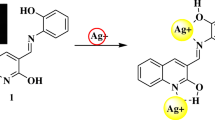
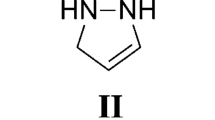
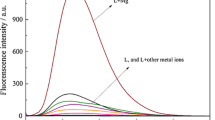
Abstract
Metal cations such as Zn2+, Al3+, Hg2+, Cd2+, Sn2+, Fe2+, Fe3+ and Cu2+ play important roles in biology, medicine, and the environment. However, when these are not maintained in proper concentration, they can be lethal to life. Therefore, selective sensing of metal cations is of great importance in understanding various metabolic processes, disease diagnosis, checking the purity of environmental samples, and detecting toxic analytes. Schiff base probes have been largely used in designing fluorescent sensors for sensing metal ions because of their easy processing, availability, fast response time, and low detection limit. Herein, an in-depth report on metal ions recognition by some Schiff base fluorescent sensors, their sensing mechanism, their practical applicability in cell imaging, building logic gates, and analysis of real-life samples has been presented. The metal ions having biological, industrial, and environmental significance are targeted. The compiled information is expected to prove beneficial in designing and synthesis of the related Schiff base fluorescent sensors.










































Similar content being viewed by others
Availability of Data and Materials
Nil.
Abbreviations
- CHEF:
-
Chelation enhanced fluorescence
- FRET:
-
Fluorescence resonance energy transfer
- CHEQ:
-
Chelation enhanced quenching
- ICT:
-
Intramolecular charge transfer
- PET:
-
Photoinduced electron transfer
- ESIPT:
-
Excited-state intramolecular proton transfer
- LOD:
-
Limit of detection
- Ka :
-
Association constant
References
Gokel GW, Leevy WM, Weber ME (2004) Crown ethers: Sensors for ions and molecular scaffolds for materials and biological models. Chem Rev 104:2723–2750. https://doi.org/10.1021/cr020080k
Chen X, Pradhan T, Wang F et al (2012) Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives. Chem Rev 112:1910–1956. https://doi.org/10.1021/cr200201z
Ren Y, Chen X, Li X et al (2010) Quantitative prediction of the thermal motion and intrinsic disorder of protein cofactors in crystalline state: A case study on halide anions. J Theor Biol 266:291–298. https://doi.org/10.1016/j.jtbi.2010.06.038
Maurya N, Bhardwaj S, Singh AK (2016) A modest colorimetric chemosensor for investigation of CN- in semi-aqueous environment with high selectivity and sensitivity. Sens Actuators B Chem 229:483–491. https://doi.org/10.1016/j.snb.2016.02.014
Michalski R, Kurzyca I (2006) Determination of nitrogen species (nitrate, nitrite and ammonia ions) in environmental samples by ion chromatography. Pol J Environ Stud 15:5–18
Upadhyay S, Singh A, Sinha R et al (2019) Colorimetric chemosensors for d-metal ions: A review in the past, present and future prospect. J Mol Struct 1193:89–102. https://doi.org/10.1016/j.molstruc.2019.05.007
Erdman JW, Macdonald IA, Zeisel SH (2012) Present Knowledge in Nutrition, 1st edn. Wiley
Ross AC, Caballero BH, Cousins RJ, Tucker KL, Ziegler TR (2012) Modern nutrition in health and disease: Eleventh edition. Wolters Kluwer Health Adis (ESP)
Ahamed M, Verma S, Kumar A, Siddiqui MKJ (2005) Environmental exposure to lead and its correlation with biochemical indices in children. Sci Total Environ 346:48–55. https://doi.org/10.1016/j.scitotenv.2004.12.019
Gaggelli E, Kozlowski H, Valensin D, Valensin G (2006) Copper homeostasis and neurodegenerative disorders (Alzheimer’s, Prion, and Parkinson’s Diseases and amyotrophic lateral sclerosis). Chem Rev 106:1995–2044. https://doi.org/10.1021/cr040410w
Jaishankar M, Tseten T, Anbalagan N et al (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7:60–72. https://doi.org/10.2478/intox-2014-0009
Matsui H, Morimoto M, Horimoto K, Nishimura Y (2007) Some characteristics of fluoride-induced cell death in rat thymocytes: Cytotoxicity of sodium fluoride. Toxicol In Vitro 21:1113–1120. https://doi.org/10.1016/j.tiv.2007.04.006
Strausak D, Mercer JFB, Dieter HH et al (2001) Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Res Bull 55:175–185. https://doi.org/10.1016/S0361-9230(01)00454-3
Botz MM, Mudder TI (2000) Modeling of natural cyanide attenuation in tailings impoundments. Mining Metall Explor 17:228–233. https://doi.org/10.1007/BF03403239
