany, A.M. Algammal, S.A. Nasef, S.A. Abo-Eillil, M. Bin-Jumah, A.E. Taha, A.A. Allam, The occurrence of the multidrug resistance (MDR) and the prevalence of virulence genes and QACs resistance genes in E. coli isolated from environmental and avian sources. AMB Exp. 9(1), 1–9 (2019)
K. Huang, H. **a, Y. Zhang, J. Li, G. Cui, F. Li, N. Wu, Elimination of antibiotic resistance genes and human pathogenic bacteria by earthworms during vermicomposting of dewatered sludge by metagenomic analysis. Bioresource Technol. 297, 122451 (2020)
H.H. Kumburu, T. Sonda, M. van Zwetselaar, P. Leekitcharoenphon, O. Lukjancenko, B.T. Mmbaga, G.S. Kibiki, Using WGS to identify antibiotic resistance genes and predict antimicrobial resistance phenotypes in MDR Acinetobacter baumannii in Tanzania. J. Antimicrobial Chemother. 74(6), 1484–1493 (2019)
S. Sanyasi, R.K. Majhi, S. Kumar, M. Mishra, A. Ghosh, M. Suar, L. Goswami, Polysaccharide-capped silver Nanoparticles inhibit biofilm formation and eliminate multi-drug-resistant bacteria by disrupting bacterial cytoskeleton with reduced cytotoxicity towards mammalian cells. Sci. Rep. 6(1), 24929 (2016)
M. Alavi, M.R. Hamblin, J.F. Kennedy, Antimicrobial applications of lichens: secondary metabolites and green synthesis of silver nanoparticles: a review. Nano Micro Biosystems 1(1), 15–21 (2022)
M. Alavi, R. Kowalski, R. Capasso, H. Douglas Melo Coutinho, I. De Rose Alencar Menezes, Various novel strategies for functionalization of gold and silver nanoparticles to hinder drug-resistant bacteria and cancer cells. Micro Nano Bio Aspects 1(1), 38–48 (2022)
M. Alavi, S. Thomas, M. Sreedharan, Modification of silica nanoparticles for antibacterial activities: mechanism of action. Micro Nano Bio Aspects 1(1), 49–58 (2022)
K. Marimuthu, H.K. Gautam, Nanobiotics against antimicrobial resistance: harnessing the power of nanoscale materials and technologies. J. Nanobiotechnol. 20(1), 375 (2022)
D.L. Green, K. Keenan, K.J. Fredricks, S.I. Huque, M.F. Mushi, C. Kansiime, M. Clarkson, The role of multidimensional poverty in antibiotic misuse: a mixed-methods study of self-medication and non-adherence in Kenya, Tanzania, and Uganda. Lancet Glob. Health 11(1), e59–e68 (2023)
V. Kimothi, R.S. Dhariyal, Antibiotic resistance: a review. Int. J. Pharmacy Res. 10(2), 4–12 (2019)
U. Nations, No time to wait: securing the future from drug-resistant infections; report to the secretary-general of the United Nations (WHO, Geneva, 2019)
N. Chakraborty, D. Jha, I. Roy, P. Kumar, S.S. Gaurav, K. Marimuthu, H.K. Gautam, Nanobiotics against antimicrobial resistance: harnessing the power of nanoscale materials and technologies. J. Nanobiotechnol. 20(1), 375 (2022)
R.M. Klevens, M.A. Morrison, J. Nadle, S. Petit, K. Gershman, S. Ray, Active Bacterial Core surveillance (ABCs) MRSA Investigators, Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298(15), 1763–1771 (2007)
World Health Organization. Antibiotic resistance (2020). https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance accessed 11 November 2022).
P.C. Appelbaum, 2012 and beyond: potential for the start of a second pre-antibiotic era? J. Antimicrob. Chemother. 67(9), 2062–2068 (2012)
E. Cox, S. Nambiar, L. Baden, Needed: antimicrobial development. N. Engl. J. Med. 380(8), 783–785 (2019)
O. Pacios, L. Blasco, I. Bleriot, L. Fernandez-Garcia, M. González Bardanca, A. Ambroa, M. Tomás, Strategies to combat multidrug-resistant and persistent infectious diseases. Antibiotics 9(2), 65 (2020)
World Health Organization, Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline (World Health Organization, 2019).
D.D. Flannery, K. Chiotos, J.S. Gerber, K.M. Puopolo, Neonatal multidrug-resistant gram-negative infection: epidemiology, mechanisms of resistance, and management. Pediatr. Res. 91(2), 380–391 (2022)
S. Mortazavi-Derazkola, M.A. Ebrahimzadeh, O. Amiri, H.R. Goli, A. Rafiei, M. Kardan, M. Salavati-Niasari, Facile green synthesis and characterization of Crataegus microphylla extract-capped silver nanoparticles (CME@ Ag-NPs) and its potential antibacterial and anticancer activities against AGS and MCF-7 human cancer cells. J. Alloy. Compd. 820, 153186 (2020)
A.A. Moussa, A.F. Md Nordin, R.A. Hamat, A.S. Jasni, High level aminoglycoside resistance and distribution of the resistance genes in Enterococcus faecalis and Enterococcus faecium from teaching hospital in Malaysia. Infection Drug Resist. (2019). https://doi.org/10.2147/IDR.S219544
C.A. Rodriguez, C.D. Mitnick, M.F. Franke, Value of observational data for multidrug-resistant tuberculosis. Lancet Infect. Dis. 19(9), 930–931 (2019)
L. Xu, H.W. Liang, Y. Yang, S.H. Yu, Stability and reactivity: positive and negative aspects for nanoparticle processing. Chem. Rev. 118(7), 3209–3250 (2018)
B. Naseer, G. Srivastava, O.S. Qadri, S.A. Faridi, R.U. Islam, K. Younis, Importance and health hazards of nanoparticles used in the food industry. Nanotechnol. Rev. 7(6), 623–641 (2018)
A.A. Menazea, A.M. Ismail, A. Samy, Novel green synthesis of zinc oxide nanoparticles using orange waste and its thermal and antibacterial activity. J. Inorg. Organomet. Polym. Mater. 31, 4250–4259 (2021)
H. Javid, S. Ahmadi, E. Mohamadian, Therapeutic applications of apigenin and its derivatives: micro and nano aspects. Micro Nano Bio Aspects 2(1), 30–38 (2023)
V. Singh, S. Shrivastava, S.K. Singh, A. Kumar, S. Saxena, Multi-scale temporal convolutional networks and continual learning based in silico discovery of alternative antibiotics to combat multi-drug resistance. Expert Syst. Appl. 215, 119295 (2023)
A. Selmani, D. Kovačević, K. Bohinc, Nanoparticles: From synthesis to applications and beyond. Adv. Coll. Interface. Sci. 303, 102640 (2022)
H. Ge, Y. Wang, X. Zhao, Research on the drug resistance mechanism of foodborne pathogens. Microb. Pathog. 162, 105306 (2022)
J.-H. Lee, Perspectives towards antibiotic resistance: from molecules to population. J. Microbiol. 57(3), 181–184 (2019)
J.H. Lee, Perspectives towards antibiotic resistance: from molecules to population. J. Microbiol. (2019). https://doi.org/10.1007/s12275-019-0718-8
A. Antonoplis, X. Zang, T. Wegner, P.A. Wender, L. Cegelski, Vancomycin–arginine conjugate inhibits growth of carbapenem-resistant E. coli and targets cell-wall synthesis. ACS Chem. Biol. 14(9), 2065–2070 (2019)
H.A. Kadhum, T.H. Hasan, The study of bacillus subtils antimicrobial activity on some of the pathological isolates. Int. J. Drug Deliv. Technol. 9(02), 193–196 (2019)
K. Klobucar, E.D. Brown, New potentiators of ineffective antibiotics: targeting the Gram-negative outer membrane to overcome intrinsic resistance. Curr. Opin. Chem. Biol. 66, 102099 (2022)
A.J. Baylay, L.J. Piddock, M.A. Webber, Molecular mechanisms of antibiotic resistance–Part I, in Bacterial resistance to antibiotics–from molecules to man. (Wiley, Hoboken, 2019), pp.1–26
A.A.J. Aljanaby, I.A.J. Aljanaby, Prevalence of aerobic pathogenic bacteria isolated from patients with burn infection and their antimicrobial susceptibility patterns in Al-Najaf City, Iraq-a three-year cross-sectional study. F1000Research 7(1157), 1157 (2018)
D.I. Andersson, N.Q. Balaban, F. Baquero, P. Courvalin, P. Glaser, U. Gophna, T. Tønjum, Antibiotic resistance: turning evolutionary principles into clinical reality. FEMS Microbiol. Rev. 44(2), 171–188 (2020)
A.H. Holmes, L.S. Moore, A. Sundsfjord, M. Steinbakk, S. Regmi, A. Karkey, L.J. Piddock, Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 387(10014), 176–187 (2016)
I. Álvarez-Rodríguez, L. Arana, B. Ugarte-Uribe, E. Gómez-Rubio, S. Martín-Santamaría, C. Garbisu, I. Alkorta, Type IV coupling proteins as potential targets to control the dissemination of antibiotic resistance. Front. Mol. Biosci. 7, 201 (2020)
A. Magallon, L. Amoureux, T. Garrigos, M. Sonois, V. Varin, C. Neuwirth, J. Bador, Role of AxyABM overexpression in acquired resistance in Achromobacter xylosoxidans. J. Antimicrob. Chemother. 77(4), 926–929 (2022)
F. Han, C. Yu, G. Fu, Warming alters elevation distributions of soil bacterial and fungal communities in alpine grasslands. Global Ecol. Conserv. 39, e02306 (2022)
K.K. Salimiyan Rizi, M. Noghondar, Adaptive antibiotic resistance: overview and perspectives. J. Infect. Dis. Ther 6, 1–3 (2018)
Criswell & Daniel. The “Evolution” of Antibiotic Resistance. Institute for Creation Research. N.p., 2004. Web. 28.)