Fang G (2001) Spectrophotometric determination of lead in foods with dibromo-p-methyl-bromosulfonazo. Talanta 54:585–589. https://doi.org/10.1016/S00399140(00)00677-9
Liu Q, Liu T, Fang Y (2020) Perylene Bisimide derivative-based fluorescent film sensors: From sensory materials to device fabrication. Langmuir 36:2155–2169. https://doi.org/10.1021/acs.langmuir.9b03919
Luo X, Han Y, Chen X et al (2020) Carbon dots derived fluorescent nanosensors as versatile tools for food quality and safety assessment: A review. Trends Food Sci Technol 95:149–161. https://doi.org/10.1016/j.tifs.2019.11.017
Shamsipur M, Barati A, Nematifar Z (2019) Fluorescent pH nanosensors: Design strategies and applications. J Photochem Photobiol C 39:76–141. https://doi.org/10.1016/j.jphotochemrev.2019.03.001
Velusamy S, Roy A, Sundaram S, Kumar Mallick T (2021) A review on heavy metal ions and containing dyes removal through graphene oxide-based adsorption strategies for textile wastewater treatment. Chem Rec 21:1570–1610. https://doi.org/10.1002/tcr.202000153
Becker JS, Matusch A, Depboylu C et al (2007) Quantitative imaging of selenium, copper, and zinc in thin sections of biological tissues (slugs−genus arion) measured by laser ablation inductively coupled plasma mass spectrometry. Anal Chem 79:6074–6080. https://doi.org/10.1021/ac0700528
Liu Y, Liang P, Guo L (2005) Nanometer titanium dioxide immobilized on silica gel as sorbent for preconcentration of metal ions prior to their determination by inductively coupled plasma atomic emission spectrometry. Talanta 68:25–30. https://doi.org/10.1016/j.talanta.2005.04.035
Wang X, Sun J, Tong J et al (2018) Paper-based sensor chip for heavy metal ion detection by SWSV. Micromachines 9:150. https://doi.org/10.3390/mi9040150
Barba-Bon A, Costero AM, Gil S et al (2012) A new selective fluorogenic probe for trivalent cations. Chem Commun 48:3000. https://doi.org/10.1039/c2cc17184h
Kaur B, Kaur N, Kumar S (2018) Colorimetric metal ion sensors – A comprehensive review of the years 2011–2016. Coord Chem Rev 358:13–69. https://doi.org/10.1016/j.ccr.2017.12.002
Sarkar M (2020) A review on 2,6-Diformyl-4-methylphenol derived schiff bases as fluorescent sensors. Asian J Chem 32:1837–1848. https://doi.org/10.14233/ajchem.2020.22644
Wright AT, Anslyn EV (2006) Differential receptor arrays and assays for solution-based molecular recognition. Chem Soc Rev 35:14–28. https://doi.org/10.1039/B505518K
Amendola V, Fabbrizzi L (2009) Anion receptors that contain metals as structural units. ChemCommun 513–531. https://doi.org/10.1039/B808264M
Prodi L (2005) Luminescent chemosensors: from molecules to nanoparticles. New J Chem 29:20. https://doi.org/10.1039/b411758a
Yoon J, Kim SK, Singh NJ, Kim KS (2006) Imidazolium receptors for the recognition of anions. Chem Soc Rev 35:355. https://doi.org/10.1039/b513733k
Czarnik AW (1994) Fluorescent chemosensors for ion and molecule recognition. Instrum Sci Technol 22:405–406. https://doi.org/10.1080/10739149408001201
Kumar A, Virender MB et al (2022) Development of 2-hydroxy-naphthaldehyde functionalized Schiff base chemosensor for spectroscopic and colorimetric detection of Cu2+ and Pd2+ ions. Microchem J 180:107561. https://doi.org/10.1016/j.microc.2022.107561
Yin P, Ma W, Liu J et al (2022) Dual functional chemosensor for nano-level detection of Al3+ and Cu2+: Application to real samples analysis, colorimetric test strips and molecular logic gates. Microchem J 180:107557. https://doi.org/10.1016/j.microc.2022.107557
Yu W, Wang L, Wang L et al (2021) Quinoline based colorimetric and “turn-off” fluorescent chemosensor for phosgene sensing in solution and vapor phase. Microchem J 168:106334. https://doi.org/10.1016/j.microc.2021.106334
Udhayakumari D, Naha S, Velmathi S (2017) Colorimetric and fluorescent chemosensors for Cu2+. A comprehensive review from the years 2013–15. Anal Methods 9:552–578. https://doi.org/10.1039/C6AY02416E
Abu-Dief AM, Mohamed IMA (2015) A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef Univ J Basic Appl Sci 4:119–133. https://doi.org/10.1016/j.bjbas.2015.05.004
Qin W, Long S, Panunzio M, Biondi S (2013) Schiff bases: a short survey on an evergreen chemistry tool. Molecules 18:12264–12289. https://doi.org/10.3390/molecules181012264