Y. Liu, Y. Cai, G. Li, W. Wang, P.K. Wong, T. An, Response mechanisms of different antibiotic-resistant bacteria with different resistance action targets to the stress from photocatalytic oxidation. Water Res. 218, 118407 (2022)
F.J. Pérez-Llarena, G. Bou, Proteomics as a tool for studying bacterial virulence and antimicrobial resistance. Front. Microbiol. 7, 410 (2016)
E. Christaki, M. Marcou, A. Tofarides, Antimicrobial resistance in bacteria: mechanisms, evolution, and persistence. J. Mol. Evol. 88(1), 26–40 (2020)
J.M. Munita, C.A. Arias, Mechanisms of antibiotic resistance. Microbiol. Spectrum 4(2), 4 (2016)
M. Cerezales, K. Xanthopoulou, J. Wille, O. Krut, H. Seifert, L. Gallego, P.G. Higgins, Mobile genetic elements harboring antibiotic resistance determinants in. Front. Microbiol. 11, 919 (2020)
J.I. Hwang, J.K. Norsworthy, F. González-Torralva, G.L. Priess, L.T. Barber, T.R. Butts, Non-target-site resistance mechanism of barnyardgrass [Echinochloa crus-galli (L.) P. Beauv] to florpyrauxifen-benzyl. Pest Manag. Sci. 78(1), 287–295 (2022)
Z.T. Laughlin et al., 50S subunit recognition and modification by the Mycobacterium tuberculosis ribosomal RNA methyltransferase TlyA. Proc. Natl. Acad. Sci. 119(14), e2120352119 (2022)
K. Prashanth, T. Vasanth, R. Saranathan, A.R. Makki, S. Pagal, Antibiotic resistance, biofilms and quorum sensing in Acinetobacter species, in Antibiotic resistant bacteria: a continuous challenge in the new millennium. ed. by V. Sjhal (InTech, Orlando, 2012), pp.179–212
M.C. Roberts, Update on acquired tetracycline resistance genes. FEMS Microbiol. Lett. 245(2), 195–203 (2005)
S.B. Southon, S.B. Beres, P. Kachroo, M.O. Saavedra, H. Erlendsdóttir, G. Haraldsson, K.G. Kristinsson, Population genomic molecular epidemiological study of macrolide-resistant Streptococcus pyogenes in Iceland, 1995 to 2016: identification of a large clonal population with a pbp2x mutation conferring reduced in vitro β-lactam susceptibility. J. Clin. Microbiol. 58(9), 10–1128 (2020)
G. Cox, G.D. Wright, Intrinsic antibiotic resistance: mechanisms, origins, challenges and solutions. Int. J. Med. Microbiol. 303(6–7), 287–292 (2013)
X.Z. Li, H. Nikaido, Efflux-mediated drug resistance in bacteria. Drugs 69(12), 1555–1623 (2009)
N.S. Macêdo, Z. de Sousa Silveira, P.P.M. Cordeiro, H.D.M. Coutinho, J.P.S. Júnior, L.J.Q. Júnior, M.V. Da Silva, Inhibition of Staphylococcus aureus efflux pump by O-eugenol and its toxicity in drosophila melanogaster animal model. BioMed Res. Int. (2022). https://doi.org/10.1155/2022/1440996
J. Stephen et al., membrane efflux pumps of pathogenic vibrio species: role in antimicrobial resistance and virulence. Microorganisms 10(2), 382 (2022)
E.B. Breidenstein, C. de la Fuente-Núñez, R.E. Hancock, Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 19(8), 419–426 (2011)
A. Sharma, R. Sharma, T. Bhattacharyya, T. Bhando, R. Pathania, Fosfomycin resistance in Acinetobacter baumannii is mediated by efflux through a major facilitator superfamily (MFS) transporter—AbaF. J. Antimicrob. Chemother. 72(1), 68–74 (2016)
M. Kok, L. Maton, M. van der Peet, T. Hankemeier, J.C. van Hasselt, Unraveling antimicrobial resistance using metabolomics. Drug Discov. Today (2022). https://doi.org/10.1016/j.drudis.2022.03.015
J. Tan, J. Tay, J. Hedrick, Y.Y. Yang, Synthetic macromolecules as therapeutics that overcome resistance in cancer and microbial infection. Biomaterials 252, 120078 (2020)
S. Santajit, N. Indrawattana, Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed Res. Int. (2016). https://doi.org/10.1155/2016/2475067
J. Wang, S. Wang, C. Chen, J. Hu, S. He, Y. Zhou, J. Lin, Treatment of hospital wastewater by electron beam technology: removal of COD, pathogenic bacteria and viruses. Chemosphere 308, 136265 (2022)
C. Aurilio, P. Sansone, M. Barbarisi, V. Pota, L.G. Giaccari, F. Coppolino, M.C. Pace, Mechanisms of action of carbapenem resistance. Antibiotics 11(3), 421 (2022)
L. Fernández, R.E. Hancock, Adaptive and mutational resistance: role of porins and efflux pumps in drug resistance. Clin. Microbiol. Rev. 25(4), 661–681 (2012)
R. Diab, B. Khameneh, O. Joubert, R. Duval, Insights in nanoparticle-bacterium interactions: new frontiers to bypass bacterial resistance to antibiotics. Curr. Pharm. Des. 21(28), 4095–4105 (2015)
H.C. Flemming, E.D. van Hullebusch, T.R. Neu, P.H. Nielsen, T. Seviour, P. Stoodley, S. Wuertz, The biofilm matrix: multitasking in a shared space. Nat. Rev. Microbiol. 21(2), 70–86 (2023)
C. Uruén, G. Chopo-Escuin, J. Tommassen, R.C. Mainar-Jaime, J. Arenas, Biofilms as promoters of bacterial antibiotic resistance and tolerance. Antibiotics 10(1), 3 (2020)
J. Yan, B.L. Bassler, Surviving as a community: antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe 26(1), 15–21 (2019)
M.S.A. Khan, M.M. Altaf, I. Ahmad, Chemical nature of biofilm matrix and its significance. Biofilms Plant Soil Health (2017). https://doi.org/10.1002/9781119246329.ch9
M. Majumdar, S.A. Khan, S.C. Biswas, D.N. Roy, A.S. Panja, T.K. Misra, In vitro and in silico investigation of anti-biofilm activity of Citrus macroptera fruit extract mediated silver nanoparticles. J. Mol. Liq. 302, 112586 (2020)
M. Majumdar, S.A. Khan, N.B. Nandi, S. Roy, A.S. Panja, D.N. Roy, T.K. Misra, Green synthesis of iron nanoparticles for investigation of biofilm inhibition property. ChemistrySelect 5(43), 13575–13583 (2020)
M. Majumdar, S. Shivalkar, A. Pal, M.L. Verma, A.K. Sahoo, D.N. Roy, Nanotechnology for enhanced bioactivity of bioactive compounds, in Biotechnological production of bioactive compounds. (Elsevier, Amsterdam, 2020), pp.433–466
S. Majumdar, S. Roy, S. Majumdar, S. Roy, talking about talking microbes, in Microbial communication: mathematical modeling, synthetic biology and the role of noise. (Springer Nature, Berlin, 2020), pp.9–24
D.N. Roy, I. Ahmad, Combating biofilm of ESKAPE pathogens from ancient plant-based therapy to modern nanotechnological combinations, in A complete guidebook on biofilm study. (Academic Press, Cambridge, 2022), pp.59–94
T. Abee, Á.T. Kovács, O.P. Kuipers, S. Van der Veen, Biofilm formation and dispersal in Gram-positive bacteria. Curr. Opin. Biotechnol. 22(2), 172–179 (2011)
C. Solano, M. Echeverz, I. Lasa, Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 18, 96–104 (2014)
M.J. Federle, B.L. Bassler, Interspecies communication in bacteria. J. Clin. Investig. 112(9), 1291–1299 (2003)
H.C. Flemming, J. Wingender, U. Szewzyk, P. Steinberg, S.A. Rice, S. Kjelleberg, Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 14(9), 563–575 (2016)
S. Vasudevan, H.A. Joseph, S.S. Swamy, A.P. Solomon, Antibiotic resistance in biofilms, in Introduction to biofilm engineering. (American Chemical Society, Washington, 2019), pp.205–224
X. Zhao, Z. Yu, T. Ding, Quorum-sensing regulation of antimicrobial resistance in bacteria. Microorganisms 8(3), 425 (2020)
W.R. Galloway, J.T. Hodgkinson, S.D. Bowden, M. Welch, D.R. Spring, Quorum sensing in Gram-negative bacteria: small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem. Rev. 111(1), 28–67 (2011)
Y. **ao, H. Zou, J. Li, T. Song, W. Lv, W. Wang, S. Tao, Impact of quorum sensing signaling molecules in gram-negative bacteria on host cells: current understanding and future perspectives. Gut Microbes 14(1), 2039048 (2022)
V.S. Bhatt, Quorum sensing mechanisms in gram positive bacteria, in Implication of quorum sensing system in biofilm formation and virulence. (Springer Singapore, Singapore, 2018), pp.297–311
G. Banerjee, A.K. Ray, Quorum-sensing network-associated gene regulation in Gram-positive bacteria. Acta Microbiol. Immunol. Hung. 64(4), 439–453 (2017)
A. Piketh, H. Alam, A. Ahmad, Quorum sensing as an alternative approach to combatting multidrug resistance, in Non-traditional approaches to combat antimicrobial drug resistance. (Springer Nature Singapore, Singapore, 2023), pp.191–220
W.H. Zhao, Z.Q. Hu, β-lactamases identified in clinical isolates of Pseudomonas aeruginosa. Crit. Rev. Microbiol. 36(3), 245–258 (2010)
M. Uwate, Y.K. Ichise, A. Shirai, T. Omasa, T. Nakae, H. Maseda, Two routes of MexS-MexT-mediated regulation of MexEF-OprN and MexAB-OprM efflux pump expression in Pseudomonas aeruginosa. Microbiol. Immunol. 57(4), 263–272 (2013)
Z. Pang, R. Raudonis, B.R. Glick, T.J. Lin, Z. Cheng, Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 37(1), 177–192 (2019)
M.H. Al-Agamy, A. Aljallal, H.H. Radwan, A.M. Shibl, Characterization of carbapenemases, ESBLs, and plasmid-mediated quinolone determinants in carbapenem-insensitive Escherichia coli and Klebsiella pneumoniae in Riyadh hospitals. J. Infect. Public Health 11(1), 64–68 (2018)
A. Stewart, P. Harris, A. Henderson, D. Paterson, Treatment of infections by OXA-48-producing enterobacteriaceae. Antimicrob. Agents Chemother. 62(11), e01195-e1218 (2018)
M.C.P. Nguyen, P.L. Woerther, M. Bouvet, A. Andremont, R. Leclercq, A. Canu, Escherichia coli as reservoir for macrolide resistance genes. Emerg. Infect. Dis. 15(10), 1648 (2009)
M. Xu, Y.N. Zhou, B.P. Goldstein, D.J. **, Cross-resistance of Escherichia coli RNA polymerases conferring rifampin resistance to different antibiotics. J. Bacteriol. 187(8), 2783–2792 (2005)
Mounsey, O. J. (2021). Characterization of Relationships Between Fluoroquinolone-Resistant E. coli from Humans, Dogs, and Dairy Cattle Living in South West England (Doctoral dissertation, University of Bristol).
L.S. Tzouvelekis, A. Markogiannakis, M. Psichogiou, P.T. Tassios, G.L. Daikos, Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin. Microbiol. Rev. 25(4), 682–707 (2012)
R.L. Ferreira, B.C. Da Silva, G.S. Rezende, R. Nakamura-Silva, A. Pitondo-Silva, E.B. Campanini, M.C.D.S. Pranchevicius, High prevalence of multidrug-resistant Klebsiella pneumoniae harboring several virulence and β-lactamase encoding genes in a Brazilian intensive care unit. Front. Microbiol. 9, 3198 (2019)
Y. Doi, Y. Arakawa, 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin. Infect. Dis. 45(1), 88–94 (2007)
M.W. Vetting, C.H. Park, S.S. Hegde, G.A. Jacoby, D.C. Hooper, J.S. Blanchard, Mechanistic and structural analysis of aminoglycoside N-acetyltransferase AAC (6′)-Ib and its bifunctional, fluoroquinolone-active AAC (6′)-Ib-cr variant. Biochemistry 47(37), 9825–9835 (2008)
S. Navon-Venezia, K. Kondratyeva, A. Carattoli, Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 41(3), 252–275 (2017)
Z.K. Sheng, F. Hu, W. Wang, Q. Guo, Z. Chen, X. Xu, M. Wang, Mechanisms of tigecycline resistance among Klebsiella pneumoniae clinical isolates. Antimicrob. Agents Chemother. 58(11), 6982–6985 (2014)
G. Wang, G. Zhao, X. Chao, L. **e, H. Wang, The characteristic of virulence, biofilm and antibiotic resistance of Klebsiella pneumoniae. Int. J. Environ. Res. Public Health 17(17), 6278 (2020)
L. Poirel, P. Nordmann, Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin. Microbiol. Infect. 12(9), 826–836 (2006)
W.F. Penwell, A.B. Shapiro, R.A. Giacobbe, R.F. Gu, N. Gao, J. Thresher, A.A. Miller, Molecular mechanisms of sulbactam antibacterial activity and resistance determinants in Acinetobacter baumannii. Antimicrobial Agents Chemother. 59(3), 1680–1689 (2015)
Q. Chen, X. Li, H. Zhou, Y. Jiang, Y. Chen, X. Hua, Y. Yu, Decreased susceptibility to tigecycline in Acinetobacter baumannii mediated by a mutation in trm encoding SAM-dependent methyltransferase. J. Antimicrob. Chemother. 69(1), 72–76 (2014)
M. Asif, I.A. Alvi, S.U. Rehman, Insight into Acinetobacter baumannii: pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infection Drug Resist. 11, 1249 (2018)
R. Vázquez-López, S.G. Solano-Gálvez, J.J. Juárez Vignon-Whaley, J.A. Abello Vaamonde, L.A. Padró Alonzo, A. Rivera Reséndiz, T. Barrientos Fortes, Acinetobacter baumannii resistance: a real challenge for clinicians. Antibiotics 9(4), 205 (2020)
S. Jamal, A. Al Atrouni, R. Rafei, F. Dabboussi, M. Hamze, M. Osman, Molecular mechanisms of antimicrobial resistance in Acinetobacter baumannii, with a special focus on its epidemiology in Lebanon. J. Global Antimicrob. Resist. 15, 154–163 (2018)
B. Jubeh, Z. Breijyeh, R. Karaman, Resistance of gram-positive bacteria to current antibacterial agents and overcoming approaches. Molecules 25(12), 2888 (2020)
W.T. Liu, E.Z. Chen, L. Yang, C. Peng, Q. Wang, Z. Xu, D.Q. Chen, Emerging resistance mechanisms for 4 types of common anti-MRSA antibiotics in Staphylococcus aureus: a comprehensive review. Microb. Pathog. 156, 104915 (2021)
A. Sommer, S. Fuchs, F. Layer, C. Schaudinn, R.E. Weber, H. Richard, B. Strommenger, Mutations in the gdpP gene are a clinically relevant mechanism for β-lactam resistance in meticillin-resistant Staphylococcus aureus lacking mec determinants. Microbial Genomics (2021). https://doi.org/10.1099/mgen.0.000623
A. Kumar, M. Kaushal, Progression of β-lactam resistance in Staphylococcus aureus, in Insights into drug resistance in Staphylococcus aureus. (IntechOpen, London, 2021)
M. Afzal, A.K. Vijay, F. Stapleton, M. Willcox, The relationship between ciprofloxacin resistance and genotypic changes in S. aureus ocular isolates. Pathogens 11(11), 1354 (2022)
S. Rajabi, A. Shivaee, M.A. Khosravi, M. Eshaghi, S. Shahbazi, F. Hosseini, Evaluation of multidrug efflux pump expression in clinical isolates of Staphylococcus aureus. Gene Reports 18, 100537 (2020)
I.Y. Yoo, O.K. Kang, H.J. Shim, H.J. Huh, N.Y. Lee, Linezolid resistance in methicillin-resistant Staphylococcus aureus in Korea: high rate of false resistance to linezolid by the VITEK 2 system. Ann. Lab. Med. 40(1), 57–62 (2020)
Y. Zhu, C. Wang, S. Schwarz, W. Liu, Q. Yang, T. Luan, W. Zhang, Identification of a novel tetracycline resistance gene, tet (63), located on a multiresistance plasmid from Staphylococcus aureus. J. Antimicrobial Chemother. 76(3), 576–581 (2021)
T.C. Dewé, J.C. D’Aeth, N.J. Croucher, Genomic epidemiology of penicillin-non-susceptible Streptococcus pneumoniae. Microbial Genomics (2019). https://doi.org/10.1099/mgen.0.000305
C.Y. Wang, Y.H. Chen, C. Fang, M.M. Zhou, H.M. Xu, C.M. **g, C.H. Zhang, Antibiotic resistance profiles and multidrug resistance patterns of Streptococcus pneumoniae in pediatrics: a multicenter retrospective study in mainland China. Medicine (2019). https://doi.org/10.1097/MD.0000000000015942
A.L. Bloemendaal, E.C. Brouwer, A.C. Fluit, Methicillin resistance transfer from Staphylocccus epidermidis to methicillin-susceptible Staphylococcus aureus in a patient during antibiotic therapy. PLoS ONE 5(7), e11841 (2010)
A. Brenciani, E. Tiberi, E. Tili, M. Mingoia, C. Palmieri, P.E. Varaldo, E. Giovanetti, Genetic determinants and elements associated with antibiotic resistance in viridans group streptococci. J. Antimicrob. Chemother. 69(5), 1197–1204 (2014)
C. Sinel, M. Cacaci, P. Meignen, F. Guérin, B.W. Davies, M. Sanguinetti, V. Cattoir, Subinhibitory concentrations of ciprofloxacin enhance antimicrobial resistance and pathogenicity of Enterococcus faecium. Antimicrobial Agents Chemother. 61(5), 10–1128 (2017)
X. Du, X. Hua, T. Qu, Y. Jiang, Z. Zhou, Y. Yu, Molecular characterization of Rifr mutations in Enterococcus faecalis and Enterococcus faecium. J. Chemother. 26(4), 217–221 (2014)
M. Khodabandeh, M. Mohammadi, M.R. Abdolsalehi, M. Hasannejad-Bibalan, M. Gholami, A. Alvandimanesh, R. Rajabnia, High-level aminoglycoside resistance in Enterococcus faecalis and Enterococcus faecium; as a serious threat in hospitals. Infectious Disorders-Drug 20(2), 223–228 (2020)
M. Georges, E. Odoyo, D. Matano, F. Tiria, C. Kyany’a, D. Mbwika, L. Musila, Determination of Enterococcus faecalis and Enterococcus faecium antimicrobial resistance and virulence factors and their association with clinical and demographic factors in Kenya. J. Pathog. (2022). https://doi.org/10.1155/2022/3129439
W. Wehbeh, R. Rojas-Diaz, X. Li, N. Mariano, L. Grenner, S. Segal-Maurer, J.J. Rahal, Fluoroquinolone-resistant Streptococcus agalactiae: epidemiology and mechanism of resistance. Antimicrob. Agents Chemother. 49(6), 2495–2497 (2005)
Nauta, K. M. (2021). The dissection of Β-lactam resistance in Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis (Doctoral dissertation, The University of Iowa).