Antony R, Arun T, Manickam STD (2019) A review on applications of chitosan-based schiff bases. Int J Biol Macromol 129:615–633. https://doi.org/10.1016/j.ijbiomac.2019.02.047
Kajal A, Bala S, Kamboj S et al (2013) Schiff bases: A versatile pharmacophore. J Catal 2013:1–14. https://doi.org/10.1155/2013/893512
Muzammil K, Trivedi P, Ketani DB (2015) Synthesis and characterization of schiff base m-nitro aniline and their complexes. Res J Chem 5:52–55
Kolapwar BG (2017) Study of Schiff base compounds and its derivatives. Anveshana’s Int J Rese Pharm Life Sci 2:15–18
Salvat A, Fortunato, et al (2001) Screening of some plants from Northern Argentina for their antimicrobial activity. Lett ApplMicrobiol 32:293–297. https://doi.org/10.1046/j.1472-765X.2001.00923.x
Fareed G, Rizwani GH, Ahmed M et al (2017) Schiff bases derived from 1-aminoanthraquinone: A new class of analgesic compounds. Pak J Sciind Res Ser A Physsci 60:122–127. https://doi.org/10.52763/PJSIR.PHYS.SCI.60.3.2017.122.127
Hanif M, Hassan M, Rafiq M et al (2018) Microwave-assisted synthesis, in vivo anti-inflammatory and in vitro anti-oxidant activities, and molecular docking study of new substituted schiff base derivatives. Pharm Chem J 52:424–437. https://doi.org/10.1007/s11094-018-1835-0
Kakanejadifard A, Khojasteh V, Zabardasti A, Azarbani F (2018) New azo-schiff base ligand capped silver and cadmium sulfide nanoparticles preparation, characterization, antibacterial and antifungal activities. Org Chem Res. https://doi.org/10.22036/org.chem.2018.133383.1149
Malik A, Goyat G, Verma KK, Garg S (2018) Synthesis, spectral and antimicrobial studies of some o-vanillin-2-aminopyridine schiff base complexes of organyltellurium (IV). Chem Sci Trans. https://doi.org/10.7598/cst2018.1480
Kalaiarasi G, Dharani S, Puschmann H, Prabhakaran R (2018) Synthesis, structural characterization, DNA/protein binding and antioxidant activities of binuclear Ni(II) complexes containing ONS chelating ligands bridged by 1,3-bis(diphenylphosphino)propane. Inorg Chem Commun 97:34–38. https://doi.org/10.1016/j.inoche.2018.09.004
Al-Shemary RK (2017) Design, synthesis and biological evaluation of schiff bases and their Co(II), Cu(II), Ni(II) chelates from derivative containing indole moiety bearing-triazole. ECB 6:433. https://doi.org/10.17628/ecb.2017.6.433-439
Luo H, **a Y, Sun B et al (2017) Synthesis and evaluation of in vitro antibacterial and antitumor activities of novel N, N-disubstituted schiff bases. Biochem Res Int 2017:1–10. https://doi.org/10.1155/2017/6257240
More G, Bootwala SZ, Mascarenhas J, Aruna K (2018) Anti-microbial and anti-tubercular activity evaluation of newly synthesized zinc complexes of aminothiophene schiff bases. Int J Pharm Sci Res 9:3029–3035. https://doi.org/10.13040/IJPSR.0975-8232.9(7)
Mahmood Yousif Al-Labban H, Mohammed Sadiq H, AbduljabbarJaloobAljanaby A (2019) Synthesis, Characterization and study biological activity of some Schiff bases derivatives from 4-amino antipyrine as a starting material. J Phys Conf Ser 1294:052007. https://doi.org/10.1088/1742-6596/1294/5/052007
Peng H, Liu Y, Huang J et al (2021) A simple fluorescent probe for selective detection of Al3+ based on furan Schiff base and its crystal structure. J Mol Struct 1229:129866. https://doi.org/10.1016/j.molstruc.2020.129866
Shin H, Jannah F, Yoo EJ, Kim J-M (2022) A colorimetric and fluorescence “turn-on” sensor for Fe(III) ion based on imidazole-functionalized polydiacetylene. Sens Actuators B Chem 350:130885. https://doi.org/10.1016/j.snb.2021.130885
Tümay SO, Yeşilot S (2021) Highly selective “turn-on” fluorescence determination of mercury ion in food and environmental samples through novel anthracene and pyrene appended Schiff bases. J Photochem Photobiol A 407:113093. https://doi.org/10.1016/j.jphotochem.2020.113093
Mohanasundaram D, Bhaskar R, Sankarganesh M et al (2022) A simple pyridine based fluorescent chemosensor for selective detection of copper ion. Spectrochim Acta Part A Mol Biomol Spectrosc 265:120395. https://doi.org/10.1016/j.saa.2021.120395
Chauhan A, Langyan R (2022) Photosensitization in highly luminescent nonmacrocyclic Samarium(III) complexes for application in light-emitting systems. J Photochem Photobiol A 424:113627. https://doi.org/10.1016/j.jphotochem.2021.113627
Ding W, Wu Y, Zhang S et al (2021) A dual-channel ‘turn-on’ fluorescent chemosensor for high selectivity and sensitivity detection of CN¯ based on a coumarin-Schiff base derivative in an aqueous system. Luminescence 36:1306–1316. https://doi.org/10.1002/bio.4058
Singh H, Bamrah A, Bhardwaj SK et al (2021) Recent advances in the application of noble metal nanoparticles in colorimetric sensors for lead ions. Environ Sci Nano 8:863–889. https://doi.org/10.1039/D0EN00963F
Sun X, Ding Y, Niu B, Chen Q (2021) Evaluation of a composite nanomaterial consist of gold nanoparticles and graphene-carbon nitride as capillary electrochromatography stationary phase for enantioseparation. Microchem J 169:106613. https://doi.org/10.1016/j.microc.2021.106613
Udhayakumari D, Inbaraj V (2020) A review on schiff base fluorescent chemosensors for cell imaging applications. J Fluoresc 30:1203–1223. https://doi.org/10.1007/s10895-020-02570-7
Junaid HM, Batool M, Harun FW et al (2022) Naked eye chemosensing of anions by schiff bases. Crit Rev Anal Chem 52:463–480. https://doi.org/10.1080/10408347.2020.1806703
Shah SS, Shah D, Khan I, Ahmad S, Ali U, Rahman AU (2020) Synthesis and antioxidant activities of schiff bases and their complexes: An updated review. Biointerface Res Appl Chem 10:6936–6963. https://doi.org/10.33263/BRIAC106.69366963
Al Zoubi W, Ko YG (2017) Schiff base complexes and their versatile applications as catalysts in oxidation of organic compounds: part I: Schiff bases and their versatile applications. Appl Organometal Chem 31:3574. https://doi.org/10.1002/aoc.3574
Gupta P, Dwivedi D, Chourey VR (2021) Review article on metal complexes derived from 2’-hydroxyacetophenone based Schiff Base. Orient J Chem 37:25–32. https://doi.org/10.13005/ojc/370102
Uddin MN, Ahmed SS, Alam SMR (2020) REVIEW: Biomedical applications of Schiff base metal complexes. J Coord Chem 73:3109–3149. https://doi.org/10.1080/00958972.2020.1854745
Shetty P (2020) Schiff bases: An overview of their corrosion inhibition activity in acid media against mild steel. Chem Eng Commun 207:985–1029. https://doi.org/10.1080/00986445.2019.1630387
Berhanu AL, Gaurav MI et al (2019) A review of the applications of Schiff bases as optical chemical sensors. TrAC, Trends Anal Chem 116:74–91. https://doi.org/10.1016/j.trac.2019.04.025
Naskar B, Modak R, Sikdar Y et al (2017) Fluorescent sensing of Al3+by benzophenone-based Schiff base chemosensor and live cell imaging applications: Impact of keto-enol tautomerism. Sens Actuators B Chem 239:1194–1204. https://doi.org/10.1016/j.snb.2016.08.148
Sen B, Sheet SK, Thounaojam R et al (2017) A coumarin based Schiff base probe for selective fluorescence detection of Al3+ and its application in live cell imaging. Spectrochim Acta Part A Mol Biomol Spectrosc 173:537–543. https://doi.org/10.1016/j.saa.2016.10.005
Sharma S, Hundal MS, Walia A et al (2014) Nanomolar fluorogenic detection of Al(III) by a series of Schiff bases in an aqueous system and their application in cell imaging. Org BiomolChem 12:4445. https://doi.org/10.1039/c4ob00329b
Fan L, Qin J, Li T et al (2014) A chromone Schiff-base as Al(III) selective fluorescent and colorimetric chemosensor. J Lumin 155:84–88. https://doi.org/10.1016/j.jlumin.2014.06.023
Kashyap KS, Kumar A, Hira SK, Dey S (2019) Recognition of Al3+ through the off-on mechanism as a proficient driving force for the hydrolysis of BODIPY conjugated Schiff base and its application in bio-imaging. Inorganica Chim Acta 498:119157. https://doi.org/10.1016/j.ica.2019.119157
Xu Y, Li L, Bai L et al (2020) Water-soluble fluorescent chemosensor based on Schiff base derivative terminated PEG for highly efficient detection of Al3+ in pure aqueous media. Tetrahedron Lett 61:152335. https://doi.org/10.1016/j.tetlet.2020.152335
Xu Y, Kong L, Bai L et al (2021) A new water-soluble polymer fluorescent chemosensor with thiophene Schiff base site for selectively sensing Al3+ ions. Tetrahedron 79:131888. https://doi.org/10.1016/j.tet.2020.131888