M. Hennart, L.G. Panunzi, C. Rodrigues, Q. Gaday, S.L. Baines, M. Barros-Pinkelnig, S. Brisse, Population genomics and antimicrobial resistance in Corynebacterium diphtheriae. Genome Med. 12, 1–18 (2020)
L.T. Matereke, A.I. Okoh, Listeria monocytogenes virulence, antimicrobial resistance and environmental persistence: a review. Pathogens 9(7), 528 (2020)
R.T. Aruleba, T.A. Adekiya, B.E. Oyinloye, P. Masamba, L.S. Mbatha, A. Pretorius, A.P. Kappo, PZQ therapy: how close are we in the development of effective alternative anti-schistosomal drugs? Infect. Disorders-Drug Targets 19(4), 337–349 (2019)
J. Dey, S.R. Mahapatra, T.K. Raj, T. Kaur, P. Jain, A. Tiwari, M. Suar, Designing a novel multi-epitope vaccine to evoke a robust immune response against pathogenic multidrug-resistant Enterococcus faecium bacterium. Gut Pathogens 14(1), 1–20 (2022)
L.D.R. Dos Santos, J.P.R. Furlan, M.S. Ramos, I.F.L. Gallo, L.V.P. de Freitas, E.G. Stehling, Co-occurrence of mcr-1, mcr-3, mcr-7 and clinically relevant antimicrobial resistance genes in environmental and fecal samples. Arch. Microbiol. 202, 1795–1800 (2020)
A. Haslam, J. Gill, V. Prasad, Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw. Open 3(3), e200423–e200423 (2020)
S. Roy, I. Hasan, B. Guo, Recent advances in nanoparticle-mediated antibacterial applications. Coord. Chem. Rev. 482, 215075 (2023)
U. Anand, M. Carpena, M. Kowalska-Góralska, P. Garcia-Perez, K. Sunita, E. Bontempi, J. Simal-Gandara, Safer plant-based nanoparticles for combating antibiotic resistance in bacteria: a comprehensive review on its potential applications, recent advances, and future perspective. Sci. Total. Environ. 821, 153472 (2022)
Y. Chen, X. Zheng, Y. **e, C. Ding, H. Ruan, C. Fan, Anti-bacterial and cytotoxic properties of plasma sprayed silver-containing HA coatings. J. Mater. Sci. - Mater. Med. 19, 3603–3609 (2008)
H. Li, H. Xu, Y.L. Yang, X.L. Yang, Y. Wu, S. Zhang, H.L. Song, Effects of graphite and Mn ore media on electro-active bacteria enrichment and fate of antibiotic and corresponding resistance gene in up flow microbial fuel cell constructed wetland. Water Res. 165, 114988 (2019)
H. Cui, A.L. Smith, Impact of engineered nanoparticles on the fate of antibiotic resistance genes in wastewater and receiving environments: a comprehensive review. Environ. Res. 204, 112373 (2022)
A. Gupta, S. Mumtaz, C.H. Li, I. Hussain, V.M. Rotello, Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 48(2), 415–427 (2019)
S. Mumtaz, S. Ali, S.A.R. Kazmi, T.A. Mughal, S. Mumtaz, H.M. Tahir, M.I. Rashid, Analysis of the antimicrobial potential of sericin-coated silver nanoparticles against human pathogens. Microscopy Res. Tech. (2022). https://doi.org/10.1002/jemt.24273
M. Summer, H.M. Tahir, S. Ali, Sonication and heat-mediated synthesis, characterization and larvicidal activity of sericin-based silver nanoparticles against dengue vector (Aedes aegypti). Microsc. Res. Tech. (2023). https://doi.org/10.1002/jemt.24333
M. Summer, H.M. Tahir, S. Ali, R. Abaidullah, S. Mumtaz, S. Nawaz, Bactericidal potential of different size sericin-capped silver nanoparticles synthesized by heat, light, and sonication. J. Basic Microbiol. (2023). https://doi.org/10.1002/jobm.202200632
H.M. Tahir, F. Saleem, S. Ali, Q.U. Ain, A. Fazal, M. Summer, G. Murtaza, Synthesis of sericin-conjugated silver nanoparticles and their potential antimicrobial activity. J. Basic Microbiol. 60(5), 458–467 (2020)
S. Muzammil, S. Hayat, M. Fakhar-E-Alam, B. Aslam, M.H. Siddique, M.A. Nisar, Z. Wang, Nanoantibiotics: future nanotechnologies to combat antibiotic resistance. Front Biosci 10, 352–374 (2018)
X. Zhao, H. Tang, X. Jiang, Deploying gold nanomaterials in combating multi-drug-resistant bacteria. ACS Nano 16(7), 10066–10087 (2022)
M.J. Hajipour, K.M. Fromm, A.A. Ashkarran, D.J. de Aberasturi, IR d. Larramendi, T. Rojo, V. Serpooshan, WJ Parak and M. Mahmoudi. Trends Biotechnol. 30, 499–511 (2012)
E.P. Ivanova, J. Hasan, H.K. Webb, G. Gervinskas, S. Juodkazis, V.K. Truong, R.J. Crawford, Bactericidal activity of black silicon. Nat. Commun. (2013). https://doi.org/10.1038/ncomms3838
S. Medici, M. Peana, V.M. Nurchi, M.A. Zoroddu, Medical uses of silver: history, myths, and scientific evidence. J. Med. Chem. 62(13), 5923–5943 (2019)
A. Hamad, K.S. Khashan, A. Hadi, Silver nanoparticles and silver ions as potential antibacterial agents. J. Inorg. Organomet. Polym. Mater. 30(12), 4811–4828 (2020)
H.H. Lara, L. Ixtepan-Turrent, M. Jose Yacaman, J. Lopez-Ribot, Inhibition of Candida auris biofilm formation on medical and environmental surfaces by silver nanoparticles. ACS Appl. Mater. Interfaces 12(19), 21183–21191 (2020)
T.A.J. de Souza, L.R.R. Souza, L.P. Franchi, Silver nanoparticles: An integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicol. Environ. Saf. 171, 691–700 (2019)
D.R. Ibraheem, N.N. Hussein, G.M. Sulaiman, H.A. Mohammed, R.A. Khan, O. Al Rugaie, Ciprofloxacin-loaded silver nanoparticles as potent nano-antibiotics against resistant pathogenic bacteria. Nanomaterials 12(16), 2808 (2022)
G.R. Tortella, O. Rubilar, N. Durán, M.C. Diez, M. Martínez, J. Parada, A.B. Seabra, Silver nanoparticles: toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 390, 121974 (2020)
S. Ali, S. Perveen, M. Ali, T. Jiao, A.S. Sharma, H. Hassan, Q. Chen, Bioinspired morphology-controlled silver nanoparticles for antimicrobial application. Mater. Sci. Eng. C 108, 110421 (2020)
M. Nilavukkarasi, S. Vijayakumar, S.P. Kumar, Biological synthesis and characterization of silver nanoparticles with Capparis zeylanica L. leaf extract for potent antimicrobial and anti-proliferation efficiency. Mater. Sci. Energy Technol. 3, 371–376 (2020)
L.H. Abdel-Rahman, B.S. Al-Farhan, D. Abou El-ezz, M.A. Abd-El Sayed, M.M. Zikry, A.M. Abu-Dief, Green biogenic synthesis of silver nanoparticles using aqueous extract of Moringa oleifera: access to a powerful antimicrobial, anticancer, pesticidal and catalytic agents. J. Inorg. Organomet. Polym. Mater. 32(4), 1422–1435 (2022)
N. Feroze, B. Arshad, M. Younas, M.I. Afridi, S. Saqib, A. Ayaz, Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity. Microsc. Res. Tech. 83(1), 72–80 (2020)
H. Ji, S. Zhou, Y. Fu, Y. Wang, J. Mi, T. Lu, C. Lü, Size-controllable preparation and antibacterial mechanism of thermo-responsive copolymer-stabilized silver nanoparticles with high antimicrobial activity. Mater. Sci. Eng. C 110, 110735 (2020)
M.J. Sweet, I. Singleton, Silver nanoparticles: a microbial perspective. Adv. Appl. Microbiol. 77, 115–133 (2011)
G. Franci, A. Falanga, S. Galdiero, L. Palomba, M. Rai, G. Morelli, M. Galdiero, Silver nanoparticles as potential antibacterial agents. Molecules 20(5), 8856–8874 (2015)
M. Ramzan, M.I. Karobari, A. Heboyan, R.N. Mohamed, M. Mustafa, S.N. Basheer, B. Zeshan, Synthesis of silver nanoparticles from extracts of wild ginger (Zingiber zerumbet) with antibacterial activity against selective multidrug resistant oral bacteria. Molecules 27(6), 2007 (2022)
P. Korshed, L. Li, D.-T. Ngo, T. Wang, Effect of storage conditions on the long-term stability of bactericidal effects for laser generated silver nanoparticles. Nanomaterials 8, 218 (2018)
W. Ma, L. **g, A. Valladares, S.L. Mehta, Z. Wang, P. Andy Li, J.J. Bang, Silver nanoparticle exposure induced mitochondrial stress, caspase-3 activation and cell death: Amelioration by sodium selenite. Int. J. Biol. Sci. 11, 860–867 (2015)
P. Aramwit, N. Bang, J. Ratanavaraporn, S. Ekgasit, Green synthesis of silk sericin-capped silver nanoparticles and their potent anti-bacterial activity. Nanoscale Res. Lett. 9(1), 1–7 (2014)
J.M. DeSimone, Practical approaches to green solvents. Science 297(5582), 799–803 (2002)
S.H. Lee, B.H. Jun, Silver nanoparticles: synthesis and application for nanomedicine. Int. J. Mol. Sci. 20(4), 865 (2019)
V. Lazar, Quorum sensing in biofilms—How to destroy the bacterial citadels or their cohesion/power? Anaerobe 17, 280–285 (2011)
S. Periasamy, H.S. Joo, A.C. Duong, T.H.L. Bach, V.Y. Tan, S.S. Chatterjee, M. Otto, How Staphylococcus aureus biofilms develop their characteristic structure. Proc. National Acad. Sci. 109(4), 1281–1286 (2012)
L. Krce, M. Šprung, T. Rončević, A. Maravić, V. ČikešČulić, D. Blažeka, I. Aviani, Probing the mode of antibacterial action of silver nanoparticles synthesized by laser ablation in water: what fluorescence and AFM data tell us. Nanomaterials 10(6), 1040 (2020)
M. Rai, K. Kon, A. Ingle, N. Duran, S. Galdiero, M. Galdiero, Broad-spectrum bioactivities of silver nanoparticles: the emerging trends and future prospects. Appl. Microbiol. Biotechnol. 98(5), 1951–1961 (2014)
A. Salleh, R. Naomi, N.D. Utami, A.W. Mohammad, E. Mahmoudi, N. Mustafa, M.B. Fauzi, The potential of silver nanoparticles for antiviral and antibacterial applications: a mechanism of action. Nanomaterials 10(8), 1566 (2020)
T.L. Collins, E.A. Markus, D.J. Hassett, J.B. Robinson, The effect of a cationic porphyrin on Pseudomonas aeruginosa biofilms. Curr. Microbiol. 61, 411–416 (2010)
D. Wu, W. Fan, A. Kishen, J.L. Gutmann, B. Fan, Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. Journal of endodontics 40(2), 285–290 (2014)
L.A. Tamayo, P.A. Zapata, N.D. Vejar, M.I. Azócar, M.A. Gulppi, X. Zhou, M.A. Páez, Release of silver and copper nanoparticles from polyethylene nanocomposites and their penetration into Listeria monocytogenes. Mater. Sci. Eng. C 40, 24–31 (2014)
Z. Chen, Z. Zhang, X. Zhai, Y. Li, L. Lin, H. Zhao, G. Lin, Rapid and sensitive detection of anti-SARS-CoV-2 IgG, using lanthanide-doped nanoparticles-based lateral flow immunoassay. Anal. Chem. 92(10), 7226–7231 (2020)
D. Muchintala, V. Suresh, D. Raju, R.B. Sashidhar, Synthesis and characterization of cecropin peptide-based silver nanocomposites: its antibacterial activity and mode of action. Mater. Sci. Eng. C 110, 110712 (2020)
Y. Cai, D. Wu, X. Zhu, W. Wang, F. Tan, J. Chen, X. Qiao, X. Qiu, Sol-gel preparation of Ag-doped MgO nanoparticles with high efficiency for bacterial inactivation. Ceram. Int. 43(1), 1066–1072 (2017)
S. Silver, Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol. Rev. 27(2–3), 341–353 (2003)
J.P. Rolim, M.A. de Melo, S.F. Guedes, F.B. Albuquerque-Filho, J.R. de Souza, N.A. Nogueira, I.C. Zanin, L.K. Rodrigues, The antimicrobial activity of photodynamic therapy against Streptococcus mutansusing different photosensitizers. J. Photochem. Photobiol. B. 106, 40–46 (2012)
N. Beyth, Y. Houri-Haddad, A. Domb, W. Khan, R. Hazan, Alternative antimicrobial approach: nano-antimicrobial materials. Evidence-Based Complement. Altern. Med. (2015). https://doi.org/10.1155/2015/246012
N.R. Bury, F. Galvez, C.M. Wood, Effects of chloride, calcium, and dissolved organic carbon on silver toxicity: comparison between rainbow trout and fathead minnows. Environ. Toxicol. Chem.: Int. J. 18(1), 56–62 (1999)
J.R. Morones, J.L. Elechiguerra, A. Camacho, K. Holt, J.B. Kouri, J.T. Ramirez, M.J. Yacaman, The bactericidal effect of silver nanoparticles. Nanotechnology 16, 2346–2353 (2005)
W.K. Jung, H.C. Koo, K.W. Kim, S. Shin, S.H. Kim, Y.H. Park, Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 74, 2171–2178 (2008)
L. Chun-Nam, C.M. Ho, R. Chen, Q.Y. He, W.Y. Yu, H.P. Sun, K.H. Tam, J.F. Chiu, C.M. Che, Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res. 5, 916–924 (2006)
P. Jena, M. Bhattacharya, G. Bhattacharjee, B. Satpati, P. Mukherjee, D. Senapati, R. Srinivasan, Bimetallic gold–silver nanoparticles mediate bacterial killing by disrupting the actin cytoskeleton MreB. Nanoscale 12(6), 3731–3749 (2020)
L. Wu, G. Zhu, X. Zhang, Y. Si, Silver nanoparticles inhibit denitrification by altering the viability and metabolic activity of Pseudomonas stutzeri. Sci. Total. Environ. 706, 135711 (2020)
M.S. Yousaf, A. Haider, A. Shahzadi, A. Ul-Hamid, M. Imran, M.A. Khan, M. Ikram, Aggrandized catalytic and bactericidal activity of silver and polyvinylpyrrolidone capped bismuth oxybromide quantum dots: in silico molecular docking studies. J. Inorg. Organomet. Polym. Mater. (2023). https://doi.org/10.1007/s10904-023-02821-7
A.M. Elgorban, A.H. Bahkali, M.A. El-Metwally, M. Elsheshtawi, M.A. Abdel-Wahab, In vitro antifungal activity of some plant essential oils. Int. J. Pharmcol. 11(1), 56–61 (2015)
R.S. Shaban, A.H. Bahkali, M.B. Marwa, Antibacterial activity of biogenic silver nanoparticles produced by Aspergillus terreus. Int. J. Pharmacol. 11, 858–863 (2015)
J. Narware, R.N. Yadav, C. Keswani, S.P. Singh, H.B. Singh, Silver nanoparticle based biopesticides for phytopathogens: scope and potential in agriculture. Nano-Biopesticides Today Future Perspect. 2019, 303–314 (2019)
N. Kaur, A. Singh, W. Ahmad, Microwave assisted green synthesis of silver nanoparticles and its application: a review. J. Inorg. Organomet. Polym. Mater. 33(3), 663–672 (2023)
A. Pal, R. Goswami, D.N. Roy, A critical assessment on biochemical and molecular mechanisms of toxicity developed by emerging nanomaterials on important microbes. Environ. Nanotechnol. Monit. Manag. 16, 100485 (2021)
M.A. Biel, C. Sievert, M. Usacheva, M. Teichert, E. Wedell, N. Loebel, …& Zimmermann, R., Reduction of endotracheal tube biofilms using antimicrobial photodynamic therapy. Lasers Surg. Med. 43(7), 586–590 (2011)
U.F. Gunputh, H. Le, K. Lawton, A. Besinis, C. Tredwin, R.D. Handy, Antibacterial properties of silver nanoparticles grown in situ and anchored to titanium dioxide nanotubes on titanium implant against Staphylococcus aureus. Nanotoxicology 14(1), 97–110 (2020)
J.S. Kim, E. Kuk, K.N. Yu, J.H. Kim, S.J. Park, H.J. Lee, S.H. Kim, Y.K. Park, Y.H. Park, C.Y. Hwang, Antimicrobial effects of silver nanoparticles. Nanomedicine 3, 95–101 (2007)
K. Shameli, M.B. Ahmad, S.D. Jazayeri, P. Shabanzadeh, P. Sangpour, H. Jahangirian, Y. Gharayebi, Investigation of antibacterial properties silver nanoparticles prepared via green method. Chem. Cent. J. 6(1), 73 (2012)
D.A. Kumar, V. Palanichamy, S.M. Roopan, Green synthesis of silver nanoparticles using alternanthera dentata leaf extract at room temperature and their antimicrobialactivity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 127, 168–171 (2014)
S. Naraginti, A. Sivakumar, Eco-friendly synthesis of silver and gold nanoparticles with enhanced bactericidal activity and study of silver catalyzed reduction of 4-nitrophenol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 128, 357–362 (2014)
P. Parvekar, J. Palaskar, S. Metgud, R. Maria, S. Dutta, The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investigations Dentistry 7(1), 105–109 (2020)
G.R. Salunke, S. Ghosh, R.J. Santosh Kumar, S. Khade, P. Vashisth, T. Kale, S. Chopade, V. Pruthi, G. Kundu, J.R. Bellare, Rapid efficient synthesis and characterization of silver, gold, and bimetallic nanoparticles from the medicinal plant Plumbago zeylanicaand their application in biofilm control. Int. J. Nanomed. 9, 2635–2653 (2014)
S. Wang, R. Su, S. Nie, M. Sun, J. Zhang, D. Wu, N. Moustaid-Moussa, Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 25(4), 363–376 (2014)
S.D. Nishu, J.H. No, T.K. Lee, Transcriptional response and plant growth promoting activity of Pseudomonas fluorescens DR397 under drought stress conditions. Microbiol. Spectrum 10(4), e00979-e1022 (2022)
A.M. Badawy, R. Silva, B. Morris, K.G. Scheckel, M.T. Suidan, T.M. Tolaymat, Surface charge-dependent toxicity of silver nanoparticles. Environ. Sci. Technol. 45, 283–287 (2011). https://doi.org/10.1021/es1034188
D. Swolana, R.D. Wojtyczka, Activity of silver nanoparticles against Staphylococcus spp. Int. J. Mol. Sci. 23(8), 4298 (2022)
X. Gu, Q. Cheng, P. He, Y. Zhang, Z. Jiang, Y. Zeng, Dihydroartemisinin-loaded chitosan nanoparticles inhibit the rifampicin-resistant mycobacterium tuberculosis by disrupting the cell wall. Front. Microbiol. 12, 735166 (2021)
Estevez, H., Palacios, A., Gil, D., Anguita, J., Vallet-Regi, M., González, B., ... & Luque-Garcia, J. L. (2020). Antimycobacterial effect of selenium nanoparticles on Mycobacterium tuberculosis. Frontiers in microbiology, 11, 800.
S. Djearamane, Z.C. Loh, J.J. Lee, L.S. Wong, R. Rajamani, P.A. Luque, S.X.T. Liang, Remedial aspect of zinc oxide nanoparticles against Serratia marcescens and Enterococcus faecalis. Front. Pharmacol. (2022). https://doi.org/10.3389/fphar.2022.891304
A. Abdelghafar, N. Yousef, M. Askoura, Zinc oxide nanoparticles reduce biofilm formation, synergize antibiotics action and attenuate Staphylococcus aureus virulence in host; an important message to clinicians. BMC Microbiol. 22(1), 1–17 (2022)
L. Shkodenko, I. Kassirov, E. Koshel, Metal oxide nanoparticles against bacterial biofilms: perspectives and limitations. Microorganisms 8(10), 1545 (2020)
A.K. Keshari, R. Srivastava, P. Singh, V.B. Yadav, G. Nath, Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integrative Med. 11(1), 37–44 (2020)
U. Halder, R.K. Roy, R. Biswas, D. Khan, K. Mazumder, R. Bandopadhyay, Synthesis of copper oxide nanoparticles using capsular polymeric substances produced by Bacillus altitudinis and investigation of its efficacy to kill pathogenic Pseudomonas aeruginosa. Chem. Eng. J. Adv. 11, 100294 (2022)
Y. Zhang, X. Pan, S. Liao, C. Jiang, L. Wang, Y. Tang, L. Chen, Quantitative proteomics reveals the mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa biofilms. J. Proteome Res. 19(8), 3109–3122 (2020)
A. Das Mahapatra, C. Patra, J. Mondal, C. Sinha, P. Chandra Sadhukhan, D. Chattopadhyay, Silver nanoparticles derived from Albizia lebbeck bark extract demonstrate killing of multidrug-resistant bacteria by damaging cellular architecture with antioxidant activity. Chem. Select 5(15), 4770–4777 (2020)
M. Saberpour, S. Najar-Peeraye, S. Shams, B. Bakhshi, Effects of chitosan nanoparticles loaded with mesenchymal stem cell conditioned media on gene expression in Vibrio cholerae and Caco-2 cells. Sci. Rep. 12(1), 1–9 (2022)
R. Manjumeena, D. Duraibabu, J. Sudha, P.T. Kalaichelvan, Biogenic nanosilver incorporated reverse osmosis membrane for antibacterial and antifungal activities against selected pathogenic strains: an enhanced eco-friendly water disinfection approach. J. Environ. Sci.: Health ToxicHazard Substance Environ. England 49, 1125–1133 (2014)
M.H. Siddique, B. Aslam, M. Imran, A. Ashraf, H. Nadeem, S. Hayat, U. Qureshi, Effect of silver nanoparticles on biofilm formation and eps production of multidrug-resistant Klebsiella pneumoniae. BioMed. Res. Int. (2020). https://doi.org/10.1155/2020/6398165
Pareek, V., Devineau, S., Sivasankaran, S. K., Bhargava, A., Panwar, J., Srikumar, S., & Fanning, S. (2020). Silver nanoparticles induce a triclosan-like antibacterial action mechanism in multi-drug resistant Klebsiella pneumoniae. bioRxiv.