Selvan GT, Kumaresan M, Sivaraj R et al (2016) Isomeric 4-aminoantipyrine derivatives as fluorescent chemosensors of Al3+ ions and their molecular logic behaviour. Sens Actuators B Chem 229:181–189. https://doi.org/10.1016/j.snb.2016.01.097
Xu H, Chen W, Ju L, Lu H (2021) A purine based fluorescent chemosensor for the selective and sole detection of Al3+ and its practical applications in test strips and bio-imaging. Spectrochim Acta Part A Mol Biomol Spectrosc 247:119074. https://doi.org/10.1016/j.saa.2020.119074
Xu J, Li H, Li L et al (2020) A highly selective fluorescent chemosensor for Al3+ Based on 2,2’:6’,2”-terpyridine with a salicylal schiff base. J Braz Chem Soc. https://doi.org/10.21577/0103-5053.20200063
Chen Y (2020) Microwave-assisted synthesis of a novel steroid-derived Schiff base chemosensor for detection of Al3+ in aqueous media. J Chem Res 44:750–755. https://doi.org/10.1177/1747519820914827
Banerjee S, Brandão P, Saha A (2016) A robust fluorescent chemosensor for aluminum ion detection based on a Schiff base ligand with an azo arm and application in a molecular logic gate. RSC Adv 6:101924–101936. https://doi.org/10.1039/C6RA21217D
Gao C, Zang P, Liu W, Tang Y (2016) A highly selective and sensitive fluorescent chemosensor for aluminum ions based on schiff base. J Fluoresc 26:2015–2021. https://doi.org/10.1007/s10895-016-1895-z
Hossain SM, Singh K, Lakma A et al (2017) A schiff base ligand of coumarin derivative as an ICT-Based fluorescence chemosensor for Al3+. Sens Actuators B Chem 239:1109–1117. https://doi.org/10.1016/j.snb.2016.08.093
Hwang IH, Choi YW, Kim KB et al (2016) A highly selective and sensitive fluorescent turn-on Al3+ chemosensor in aqueous media and living cells: experimental and theoretical studies. New J Chem 40:171–178. https://doi.org/10.1039/C5NJ02334C
Alyaninezhad Z, Bekhradnia A, Feizi N et al (2019) A novel aluminum-sensitive fluorescent chemosensor based on 4-aminoantipyrine: An experimental and theoretical study. Spectrochim Acta Part A Mol Biomol Spectrosc 212:32–41. https://doi.org/10.1016/j.saa.2018.12.035
Manna AK, Chowdhury S, Patra GK (2020) Combined experimental and theoretical studies on a phenyl thiadiazole-based novel turn-on fluorescent colorimetric Schiff base chemosensor for the selective and sensitive detection of Al3+. New J Chem 44:10819–10832. https://doi.org/10.1039/D0NJ01954B
Kim SY, Lee SY, Kang JH et al (2018) Colorimetric detection of Fe3+/2+ and fluorescent detection of Al3+ in aqueous media: applications and DFT calculations. J Coord Chem 71:2401–2414. https://doi.org/10.1080/00958972.2018.1478086
Kurbah SD, Kumar A, Shangpung S et al (2017) Synthesis, characterization, and fluorescence chemosensor properties of a cis -dioxomolybdenum (VI) complex containing multidentate hydrazone ligands. Z Anorg Allg Chem 643:794–801. https://doi.org/10.1002/zaac.201700100
Li H, Wang J, Zhang S et al (2018) A novel off-on fluorescent chemosensor for Al3+ derived from a 4,5-diazafluorene Schiff base derivative. RSC Adv 8:31889–31894. https://doi.org/10.1039/C8RA05280H
Liu T, Wan X, Dong Y et al (2017) Facile synthesis of a water-soluble fluorescence sensor for Al3+ in aqueous solution and on paper substrate. Spectrochim Acta Part A Mol Biomol Spectrosc 173:625–629. https://doi.org/10.1016/j.saa.2016.10.004
Naskar B, Modak R, Sikdar Y et al (2017) Fluorescent sensing of Al3+ by benzophenone-based Schiff base chemosensor and live cell imaging applications: Impact of keto-enol tautomerism. Sens Actuators B Chem 239:1194–1204. https://doi.org/10.1016/j.snb.2016.08.148
Selvan GT, Kumaresan M, Sivaraj R et al (2016) Isomeric 4-aminoantipyrine derivatives as fluorescent chemosensors of Al3+ ions and their molecular logic behavior. Sens Actuators B Chem 229:181–189. https://doi.org/10.1016/j.snb.2016.01.097
Tian H, Qiao X, Zhang Z-L et al (2019) A high performance 2-hydroxynaphthalene schiff base fluorescent chemosensor for Al3+ and its applications in imaging of living cells and zebrafish in vivo. Spectrochim Acta Part A Mol Biomol Spectrosc 207:31–38. https://doi.org/10.1016/j.saa.2018.08.063
Andreini C, Banci L, Bertini I, Rosato A (2006) Zinc through the three domains of life. J Proteome Res 5:3173–3178. https://doi.org/10.1021/pr0603699