M.E. Lysakowska, A. Ciebiada-Adamiec, L. Klimek, M. Sienkiewicz, The activity of silver nanoparticles (Axonnite) on clinical and environmental strains of Acinetobacter spp. Burns 41, 364–371 (2015)
T.A. Salih, K.T. Hassan, S.R. Majeed, I.J. Ibraheem, O.M. Hassan, A.S. Obaid, In vitro scolicidal activity of synthesised silver nanoparticles from aqueous plant extract against Echinococcus granulosus. Biotechnol. Rep. 28, e00545 (2020)
D.K. Singaravelu, D.N. Binjawhar, F. Ameen, A. Veerappan, Lectin-fortified cationic copper sulfide nanoparticles gain dual targeting capabilities to treat carbapenem-resistant Acinetobacter baumannii infection. ACS Omega 7(48), 43934–43944 (2022)
S. Sharma, V.K. Singh, A. Kumar, S. Mallubhotla, Effect of nanoparticles on oxidative damage and antioxidant defense system in plants. Mole. Plant Abiotic Stress: Biol. Biotechnol. (2019). https://doi.org/10.1002/9781119463665.ch17
E. Tvrdá, F. Benko, Free radicals: what they are and what they do, in Pathology. (Academic Press, Cambridge, 2020), pp.3–13
N. Ghosh, A. Das, S. Chaffee, S. Roy, C.K. Sen, Reactive oxygen species, oxidative damage and cell death, in Immunity and inflammation in health and disease. (Academic Press, Cambridge, 2018), pp.45–55
R.Z. Zhao, S. Jiang, L. Zhang, Z.B. Yu, Mitochondrial electron transport chain, ROS generation and uncoupling. Int. J. Mol. Med. 44(1), 3–15 (2019)
R. Canaparo, F. Foglietta, T. Limongi, L. Serpe, Biomedical applications of reactive oxygen species generation by metal nanoparticles. Materials 14(1), 53 (2020)
M. Alavi, R. Yarani, ROS and RNS modulation: the main antimicrobial, anticancer, antidiabetic, and antineurodegenerative mechanisms of metal or metal oxide nanoparticles. Nanotheranostics Treat. Nano Micro Biosyst. 2, 22–30 (2023)
K.S. Siddiqi, A. Husen, R.A. Rao, A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 16(1), 1–28 (2018)
A. Abdal Dayem, M.K. Hossain, S.B. Lee, K. Kim, S.K. Saha, G.M. Yang, S.G. Cho, The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 18(1), 120 (2017)
O. Metryka, D. Wasilkowski, A. Mrozik, Evaluation of the effects of Ag, Cu, ZnO and TiO2 nanoparticles on the expression level of oxidative stress-related genes and the activity of antioxidant enzymes in Escherichia coli, Bacillus cereus and Staphylococcus epidermidis. Int. J. Mol. Sci. 23(9), 4966 (2022)
D. Manzanares, V. Ceña, Endocytosis: the nanoparticle and submicron nanocompounds gateway into the cell. Pharmaceutics 12(4), 371 (2020)
J. Mosquera, I. García, L.M. Liz-Marzán, Cellular uptake of nanoparticles versus small molecules: a matter of size. Acc. Chem. Res. 51(9), 2305–2313 (2018)
Q. Chen, N. Wang, M. Zhu, J. Lu, H. Zhong, X. Xue, H. Yin, TiO2 nanoparticles cause mitochondrial dysfunction, activate inflammatory responses, and attenuate phagocytosis in macrophages: a proteomic and metabolomic insight. Redox Biol. 15, 266–276 (2018)
T. **a, M. Kovochich, J. Brant, M. Hotze, J. Sempf, T. Oberley, A.E. Nel, Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 6(8), 1794–1807 (2006)
S.J. Soenen, P. Rivera-Gil, J.M. Montenegro, W.J. Parak, S.C. De Smedt, K. Braeckmans, Cellular toxicity of inorganic nanoparticles: common aspects and guidelines for improved nanotoxicity evaluation. Nano Today 6(5), 446–465 (2011)
P.V. AshaRani, G. Low Kah Mun, M.P. Hande, S. Valiyaveettil, Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3(2), 279–290 (2009)
K.B. Holt, A.J. Bard, Interaction of silver (I) ions with the respiratory chain of Escherichia coli: an electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+. Biochemistry 44(39), 13214–13223 (2005)
A. Manke, L. Wang, Y. Rojanasakul, Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. (2013). https://doi.org/10.1155/2013/942916
S. Zhang, T. Ouyang, B.M. Reinhard, Multivalent ligand-nanoparticle conjugates amplify reactive oxygen species second messenger generation and enhance epidermal growth factor receptor phosphorylation. Bioconjug. Chem. 33(9), 1716–1728 (2022)
T.C. Dakal, A. Kumar, R.S. Majumdar, V. Yadav, Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 7, 1831 (2016)
Y. Gao, M.A.V. Anand, V. Ramachandran, V. Karthikkumar, V. Shalini, S. Vijayalakshmi, D. Ernest, Biofabrication of zinc oxide nanoparticles from Aspergillus niger, their antioxidant, antimicrobial and anticancer activity. J. Cluster Sci. 30(4), 937–946 (2019)
K.S. Khashan, G.M. Sulaiman, S.A. Hussain, T.R. Marzoog, M.S. Jabir, Synthesis, characterization and evaluation of anti-bacterial, anti-parasitic and anti-cancer activities of aluminum-doped zinc oxide nanoparticles. J. Inorg. Organomet. Polym. Mater. 30(9), 3677–3693 (2020)
S. Shahid, S.A. Khan, W. Ahmad, U. Fatima, S. Knawal, Size-dependent bacterial growth inhibition and antibacterial activity of Ag-doped ZnO nanoparticles under different atmospheric conditions. Indian J. Pharm. Sci. 80(1), 173–180 (2018)
H.M. Yusof, R. Mohamad, U.H. Zaidan, Abdul Rahman NA Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: a review. J. Anim. Sci. Biotechnol 10, 57 (2019)
N.A.A. Yusof, N.M. Zain, N. Pauzi, Synthesis of ZnO nanoparticles with chitosan as stabilizing agent and their antibacterial properties against Gram-positive and Gram-negative bacteria. Int. J. Biol. Macromol. 124, 1132–1136 (2019)
X. Zhu, J. Wang, L. Cai, Y. Wu, M. Ji, H. Jiang, J. Chen, Dissection of the antibacterial mechanism of zinc oxide nanoparticles with manipulable nanoscale morphologies. J. Hazard. Mater. 430, 128436 (2022)
S.E. **, H.E. **, Synthesis, characterization, and three-dimensional structure generation of zinc oxide-based nanomedicine for biomedical applications. Pharmaceutics 11(11), 575 (2019)
E. Sánchez-López, D. Gomes, G. Esteruelas, L. Bonilla, A.L. Lopez-Machado, R. Galindo, E.B. Souto, Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials 10(2), 292 (2020)
A. Sirelkhatim, S. Mahmud, A. Seeni, N.H.M. Kaus, L.C. Ann, S.K.M. Bakhori, D. Mohamad, Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett. 7(3), 219–242 (2015)
M. Moharramnejad, A. Ehsani, S. Salmani, M. Shahi, R.E. Malekshah, Z.S. Robatjazi, H. Parsimehr, Zinc-based metal-organic frameworks: synthesis and recent progress in biomedical application. J. Inorg. Organomet. Polym. Mater. 32(9), 3339–3354 (2022)
M. Pal, Nanotechnology: a new approach in food packaging. J Food Microbiol Safety Hyg 2, 121 (2017)
V. Tiwari, N. Mishra, K. Gadani, P.S. Solanki, N.A. Shah, M. Tiwari, Mechanism of anti-bacterial activity of zinc oxide nanoparticle against carbapenem-resistant Acinetobacter baumannii. Front. Microbiol. 9, 1218 (2018)
D.K. Tiwari, J. Behari, P. Sen, Time and dose-dependent antimicrobial potential of Ag nanoparticles synthesized by top-down approach. Curr. Sci. 95, 647–655 (2008)
P.J.P. Espitia, N.D.F.F. Soares, J.S. dos Reis Coimbra, N.J. de Andrade, R.S. Cruz, E.A.A. Medeiros, Food Bioprocess Tech. 5, 1447–1464 (2012)
B. Abebe, H.A. Murthy, E. Amare, Enhancing the photocatalytic efficiency of ZnO: defects, heterojunction, and optimization. Environ. Nanotechnol. Monit. Manag. 14, 100336 (2020)
B.A. Fahimmunisha, R. Ishwarya, M.S. AlSalhi, S. Devanesan, M. Govindarajan, B. Vaseeharan, Green fabrication, characterization and antibacterial potential of zinc oxide nanoparticles using Aloe socotrina leaf extract: a novel drug delivery approach. J. Drug Deliv. Sci. Technol. 55, 101465 (2020)
B.L. da Silva, B.L. Caetano, B.G. Chiari-Andréo, R.C.L.R. Pietro, L.A. Chiavacci, Increased antibacterial activity of ZnO nanoparticles: Influence of size and surface modification. Colloids Surf. B 177, 440–447 (2019)
K. Dulta, G. Koşarsoy Ağçeli, P. Chauhan, R. Jasrotia, P.K. Chauhan, A novel approach of synthesis zinc oxide nanoparticles by bergenia ciliata rhizome extract: antibacterial and anticancer potential. J. Inorg. Organomet. Polym. Mater. 31, 180–190 (2021)
S.S.N. Fernando, T.D.C.P. Gunasekara, J. Holton, Antimicrobial nanoparticles: applications and mechanisms of action. Sri Lankan J. Infec. Dis. (2018). https://doi.org/10.4038/sljid.v8i1.8167
P.T.L. Huong, N. Van Quang, M.T. Tran, D.Q. Trung, D.T.B. Hop, T.T.H. Tam, V.D. Dao, Excellent visible light photocatalytic degradation and mechanism insight of Co2+-doped ZnO nanoparticles. Appl. Phys. A 128(1), 1–16 (2022)
A. Joe, S.H. Park, K.D. Shim, D.J. Kim, K.H. Jhee, H.W. Lee, E.S. Jang, Antibacterial mechanism of ZnO nanoparticles under dark conditions. J. Ind. Eng. Chem. 45, 430–439 (2017)
K.R. Raghupathi, R.T. Koodali, A.C. Manna, Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 27(7), 4020–4028 (2011)
L.K. Adams, D.Y. Lyon, P.J. Alvarez, Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 40(19), 3527–3532 (2006)
K. Hirota, M. Sugimoto, M. Kato, K. Tsukagoshi, T. Tanigawa, H. Sugimoto, Preparation of zinc oxide ceramics with a sustainable antibacterial activity under dark conditions. Ceram. Int. 36(2), 497–506 (2010)
V. Lakshmi Prasanna, R. Vijayaraghavan, Insight into the mechanism of antibacterial activity of ZnO: surface defects mediated reactive oxygen species even in the dark. Langmuir 31(33), 9155–9162 (2015)
E. Jeong, C.U. Kim, J. Byun, J. Lee, H.E. Kim, E.J. Kim, S.W. Hong, Quantitative evaluation of the antibacterial factors of ZnO nanorod arrays under dark conditions: physical and chemical effects on Escherichia coli inactivation. Sci. Total. Environ. 712, 136574 (2020)
H.T. Hoang, T.T.T. Nguyen, H.M. Do, T.K.N. Nguyen, H.T. Pham, A novel finding of intra-genus inhibition of quorum sensing in Vibrio bacteria. Sci. Rep. 12(1), 15203 (2022)
S. Khanna, D.S. Pardi, C.R. Kelly, C.S. Kraft, T. Dhere, M.R. Henn, E.L. Hohmann, A novel microbiome therapeutic increases gut microbial diversity and prevents recurrent Clostridium difficile infection. J. Infectious Dis. 214(2), 173–181 (2016)
N. Thakur, P. Manna, J. Das, Synthesis and biomedical applications of nanoceria, a redox active nanoparticle. J. Nanobiotechnol. 17(1), 1–27 (2019)
S. Tang, J. Zheng, Antibacterial activity of silver nanoparticles: structural effects. Adv. Healthcare Mater. 7(13), 1701503 (2018)
Y. **e, Y. He, P.L. Irwin, T. **, X. Shi, Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 77(7), 2325–2331 (2011)
E.A. Campbell, R. Greenwell, J.R. Anthony, S. Wang, L. Lim, K. Das, S.A. Darst, A conserved structural module regulates transcriptional responses to diverse stress signals in bacteria. Mole. Cell 27(5), 793–805 (2007)
M. Cerasi, S. Ammendola, A. Battistoni, Competition for zinc binding in the host-pathogen interaction. Front. Cell. Infect. Microbiol. 3, 108 (2013)
M.J. Hakeem, J. Feng, A. Nilghaz, L. Ma, H.C. Seah, M.E. Konkel, X. Lu, Active packaging of immobilized zinc oxide nanoparticles controls Campylobacter jejuni in raw chicken meat. Appl. Environ. Microbiol. 86(22), e01195-e1220 (2020)
U. Kadiyala, E.S. Turali-Emre, J.H. Bahng, N.A. Kotov, J.S. VanEpps, Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus (MRSA). Nanoscale 10(10), 4927–4939 (2018)
B. Lallo da Silva, M.P. Abuçafy, E. Berbel Manaia, J.A. Oshiro Junior, B.G. Chiari-Andréo, R.C.R. Pietro, L.A. Chiavacci, Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: an overview. Int. J. Nanomed. (2019). https://doi.org/10.2147/IJN.S216204
D.E. Navarro-López, R. Garcia-Varela, O. Ceballos-Sanchez, A. Sanchez-Martinez, G. Sanchez-Ante, K. Corona-Romero, E.R. López-Mena, Effective antimicrobial activity of ZnO and Yb-doped ZnO nanoparticles against Staphylococcus aureus and Escherichia coli. Mater. Sci. Eng. C 123, 112004 (2021)
M. Li, L. Zhu, D. Lin, Toxicity of ZnO nanoparticles to Escherichia coli: mechanism and the influence of medium components. Environ. Sci. Technol. 45(5), 1977–1983 (2011)
J. Pasquet, Y. Chevalier, E. Couval, D. Bouvier, M.A. Bolzinger, Zinc oxide as a new antimicrobial preservative of topical products: Interactions with common formulation ingredients. Int. J. Pharm. 479(1), 88–95 (2015)
Y. Li, W. Zhang, J. Niu, Y. Chen, Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 6(6), 5164–5173 (2012)
A. Akbar, M.B. Sadiq, I. Ali, N. Muhammad, Z. Rehman, M.N. Khan, A.K. Anal, Synthesis and antimicrobial activity of zinc oxide nanoparticles against foodborne pathogens Salmonella typhimurium and Staphylococcus aureus. Biocatal. Agric. Biotechnol. 17, 36–42 (2019)
L. Palanikumar, S.N. Ramasamy, C. Balachandran, Size-dependent antimicrobial response of zinc oxide nanoparticles. IET Nanobiotechnol. 8(2), 111–117 (2014)
M.A. Al-Holy, L.F. Castro, H.M. Al-Qadiri, Inactivation of Cronobacter spp. (Enterobacter sakazakii) in infant formula using lactic acid, copper sulfate and monolaurin. Lett. Appl. Microbiol. 50(3), 246–251 (2010)
G. Faúndez, M. Troncoso, P. Navarrete, G. Figueroa, Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni. BMC Microbiol. 4(1), 1–7 (2004)
D.S. Idris, A. Roy, Biogenic synthesis of Ag–CuO nanoparticles and its antibacterial, antioxidant, and catalytic activity. J. Inorg. Organomet. Polym. Mater. (2023). https://doi.org/10.1007/s10904-023-02873-9
L. Weaver, H.T. Michels, C.W. Keevil, Potential for preventing spread of fungi in air-conditioning systems constructed using copper instead of aluminum. Lett. Appl. Microbiol. 50(1), 18–23 (2010)
M. Vincent, R.E. Duval, P. Hartemann, M. Engels-Deutsch, Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 124(5), 1032–1046 (2018)
M.I. Devi, N. Nallamuthu, N. Ra**i, T.S.M. Kumar, S. Siengchin, A.V. Rajulu, N. Ayrilmis, Biodegradable poly (propylene) carbonate using in-situ generated CuNPs coated Tamarindus indica filler for biomedical applications. Mater. Today Commun. 19, 106–113 (2019)
M. Hasanin, M.A. Al Abboud, M.M. Alawlaqi, T.M. Abdelghany, A.H. Hashem, Ecofriendly synthesis of biosynthesized copper nanoparticles with starch-based nanocomposite: antimicrobial, antioxidant, and anticancer activities. Biol. Trace Elem. Res. 200(5), 2099–2112 (2022)
A.P. Ingle, N. Duran, M. Rai, Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: a review. Appl. Microbiol. Biotechnol. 98(3), 1001–1009 (2014)
G. Wang, W. **, A.M. Qasim, A. Gao, X. Peng, W. Li, P.K. Chu, Antibacterial effects of titanium embedded with silver nanoparticles based on electron-transfer-induced reactive oxygen species. Biomaterials 124, 25–34 (2017)
L. Wang, C. Hu, L. Shao, The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomed. 12, 1227 (2017)
M.F. Gutiérrez, P. Malaquias, V. Hass, T.P. Matos, L. Lourenço, A. Reis, P.V. Farago, The role of copper nanoparticles in an etch-and-rinse adhesive on antimicrobial activity, mechanical properties and the durability of resin-dentine interfaces. J. Dent. 61, 12–20 (2017)
N. Jayarambabu, A. Akshaykranth, T.V. Rao, K.V. Rao, R.R. Kumar, Green synthesis of Cu nanoparticles using Curcuma longa extract and their application in antimicrobial activity. Mater. Lett. 259, 126813 (2020)
Q. Maqbool, S. Iftikhar, M. Nazar, F. Abbas, A. Saleem, T. Hussain, N. Jabeen, Green fabricated CuO nanobullets via Olea europaea leaf extract shows auspicious antimicrobial potential. IET Nanobiotechnol. 11(4), 463–468 (2017)
Y. Yuan, Y. Wu, V. Chinnadurai, M. Saravanan, A. Chinnathambi, S.A. Alharbi, A. Pugazhendhi, In vitro analysis of green synthesized copper nanoparticles using Chloroxylon swietenia leaves for dye degradation and antimicrobial application. Food Chem. Toxicol. 168, 113367 (2022)
K.Y. Yoon, J.H. Byeon, J.H. Park, J. Hwang, Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci. Total. Environ. 373(2–3), 572–575 (2007)
M.S. Usman, M.E. El Zowalaty, K. Shameli, N. Zainuddin, M. Salama, N.A. Ibrahim, Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomedicine 8, 4467–4479 (2013)
K. Cheirmadurai, S. Biswas, R. Murali, P. Thanikaivelan, Green synthesis of copper nanoparticles and conducting nanobiocomposites using plant and animal sources. RSC Adv. 4(37), 19507–19511 (2014)
M. Pérez-Alvarez, G. Cadenas-Pliego, O. Pérez-Camacho, V.E. Comparán-Padilla, C.J. Cabello-Alvarado, E. Saucedo-Salazar, Green synthesis of copper nanoparticles using cotton. Polymers 13(12), 1906 (2021)
K.S. Siddiqi, A. Husen, Current status of plant metabolite-based fabrication of copper/copper oxide nanoparticles and their applications: a review. Biomater. Res. 24, 1–15 (2020)
J.Y. Song, H.K. Jang, B.S. Kim, Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 44(10), 1133–1138 (2009)
K. Gopalakrishnan, C. Ramesh, V. Ragunathan, M. Thamilselvan, Antibacterial activity of Cu2O nanoparticles on E. coli synthesized from Tridax procumbens leaf extract and surface coating with polyaniline. Digest J. Nanomater. Biostruct. 7(2), 833–839 (2012)
M. Raffi, S. Mehrwan, T.M. Bhatti, J.I. Akhter, A. Hameed, W. Yawar, Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Annals of microbiology 60(1), 75–80 (2010)
S. Rajeshkumar, S. Menon, S.V. Kumar, M.M. Tambuwala, H.A. Bakshi, M. Mehta, K. Dua, Antibacterial and antioxidant potential of biosynthesized copper nanoparticles mediated through Cissus arnotiana plant extract. J. Photochem. Photobiol. B: Biol. 197, 111531 (2019)
S. Saleem, B. Ahmed, M.S. Khan, M. Al-Shaeri, J. Musarrat, Inhibition of growth and biofilm formation of clinical bacterial isolates by NiO nanoparticles synthesized from Eucalyptus globulus plants. Microb. Pathog. 111, 375–387 (2017)
T. Foteva, N. Georgieva, Antimicrobial properties of silica/hydroxypropylcellulose hybrids doped with copper ions. J. Chem. Metall. 57(5), 930–936 (2022)
M. Rai, A.P. Ingle, R. Pandit, P. Paralikar, S. Shende, I. Gupta, S.S. da Silva, Copper and copper nanoparticles: role in management of insect-pests and pathogenic microbes. Nanotechnol. Rev. 7(4), 303–315 (2018)
G. Ren, D. Hu, E.W. Cheng, M.A. Vargas-Reus, P. Reip, R.P. Allaker, Characterization of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 33(6), 587–590 (2009)
A.K. Chatterjee, R. Chakraborty, T. Basu, Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 25(13), 135101 (2014)
J.H. Kim, H. Cho, S.E. Ryu, M.U. Choi, Effects of metal ions on the activity of protein tyrosine phosphatase VHR: highly potent and reversible oxidative inactivation by Cu2+ ion. Arch. Biochem. Biophys. 382(1), 72–80 (2000)
Q. Lv, B. Zhang, X. **ng, Y. Zhao, R. Cai, W. Wang, Q. Gu, Biosynthesis of copper nanoparticles using Shewanella loihica PV-4 with antibacterial activity: novel approach and mechanisms investigation. J. Hazard. Mater. 347, 141–149 (2018)
H. Zhang, Z. Ji, T. **a, H. Meng, C. Low-Kam, R. Liu, A.E. Nel, Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano 6(5), 4349–4368 (2012)
W. **e, S. Zhang, F. Pan, S. Chen, L. Zhong, J. Wang, X. Pei, Nanomaterial-based ROS-mediated strategies for combating bacteria and biofilms. J. Mater. Res. 36, 822–845 (2021)
G. Applerot, J. Lellouche, A. Lipovsky, Y. Nitzan, R. Lubart, A. Gedanken, E. Banin, Understanding the antibacterial mechanism of CuO nanoparticles: revealing the route of induced oxidative stress. Small 8(21), 3326–3337 (2012)
S. Banerjee, K. Vishakha, S. Das, P.D. Sangma, S. Mondal, A. Ganguli, Oxidative stress, DNA, and membranes targets as modes of antibacterial and antibiofilm activity of facile synthesized biocompatible keratin-copper nanoparticles against multidrug resistant uro-pathogens. World J. Microbiol. Biotechnol. 38(2), 1–16 (2022)
R.K. Swarnkar, J.K. Pandey, K.K. Soumya, P. Dwivedi, S. Sundaram, S. Prasad, R. Gopal, Enhanced antibacterial activity of copper/copper oxide nanowires prepared by pulsed laser ablation in water medium. Appl. Phys. A 122(7), 1–7 (2016)
C. Kaweeteerawat, P. Na Ubol, S. Sangmuang, S. Aueviriyavit, R. Maniratanachote, Mechanisms of antibiotic resistance in bacteria mediated by silver nanoparticles. J. Toxicol. Environ. Health A 80(23–24), 1276–1289 (2017)
O. Metryka, D. Wasilkowski, A. Mrozik, Insight into the antibacterial activity of selected metal nanoparticles and alterations within the antioxidant defence system in Escherichia coli, Bacillus cereus and Staphylococcus epidermidis. Int. J. Mol. Sci. 22(21), 11811 (2021)
P. Sharma, D. Goyal, B. Chudasama, Antibacterial activity of colloidal copper nanoparticles against Gram-negative (Escherichia coli and Proteus vulgaris) bacteria. Lett. Appl. Microbiol. 74(5), 695–706 (2022)
J. Li, K. Rong, H. Zhao, F. Li, Z. Lu, R. Chen, Highly selective antibacterial activities of silver nanoparticles against Bacillus subtilis. J. Nanosci. Nanotechnol. 13(10), 6806–6813 (2013)
D.N. Phan, N. Dorjjugder, Y. Saito, M.Q. Khan, A. Ullah, X. Bie, I.S. Kim, Antibacterial mechanisms of various copper species incorporated in polymeric nanofibers against bacteria. Mater. Today Commun. 25, 101377 (2020)
H. Li, Q. Chen, J. Zhao, K. Urmila, Enhancing the antimicrobial activity of natural extraction using the synthetic ultrasmall metal nanoparticles. Sci. Rep. 5(1), 1–13 (2015)
L. Yadav, R.M. Tripathi, R. Prasad, R.N. Pudake, J. Mittal, Antibacterial activity of Cu nanoparticles against E. coli, Staphylococcus aureus and Pseudomonas aeruginosa. Nano Biomed. Eng 9(1), 9–14 (2017)
C.L. de Dicastillo, M.G. Correa, F.B. Martínez, C. Streitt, M.J. Galotto, Antimicrobial effect of titanium dioxide nanoparticles, in Antimicrobial resistance-a one health perspectivem. (IntechOpen, London, 2020)
A. Kösemen, Z.A. Kösemen, B. Canimkubey, M. Erkovan, F. Başarir, S.E. San, A.V. Tunç, Fe doped TiO2 thin film as electron selective layer for inverted solar cells. Sol. Energy 132, 511–517 (2016)
S. Sagadevan, S. Vennila, P. Singh, J.A. Lett, W.C. Oh, S. Paiman, P.K. Obulapuram, Exploration of the antibacterial capacity and ethanol sensing ability of Cu-TiO2 nanoparticles. J. Exp. Nanosci. 15(1), 337–349 (2020)
M.A. Sebak, T.F. Qahtan, G.M. Asnag, E.M. Abdallah, The role of TiO2 nanoparticles in the structural, thermal and electrical properties and antibacterial activity of PEO/PVP blend for energy storage and antimicrobial application. J. Inorg. Organomet. Polym. Mater. 32(12), 4715–4728 (2022)
J.T. Seil, T.J. Webster, Antimicrobial applications of nanotechnology: methods and literature. Int. J. Nanomed. 7, 2767 (2012)