Bhowmick R, Alam R, Mistri T et al (2016) A thiosemicarbazone based chemo and fluorogenic sensor for Zn 2+ with CHEF and ESIPT behavior: computational studies and cell imaging application. RSC Adv 6:11388–11399. https://doi.org/10.1039/C5RA25653D
Mantyh PW, Ghilardi JR, Rogers S et al (1993) Aluminum, iron, and zinc ions promote aggregation of physiological concentrations of β-amyloid peptide. J Neurochem 61:1171–1174. https://doi.org/10.1111/j.1471-4159.1993.tb03639.x
Yanagisawa H, Miyakoshi Y, Kobayashi K et al (2009) Long-term intake of a high zinc diet causes iron deficiency anemia accompanied by reticulocytosis and extra-medullary erythropoiesis. Toxicol Lett 191:15–19. https://doi.org/10.1016/j.toxlet.2009.07.024
Ahmed N, Zareen W, Zhang D et al (2020) A DCM-based NIR sensor for selective and sensitive detection of Zn2+ in living cells. Spectrochim Acta Part A Mol Biomol Spectrosc 243:118758. https://doi.org/10.1016/j.saa.2020.118758
Choi YW, You GR, Lee JJ, Kim C (2016) Turn-on fluorescent chemosensor for selective detection of Zn2+ in an aqueous solution: Experimental and theoretical studies. Inorg Chem Commun 63:35–38. https://doi.org/10.1016/j.inoche.2015.11.012
Gomathi A, Vasanthi M, Viswanthamurthi P et al (2018) A simple perceptive diphenyl-imidazole-based dipodal schiff-base chemosensor for Zn2+ and PPi ions and its live-cell imaging applications. ChemistrySelect 3:11809–11815. https://doi.org/10.1002/slct.201802233
Berrones-Reyes JC, Muñoz-Flores BM, Cantón-Diáz AM et al (2019) Quantum chemical elucidation of the turn-on luminescence mechanism in two new Schiff bases as selective chemosensors of Zn2+ : synthesis, theory and bioimaging applications. RSC Adv 9:30778–30789. https://doi.org/10.1039/C9RA05010H
Feng E, Tu Y, Fan C et al (2017) A highly selective and sensitive fluorescent chemosensor for Zn2+ based on a diarylethene derivative. RSC Adv 7:50188–50194. https://doi.org/10.1039/C7RA09966E
Sarkar D, Pramanik A, Jana S et al (2015) Quinoline based reversible fluorescent ‘turn-on’ chemosensor for the selective detection of Zn2+: Application in living cell imaging and as INHIBIT logic gate. Sens Actuators B Chem 209:138–146. https://doi.org/10.1016/j.snb.2014.11.097
Rout K, Manna AK, Sahu M, Patra GK (2019) A guanidine based bis schiff base chemosensor for colorimetric detection of Hg(II) and fluorescent detection of Zn(II) ions. Inorganica Chim Acta 486:733–741. https://doi.org/10.1016/j.ica.2018.11.021
Kim MS, Jo TG, Yang M et al (2019) A fluorescent and colorimetric Schiff base chemosensor for the detection of Zn2+ and Cu2+: Application in live cell imaging and colorimetric test kit. Spectrochim Acta Part A Mol Biomol Spectrosc 211:34–43. https://doi.org/10.1016/j.saa.2018.11.058
Kumar M, Kumar A, Singh MK et al (2017) A novel benzidine based Schiff base “turn-on” fluorescent chemosensor for selective recognition of Zn2+. Sens Actuators B Chem 241:1218–1223. https://doi.org/10.1016/j.snb.2016.10.008
Li H, Zhang S, Gong C et al (2016) A turn-on and reversible fluorescence sensor for zinc ion based on 4,5-diazafluorene schiff base. J Fluoresc 26:1555–1561. https://doi.org/10.1007/s10895-016-1877-1
Shamel A, Salemnoush T (2016) Synthesis and fluorescence study of the grafted salicylidene schiff base onto SBA-15 mesoporous silica for detecting Zn2+ traces in aqueous medium. Russ J Appl Chem 89:500–504. https://doi.org/10.1134/S10704272160030228
Wang W, Li R, Song T et al (2016) Study on the fluorescent chemosensors based on a series of bis-Schiff bases for the detection of zinc(II). Spectrochim Acta Part A Mol Biomol Spectrosc 164:133–138. https://doi.org/10.1016/j.saa.2016.04.016
Yan J, Fan L, Qin J et al (2016) A novel and resumable schiff-base fluorescent chemosensor for Zn(II). Tetrahedron Lett 57:2910–2914. https://doi.org/10.1016/j.tetlet.2016.05.079
Wang X, Ding G, Duan Y et al (2020) Novel ‘naked-eye’ bis-schiff base fluorescent chemosensors for sensitive detection of Zn2+ and bio-imaging in living cells. Tetrahedron 76:131108. https://doi.org/10.1016/j.tet.2020.131108
Lee SY, Bok KH, Kim JA et al (2016) Simultaneous detection of Cu2+ and Cr3+ by a simple Schiff-base colorimetric chemosensor bearing NBD (7-nitrobenzo-2-oxa-1,3-diazolyl) and julolidine moieties. Tetrahedron 72:5563–5570. https://doi.org/10.1016/j.tet.2016.07.051