A. Ansari, V.U. Siddiqui, W.U. Rehman, M.K. Akram, W.A. Siddiqi, A.M. Alosaimi, M.A. Hussein, M. Rafatullah, Green synthesis of TiO2 nanoparticles using acorus calamus leaf extract and evaluating its photocatalytic and in vitro antimicrobial activity. Catalysts 12, 181 (2022). https://doi.org/10.3390/catal12020181
I. Santana, H. Wu, P. Hu, J.P. Giraldo, Targeted delivery of nanomaterials with chemical cargoes in plants enabled by a biorecognition motif. Nat. Commun. 11(1), 1–12 (2020)
S. Albukhaty, L. Al-Bayati, H. Al-Karagoly, S. Al-Musawi, Preparation and characterization of titanium dioxide nanoparticles and in vitro investigation of their cytotoxicity and antibacterial activity against Staphylococcus aureus and Escherichia coli. Animal Biotechnol. (2020). https://doi.org/10.1080/10495398.2020.1842751
M.N. Alomary, M.A. Ansari, proanthocyanin-capped biogenic TiO2 nanoparticles with enhanced penetration, antibacterial and ROS mediated inhibition of bacteria proliferation and biofilm formation: a comparative approach. Chem. European J. 27(18), 5817–5829 (2021)
U.L.N.H. Senarathna, S.S.N. Fernando, T.D.C.P. Gunasekara, M.M. Weerasekera, H.G.S.P. Hewageegana, N.D.H. Arachchi, P.M. Jayaweera, Enhanced antibacterial activity of TiO2 nanoparticle surface modified with Garcinia zeylanica extract. Chem. Central J. 11(1), 1–8 (2017)
M. Azizi-Lalabadi, A. Ehsani, B. Divband, M. Alizadeh-Sani, Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci. Rep. 9(1), 1–10 (2019)
C. López de Dicastillo, C. Patiño, M.J. Galotto, J.L. Palma, D. Alburquenque, J. Escrig, Novel antimicrobial titanium dioxide nanotubes obtained through a combination of atomic layer deposition and electrospinning technologies. Nanomaterials 8(2), 128 (2018)
M.F. Song, Y.S. Li, H. Kasai, K. Kawai, Metal nanoparticle-induced micronuclei and oxidative DNA damage in mice. J. Clin. Biochem. Nutr. 50(3), 211–216 (2012)
T.J. Battin, F.V. Kammer, A. Weilhartner, S. Ottofuelling, T. Hofmann, Nanostructured TiO2: transport behavior and effects on aquatic microbial communities under environmental conditions. Environ. Sci. Technol. 43(21), 8098–8104 (2009)
A. Kumar, A.K. Pandey, S.S. Singh, R. Shanker, A. Dhawan, Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radical Biol. Med. 51(10), 1872–1881 (2011)
C. Pagnout, S. Jomini, M. Dadhwal, C. Caillet, F. Thomas, P. Bauda, Role of electrostatic interactions in the toxicity of titanium dioxide nanoparticles toward Escherichia coli. Colloids Surf. B 92, 315–321 (2012)
J. Rajkumari, C.M. Magdalane, B. Siddhardha, J. Madhavan, G. Ramalingam, N.A. Al-Dhabi, K. Kaviyarasu, Synthesis of titanium oxide nanoparticles using Aloe barbadensis mill and evaluation of its antibiofilm potential against Pseudomonas aeruginosa PAO1. J. Photochem. Photobiol. B: Biol. 201, 111667 (2019)
P. Maheswari, S. Ponnusamy, S. Harish, M.R. Ganesh, Y. Hayakawa, Hydrothermal synthesis of pure and bio modified TiO2: characterization, evaluation of antibacterial activity against gram positive and gram negative bacteria and anticancer activity against KB Oral cancer cell line. Arab. J. Chem. 13(1), 3484–3497 (2020)
M. Ovais, A.T. Khalil, M. Ayaz, I. Ahmad, S.K. Nethi, S. Mukherjee, Biosynthesis of metal nanoparticles via microbial enzymes: a mechanistic approach. Int. J. Mol. Sci. 19(12), 4100 (2018)
J. Hou, L. Wang, C. Wang, S. Zhang, H. Liu, S. Li, X. Wang, Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 75, 40–53 (2019)
E.B. Kurutas, The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J. 15(1), 1–22 (2015)
S. Sagadevan, S. Imteyaz, B. Murugan, J.A. Lett, N. Sridewi, G.K. Weldegebrieal, W.C. Oh, A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications. Green Proc. Synthesis 11(1), 44–63 (2022)
B. Sohm, F. Immel, P. Bauda, C. Pagnout, Insight into the primary mode of action of TiO2 nanoparticles on Escherichia coli in the dark. Proteomics 15(1), 98–113 (2015)
M. Nemattalab, M. Rohani, M. Evazalipour, Z. Hesari, Formulation of Cinnamon (Cinnamomum verum) oil loaded solid lipid nanoparticles and evaluation of its antibacterial activity against multi-drug resistant Escherichia coli. BMC Complement. Med. Therapies 22(1), 1–10 (2022)
C. Pagnout, A. Razafitianamaharavo, B. Sohm, C. Caillet, A. Beaussart, E. Delatour, J.F. Duval, Osmotic stress and vesiculation as key mechanisms controlling bacterial sensitivity and resistance to TiO2 nanoparticles. Commun. Biol. 4(1), 1–15 (2021)
S. Khan, M. Ul-Islam, W.A. Khattak, M.W. Ullah, J.K. Park, Bacterial cellulose-titanium dioxide nanocomposites: nanostructural characteristics, antibacterial mechanism, and biocompatibility. Cellulose 22(1), 565–579 (2015)
S. Shaikh, N. Nazam, S.M.D. Rizvi, K. Ahmad, M.H. Baig, E.J. Lee, I. Choi, Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int. J. Mol. Sci. 20(10), 2468 (2019)
N.B.A. Abdulrahman, Z.M. Nssaif, Antimicrobial activity of zinc oxide, titanium dioxide and silver nanoparticles against mithicillin-resistant Staphylococcus aureus Isolates. Tikrit J. Pure Sci. 21(3), 49–53 (2016)
E.T. Bekele, B.A. Gonfa, O.A. Zelekew, H.H. Belay, F.K. Sabir, Synthesis of titanium oxide nanoparticles using root extract of Kniphofia foliosa as a template, characterization, and its application on drug resistance bacteria. J. Nanomater. (2020). https://doi.org/10.1155/2020/2817037
S. Albukhaty, L. Al-Bayati, H. Al-Karagoly, S. Al-Musawi, Preparation and characterization of titanium dioxide nanoparticles and in vitro investigation of their cytotoxicity and antibacterial activity against Staphylococcus aureus and Escherichia coli. Anim. Biotechnol. 33(5), 864–870 (2022)
T. Saito, T. Iwase, J. Horie, T. Morioka, Mode of photocatalytic bactericidal action of powdered semiconductor TiO2 on mutans streptococci. J. Photochem. Photobiol. B. 14(4), 369–379 (1992)
A. Besinis, T. De Peralta, R.D. Handy, The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology 8(1), 1–16 (2014)
M. Pourhajibagher, A. Bahador, Synergistic biocidal effects of metal oxide nanoparticles-assisted ultrasound irradiation: antimicrobial sonodynamic therapy against Streptococcus mutans biofilms. Photodiagn. Photodyn. Ther. 35, 102432 (2021)
C. Miron, A. Roca, S. Hoisie, P. Cozorici, L. Sirghi, Photoinduced bactericidal activity of TiO2 thin films obtained by radiofrequency magnetron sputtering deposition. J. Optoelectron. Adv. Mater. 7, 915–919 (2004)
F. Khan, D.T.N. Pham, S.F. Oloketuyi, P. Manivasagan, J. Oh, Y.M. Kim, Chitosan and their derivatives: antibiofilm drugs against pathogenic bacteria. Colloids Surf. B 185, 110627 (2020)
P. Amezaga-Madrid, R. Silveyra-Morales, L. Cordoba-Fierro, G.V. Nevarez-Moorillon, M. Miki-Yoshida, E. Orrantia-Borunda, F.J. Solıs, TEM evidence of ultrastructural alteration on Pseudomonas aeruginosa by photocatalytic TiO2 thin films. J. Photochem. Photobiol. B. 70(1), 45–50 (2003)
S. Arya, H. Sonawane, S. Math, P. Tambade, M. Chaskar, D. Shinde, Biogenic titanium nanoparticles (TiO2 NPs) from Tricoderma citrinoviride extract: synthesis, characterization and antibacterial activity against extremely drug-resistant Pseudomonas aeruginosa. Int. Nano Lett. 11, 35–42 (2021)
W.J. Parak, L. Manna, F.C. Simmel, D. Gerion, P. Alivisatos, Nanoparticles: from theory to application (Wiley-VCH, Weinheim, 2004)
S.J. Hoseyni, M. Manoochehri, M.D. Asli, Synthesis of cobalt nanoparticles by complex demolition method using the reaction between organic ligand Schiff base and cobalt chloride by ultrasonication. Bull. Soc. Roy. Sci. Liège 86, 325–331 (2017)
O.U. Igwe, E.S. Ekebo, Biofabrication of cobalt nanoparticle odorata and their potential. Res. J. Chem. 8(1), 11–17 (2018)
M. Azharuddin, G.H. Zhu, D. Das, E. Ozgur, L. Uzun, A.P. Turner, H.K. Patra, A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 55(49), 6964–6996 (2019)
A.K. Singh, A review on plant extract-based route for synthesis of cobalt nanoparticles: photocatalytic, electrochemical sensing and antibacterial applications. Curr. Res. Green Sustain. Chem. (2022). https://doi.org/10.1016/j.crgsc.2022.100270
N.E. Eleraky, A. Allam, S.B. Hassan, M.M. Omar, Nanomedicine fight against antibacterial resistance: an overview of the recent pharmaceutical innovations. Pharmaceutics 12(2), 142 (2020)
T. Varaprasad, B. Govindh, B.V. Rao, Green synthesized cobalt nanoparticles using Asparagus racemosus root extract & evaluation of antibacterial activity. Int. J. Chem. Tech. Res. 10(9), 339–345 (2017)
C.T. Anuradha, P. Raji, Effect of annealing temperature on antibacterial, antifungal and structural properties of bio-synthesized Co3O4 nanoparticles using Hibiscus Rosa-sinensis. Mater. Res. Exp. 6(9), 095063 (2019)
M.M. Naik, H.B. Naik, G. Nagaraju, M. Vinuth, K. Vinu, R. Viswanath, Green synthesis of zinc doped cobalt ferrite nanoparticles: structural, optical, photocatalytic and antibacterial studies. Nano-Structures & Nano-Objects 19, 100322 (2019)
D. Kharade Suvarta, H. Nikam Gurunath, J. Mane Gavade Shubhangi, R. Patil Sachinkumar, V. Gaikwad Kishor, Biogenic synthesis of cobalt nanoparticles using Hibiscus cannabinus leaf extract and their antibacterial activity. Res. J. Chem. Environ 24(5), 9–13 (2020)
P.P. Shriniwas, T.K. Subhash, Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. leaves. Biochem. Biophys. Rep 10, 76–81 (2017)
H. Guan, W. Dong, Y. Lu, M. Jiang, D. Zhang, Y. Aobuliaximu, S. Lu, Distribution and antibiotic resistance patterns of pathogenic bacteria in patients with chronic cutaneous wounds in China. Front. Med. 8, 609584 (2021)
M. Hafeez, R. Shaheen, B. Akram, S. Haq, S. Mahsud, S. Ali, R.T. Khan, Green synthesis of cobalt oxide nanoparticles for potential biological applications. Mater. Res. Exp. 7(2), 025019 (2020)
S. Iravani, R.S. Varma, Sustainable synthesis of cobalt and cobalt oxide nanoparticles and their catalytic and biomedical applications. Green Chem. 22(9), 2643–2661 (2020)
G. Satpathy, E. Manikandan, Cobalt nanoparticle as the antibacterial tool. Int. J. Eng. Adv. Technol. (IJEAT) 8, 3684–3687 (2019)
V. Dogra, G. Kaur, S. **dal, R. Kumar, S. Kumar, N.K. Singhal, Bactericidal effects of metallosurfactants based cobalt oxide/hydroxide nanoparticles against Staphylococcus aureus. Sci. Total. Environ. 681, 350–364 (2019)
S. Chattopadhyay, S.K. Dash, S. Tripathy, B. Das, D. Mandal, P. Pramanik, S. Roy, Toxicity of cobalt oxide nanoparticles to normal cells; an in vitro and in vivo study. Chem. Biol. Interact. 226, 58–71 (2015)
J.S. Ajarem, S.N. Maodaa, A.A. Allam, M.M. Taher, M. Khalaf, Benign synthesis of cobalt oxide nanoparticles containing red algae extract: antioxidant, antimicrobial, anticancer, and anticoagulant activity. J. Clust. Sci. (2021). https://doi.org/10.1007/s10876-021-02004-9
M. Sivachidambaram, J.J. Vijaya, K. Kaviyarasu, L.J. Kennedy, H.A. Al-Lohedan, R.J. Ramalingam, A novel synthesis protocol for Co3O4 nanocatalysts and their catalytic applications. RSC Adv. 7(62), 38861–38870 (2017)
L. Du, S. Ahmad, L. Liu, L. Wang, J. Tang, A review of antibiotics and antibiotic resistance genes (ARGs) adsorption by biochar and modified biochar in water. Sci. Total. Environ. 858, 159815 (2023)
C.J. Murray, K.S. Ikuta, F. Sharara, L. Swetschinski, G.R. Aguilar, A. Gray, N. Tasak, Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399(10325), 629–655 (2022)
A.R. Mahoney, M.M. Safaee, W.M. Wuest, A.L. Furst, The silent pandemic: emergent antibiotic resistances following the global response to SARS-CoV-2. IScience 24(4), 102304 (2021)
R.F. O’Toole, K.W. Leong, V. Cumming, S.J. van Hal, Vancomycin-resistant Enterococcus faecium and the emergence of new sequence types associated with hospital infection. Res. Microbiol. (2023). https://doi.org/10.1016/j.resmic.2023.104046
J. Tabcheh, J. Vergalli, A. Davin-Régli, N. Ghanem, C. Al-Bayssari, J.M. Brunel, Rejuvenating the activity of usual antibiotics on resistant gram-negative bacteria: recent issues and perspectives. Int. J. Mol. Sci. 24(2), 1515 (2023)
J.M. Morris, K. Mercoulia, M. Valcanis, C.L. Gorrie, N.L. Sherry, B.P. Howden, Hidden resistances: how routine whole-genome sequencing uncovered an otherwise undetected bla NDM-1 gene in vibrio alginolyticus from imported seafood. Microbiol. Spectrum (2023). https://doi.org/10.1128/spectrum.04176-22
E. Altun, M.O. Aydogdu, E. Chung, G. Ren, S. Homer-Vanniasinkam, M. Edirisinghe, Metal-based nanoparticles for combating antibiotic resistance. Appl. Phys. Rev. (2021). https://doi.org/10.1063/5.0060299
A. Frei, A.D. Verderosa, A.G. Elliott, J. Zuegg, M.A. Blaskovich, Metals to combat antimicrobial resistance. Nat. Rev. Chem. (2023). https://doi.org/10.1038/s41570-023-00463-4
A. Chahardoli, M. Mavaei, Y. Shokoohinia, A. Fattahi, Galbanic acid, a sesquiterpene coumarin as a novel candidate for the biosynthesis of silver nanoparticles: in vitro hemocompatibility, antiproliferative, antibacterial, antioxidant, and anti-inflammatory properties. Adv. Powder Technol. 34(1), 103928 (2023)
I.H. Ifijen, M. Maliki, N.U. Udokpoh, I.J. Odiachi, B. Atoe, A concise review of the antibacterial action of gold nanoparticles against various bacteria, in TMS annual meeting & exhibition. (Springer Nature, Cham, 2023), pp.655–664
M. Alherek, O.D. Basu, Impact of low-levels of silver, zinc and copper nanoparticles on bacterial removal and potential synergy in water treatment applications. J. Chem. Technol. Biotechnol. 98(5), 1137–1146 (2023)
A.I. Doulgeraki, C.S. Kamarinou, G.J.E. Nychas, A.A. Argyri, C.C. Tassou, G. Moulas, N. Chorianopoulos, Role of microbial interactions across food-related bacteria on biofilm population and biofilm decontamination by a TiO2-nanoparticle-based surfactant. Pathogens 12(4), 573 (2023)
S.S. Hassan, K.A. Hubeatir, R.M.S. Al-Haddad, Characterization and antibacterial activity of silica-coated bismuth (Bi@ SiO2) nanoparticles synthesized by pulsed laser ablation in liquid. Optik (2023). https://doi.org/10.1016/j.ijleo.2022.170453
P.R. More, S. Pandit, A.D. Filippis, G. Franci, I. Mijakovic, M. Galdiero, Silver nanoparticles: bactericidal and mechanistic approach against drug resistant pathogens. Microorganisms 11(2), 369 (2023)
M. Paesa, C.R. de Ganuza, T. Alejo, C. Yus, S. Irusta, M. Arruebo, G. Mendoza, Elucidating the mechanisms of action of antibiotic-like ionic gold and biogenic gold nanoparticles against bacteria. J. Colloid Interface Sci. 633, 786–799 (2023)
R. Abreu, T. Semedo-Lemsaddek, E. Cunha, L. Tavares, M. Oliveira, Antimicrobial drug resistance in poultry production: current status and innovative strategies for bacterial control. Microorganisms 11(4), 953 (2023)
Y. Zaman, M.Z. Ishaque, S. Ajmal, M. Shahzad, A.B. Siddique, M.U. Hameed, G. Yasin, Tamed synthesis of AgNPs for photodegradation and anti-bacterial activity: effect of size and morphology. Inorg. Chem. Commun. 150, 110523 (2023)
V. Mageshwaran, P. Sivasubramanian, P. Kumar, Y. Nagaraju, Antibacterial response of nanostructured chitosan hybrid materials. Chitosan Nanocomposites: Bionanomech. Appl. (2023). https://doi.org/10.1007/978-981-19-9646-7_7
J. Ge, D. Li, J. Ding, X. **ao, Y. Liang, Microbial coexistence in the rhizosphere and the promotion of plant stress resistance: a review. Environ. Res. (2023). https://doi.org/10.1016/j.envres.2023.115298
A.K. Halder, A.S. Moura, M.N.D. Cordeiro, Predicting the ecotoxicity of endocrine disruptive chemicals: multitasking in silico approaches towards global models. Sci. Total. Environ. 889, 164337 (2023)
W. Hu, C. Wang, D. Gao, Q. Liang, Toxicity of transition metal nanoparticles: a review of different experimental models in the gastrointestinal tract. J. Appl. Toxicol. 43(1), 32–46 (2023)
D. Nath Roy, R. Goswami, A. Pal, Nanomaterial and toxicity: what can proteomics tell us about the nanotoxicology? Xenobiotica 47(7), 632–643 (2017)
A.M. Schrand, M.F. Rahman, S.M. Hussain, J.J. Schlager, D.A. Smith, A.F. Syed, Metal-based nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2(5), 544–568 (2010)
A. Sukhanova, S. Bozrova, P. Sokolov, M. Berestovoy, A. Karaulov, I. Nabiev, Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 13(1), 44 (2018). https://doi.org/10.1186/s11671-018-2457-x
S. Hua, M.B. De Matos, J.M. Metselaar, G. Storm, Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization. Front. Pharmacol. 9, 790 (2018)
A. Elsaesser, C.V. Howard, Toxicology of nanoparticles. Adv. Drug Deliv. Rev. 64(2), 129–137 (2012)
OECD, Publications in the series on the safety of manufactured nanomaterials, www.oecd.org, 2019
S.J. Choi, J.K. Lee, J. Jeong, J.H. Choy, Toxicity evaluation of inorganic nanoparticles: considerations and challenges. Mol. Cell. Toxicol. 9, 205–210 (2013)
S.C. Gad, Drug safety evaluation (John Wiley, New York, 2002)
V. De Matteis, Exposure to inorganic nanoparticles: routes of entry, immune response, biodistribution and in vitro/in vivo toxicity evaluation. Toxics 5(4), 29 (2017)
R. Mohammadpour, M.A. Dobrovolskaia, D.L. Cheney, K.F. Greish, H. Ghandehari, Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 144, 112–132 (2019)
C.D. Klaassen, Toxic responses of the respiratory system, in Casarett and & Doull’s toxicology: the basic science of poisons, 5th edn. (McGraw-Hill Companies Inc, New York, 1996), pp.515–534
E.O. Erhirhie, C.P. Ihekwereme, E.E. Ilodigwe, Advances in acute toxicity testing: strengths, weaknesses and regulatory acceptance. Interdiscip. Toxicol. 11, 5–12 (2018)
S. Parasuraman, Toxicological screening. J. Pharmacol. Pharmacother. 2, 74 (2011)
C. Bai, M. Tang, Toxicological study of metal and metal oxide nanoparticles in zebrafish. J. Appl. Toxicol. 40(1), 37–63 (2020)
H. Nehoff, S. Taurin, K. Greish, Toxicological assessment of nanomedicine (Wiley, Hoboken, 2013)
K. Greish, G. Thiagarajan, H. Ghandehari, In vivo methods of nanotoxicology. Methods Mol. Biol. 926, 235–253 (2012)
S. Haldar, Y. Muralidaran, D. Míguez, S.I. Mulla, P. Mishra, Eco-toxicity of nano-plastics and its implication on human metabolism: current and future perspective. Sci. Total. Environ. 861, 160571 (2023)
J. Bahamonde, B. Brenseke, M.Y. Chan, R.D. Kent, P.J. Vikesland, M.R. Prater, Gold nanoparticle toxicity in mice and rats: species differences. Toxicol. Pathol. 46(4), 431–443 (2018). https://doi.org/10.1177/0192623318770608
Y. Cao, S. Li, J. Chen, Modeling better in vitro models for the prediction of nanoparticle toxicity: a review. Toxicol. Mech. Methods 31(1), 1–17 (2021). https://doi.org/10.1080/15376516.2020.1828521
M. Xu, G. Halimu, Q. Zhang, Y. Song, X. Fu, Y. Li et al., Internalization and toxicity: a preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total. Environ. 694, 133794 (2019). https://doi.org/10.1016/j.scitotenv.2019.133794
P. Khanna, C. Ong, B.H. Bay, G.H. Baeg, Nanotoxicity: an interplay of oxidative stress, inflammation and cell death. Nanomaterials 5(3), 1163–1180 (2015)
P. Makhdoumi, H. Karimi, M. Khazaei, Review on metal-based nanoparticles: role of reactive oxygen species in renal toxicity. Chem. Res. Toxicol. 33(10), 2503–2514 (2020). https://doi.org/10.1021/acs.chemrestox.9b00438
M. Mishra, M. Panda, Reactive oxygen species: The root cause of nanoparticle-induced toxicity in Drosophila melanogaster. Free Radic. Res. 55(6), 919–935 (2021). https://doi.org/10.1080/10715762.2021.1914335
W. Yang, L. Wang, E.M. Mettenbrink, P.L. DeAngelis, S. Wilhelm, Nanoparticle toxicology. Annu. Rev. Pharmacol. Toxicol. 61, 269–289 (2021)
S. Yu, J. Liu, Y. Yin, M. Shen, Interactions between engineered nanoparticles and dissolved organic matter: a review on mechanisms and environmental effects. J. Environ. Sci. 63, 198–217 (2018)
M. Horie, Y. Tabei, Role of oxidative stress in nanoparticles toxicity. Free Radical Res. 55(4), 331–342 (2021)
S.J. Forrester, D.S. Kikuchi, M.S. Hernandes, Q. Xu, K.K. Griendling, Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 122(6), 877–902 (2018). https://doi.org/10.1161/circresaha.117.311401
K. Jakubczyk, K. Dec, J. Kaldunska, D. Kawczuga, J. Kochman, K. Janda, Reactive oxygen species - sources, functions, oxidative damage. Pol. Merkur. Lek. 48(284), 124–127 (2020)
P.D. Ray, B.W. Huang, Y. Tsuji, Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 24(5), 981–990 (2012). https://doi.org/10.1016/j.cellsig.2012.01.008
R. Wang, B. Song, J. Wu, Y. Zhang, A. Chen, L. Shao, Potential adverse effects of nanoparticles on the reproductive system. Int. J. Nanomed. 13, 8487 (2018)
C. Egbuna, V.K. Parmar, J. Jeevanandam, S.M. Ezzat, K.C. Patrick-Iwuanyanwu, C.O. Adetunji, C.G. Ibeabuchi, Toxicity of nanoparticles in biomedical application: nanotoxicology. J. Toxicol. 2021, 1–21 (2021)
C. Liao, Y. Li, S.C. Tjong, Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 20(2), 449 (2019)
Funding
Funding was not available for this study.
Author information
Authors and Affiliations
Contributions
MS wrote and edited the manuscript and did the software work. SA edited and evaluated the manuscript. HMT edited and evaluated the manuscript. RA edited the manuscript and help in data acquisition regarding mechanism of NPs action. UF did the graphical and pictorial work. SM did the reference management several times. AH provided the data for manuscript when required. TAM evaluated the data. MAF assisted in software work. HF provided the information regarding antibiotics resistance mechanism.
Corresponding author
Ethics declarations
Competing Interests
There were no competing interests declared unanimously by the authors.
Ethical Approval
Not applicable.
Consent to Participate
All authors have shown consent to participate.
Consent to Publication
All authors showed consent to publish the current review article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Summer, M., Ali, S., Tahir, H.M. et al. Mode of Action of Biogenic Silver, Zinc, Copper, Titanium and Cobalt Nanoparticles Against Antibiotics Resistant Pathogens. J Inorg Organomet Polym 34, 1417–1451 (2024). https://doi.org/10.1007/s10904-023-02935-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02935-y