Murugesan K, Jeyasingh V, Lakshminarayanan S et al (2019) Simple and highly electron deficient schiff-base host for anions: First turn-on colorimetric bifluoride sensor. Spectrochim Acta Part A Mol Biomol Spectrosc 209:165–169. https://doi.org/10.1016/j.saa.2018.10.043
Udhayakumari D, Velmathi S (2015) Azo linked polycyclic aromatic hydrocarbons-based dual chemosensor for Cu2+ and Hg2+ ions. Ind Eng Chem Res 54:3541–3547. https://doi.org/10.1021/acs.iecr.5b00775
Basaki M, Saeb M, Nazifi S, Shamsaei HA (2012) Zinc, copper, iron, and chromium concentrations in young patients with type 2 diabetes mellitus. Biol Trace Elem Res 148:161–164. https://doi.org/10.1007/s12011-012-9360-6
De Romaña DL, Olivares M, Uauy R, Araya M (2011) Risks and benefits of copper in light of new insights of copper homeostasis. J Trace Elem Med Biol 25:3–13. https://doi.org/10.1016/j.jtemb.2010.11.004
Zhang Y, Cao X, Zhen L, Wang X (2021) A mesoporous silica-based fluorescent chemosensor bearing bis-Schiff base for the sensitive detection of Cu2+ ions. J Solid-State Chem 297:122093. https://doi.org/10.1016/j.jssc.2021.122093
Moradinia E, Mansournia M, Notash B (2019) A turn-off fluorescent chemosensor for Cu(II) based on sensitive Schiff base derived from 4-tert-Butyl-2,6-diformylphenol and p-toluic hydrazide. J Photochem Photobiol A 382:111963. https://doi.org/10.1016/j.jphotochem.2019.111963
Liang S, Tong Q, Qin X et al (2020) A hydrophilic naphthalimide-based fluorescence chemosensor forCu2+ ion: Sensing properties, cell imaging and molecular logic behavior. Spectrochim Acta Part A Mol Biomol Spectrosc 230:118029. https://doi.org/10.1016/j.saa.2020.118029
Rathod RV, Bera S, Singh M, Mondal D (2016) A colorimetric and fluorometric investigation of Cu(ii) ion in aqueous medium with a fluorescein-based chemosensor. RSC Adv 6:34608–34615. https://doi.org/10.1039/C6RA03021A
Sadia M, Naz R, Khan J, Khan R (2018) Synthesis and evaluation of a schiff-based fluorescent chemosensors for the selective and sensitive detection of Cu2+ in aqueous media with fluorescence off-on responses. J Fluoresc 28:1281–1294. https://doi.org/10.1007/s10895-018-2278-4
Wang Y, Hao X, Liang L et al (2020) A coumarin-containing Schiff base fluorescent probe with AIE effect for the copper (ii) ion. RSC Adv 10:6109–6113. https://doi.org/10.1039/C9RA10632D
Kowser Z, ** C-C, Jiang X et al (2016) Fluorescent turn-on sensors based on pyrene-containing Schiff base derivatives for Cu2+ recognition: spectroscopic and DFT computational studies. Tetrahedron 72:4575–4581. https://doi.org/10.1016/j.tet.2016.06.017
Slassi S, Aarjane M, El-Ghayoury A, Amine A (2019) A highly turn-on fluorescent CHEF-type chemosensor for selective detection of Cu2+ in aqueous media. Spectrochim Acta Part A Mol Biomol Spectrosc 215:348–353. https://doi.org/10.1016/j.saa.2019.02.099
Uyanik I, Oguz M, Bhatti AA et al (2017) A new piperidine derivatized-schiff base based “turn-on” Cu2+ chemo-sensor. J Fluoresc 27:791–797. https://doi.org/10.1007/s10895-016-2013-y
Wu W-N, Mao P-D, Wang Y et al (2018) AEE active Schiff base-bearing pyrene unit and further Cu2+–induced self-assembly process. Sens Actuators B Chem 258:393–401. https://doi.org/10.1016/j.snb.2017.11.114
Xu Y, Aderinto SO, Wu H et al (2017) A highly selective fluorescent chemosensor based on naphthalimide and Schiff base units for Cu2+ detection in aqueous medium. Z Naturforsch B 72:35–41. https://doi.org/10.1515/znb-2016-0138
Zhang Y-M, Zhu W, Qu W-J et al (2018) Novel chemosensor for ultrasensitive dual-channel detection of Cu2+ and its application in IMPLICATION logic gate. J Lumin 202:225–231. https://doi.org/10.1016/j.jlumin.2018.05.064
Zhang X-B, Han Z-X, Fang Z-H et al (2006) 5,10,15-Tris(pentafluorophenyl)corrole as highly selective neutral carrier for a silver ion-sensitive electrode. Anal Chim Acta 562:210–215. https://doi.org/10.1016/j.aca.2006.01.056
He ZL, Yang XE, Stoffella PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol 19:125–140. https://doi.org/10.1016/j.jtemb.2005.02.010
Ratte HT (1999) Bioaccumulation and toxicity of silver compounds: A review. Environ Toxicol Chem 18:89–108. https://doi.org/10.1002/etc.5620180112
Chopra I (2007) The increasing use of silver-based products as antimicrobial agents: a useful development or a cause for concern? J Antimicrob Chemother 59:587–590. https://doi.org/10.1093/jac/dkm006
Purcell TW, Peters JJ (1998) Sources of silver in the environment. Environ Toxicol Chem 17:539–546. https://doi.org/10.1002/etc.5620170404
Bhuvanesh N, Suresh S, Kumar PR et al (2018) Small molecule “turn on” fluorescent probe for silver ion and application to bioimaging. J Photochem Photobiol A 360:6–12. https://doi.org/10.1016/j.jphotochem.2018.04.027
Sahu M, Kumar Manna A, Rout K et al (2020) A highly selective thiosemicarbazone based Schiff base chemosensor for colorimetric detection of Cu2+ and Ag+ ions and turn-on fluorometric detection of Ag+ ions. Inorganica Chim Acta 508:119633. https://doi.org/10.1016/j.ica.2020.119633
Satarug S, Baker JR, Urbenjapol S et al (2003) A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett 137:65–83. https://doi.org/10.1016/S0378-4274(02)00381-8
Singh BR, McLaughlin MJ (1999) Cadmium in soils and plants. In: McLaughlin MJ, Singh BR (eds) Cadmium in Soils and Plants. Springer, Netherlands, Dordrecht, pp 257–267
Dobson S (1992) Cadmium-environmental aspects. Environ Health Criteria 135:1–156
Mohanasundaram D, Bhaskar R, GangatharanVinoth Kumar G et al (2021) A quinoline based Schiff base as a turn-on fluorescence chemosensor for selective and robust detection of Cd2+ ion in semi-aqueous medium. Microchem J 164:106030. https://doi.org/10.1016/j.microc.2021.106030
Khan SA, Ullah Q, Almalki ASA et al (2021) Synthesis and photophysical investigation of (BTHN) Schiff base as off-on Cd2+ fluorescent chemosensor and its live cell imaging. J Mol Liq 328:115407. https://doi.org/10.1016/j.molliq.2021.115407
Singh AK, Gupta VK, Gupta B (2007) Chromium(III) selective membrane sensors based on Schiff bases as chelating ionophores. Anal Chim Acta 585:171–178. https://doi.org/10.1016/j.aca.2006.11.074
Latva S, Jokiniemi J, Peräniemi S, Ahlgrén M (2003) Separation of picogram quantities of Cr(iii) and Cr(vi) species in aqueous solutions and determination by graphite furnace atomic absorption spectrometry. J Anal AtSpectrom 18:84–86. https://doi.org/10.1039/B207941K
McRae R, Bagchi P, Sumalekshmy S, Fahrni CJ (2009) In situ imaging of metals in cells and tissues. Chem Rev 109:4780–4827. https://doi.org/10.1021/cr900223a
Abbaspour A (2001) Chromium(III) ion-selective electrode based on 4-dimethylaminoazobenzene. Talanta 53:1009–1013. https://doi.org/10.1016/S0039-9140(00)00593-2
O’Brien T, Mandel HG, Pritchard DE, Patierno SR (2002) Critical role of chromium (Cr)−DNA interactions in the formation of Cr-induced polymerase arresting lesions. Biochemistry 41:12529–12537. https://doi.org/10.1021/bi020452j
Chalmardi GB, Tajbakhsh M, Bekhradnia A, Hosseinzadeh R (2017) A highly sensitive and selective novel fluorescent chemosensor for detection of Cr3+ based on a schiff base. Inorganica Chim Acta 462:241–248. https://doi.org/10.1016/j.ica.2017.03.041
Chalmardi GB, Tajbakhsh M, Hasani N, Bekhradnia A (2018) A new Schiff-base as fluorescent chemosensor for selective detection of Cr3+: An experimental and theoretical study. Tetrahedron 74:2251–2260. https://doi.org/10.1016/j.tet.2018.03.046
Sivaraman G, Sathiyaraja V, Chellappa D (2014) Turn-on fluorogenic and chromogenic detection of Fe(III) and its application in living cell imaging. J Lumin 145:480–485. https://doi.org/10.1016/j.jlumin.2013.08.018
Hentze MW, Muckenthaler MU, Galy B, Camaschella C (2010) Two to tango: Regulation of mammalian iron metabolism. Cell 142:24–38. https://doi.org/10.1016/j.cell.2010.06.028
Chen M, **ong H, Wen W et al (2013) Electrochemical biosensors for the assay of DNA damage initiated by ferric ions catalyzed oxidation of dopamine in room temperature ionic liquid. Electrochim Acta 114:265–270. https://doi.org/10.1016/j.electacta.2013.10.122
Andersen JET (2005) A novel method for the filterlesspreconcentration of iron. Analyst 130:385. https://doi.org/10.1039/b412061b
Halliwell B, Gutteridge JMC (1992) Biologically relevant metal ion-dependent hydroxyl radical generation an update. FEBS Lett 307:108–112. https://doi.org/10.1016/0014-5793(92)80911-Y
Yamazaki I, Piette LH (1990) ESR spin-trap** studies on the reaction of Fe2+ ions with H2O2-reactive species in oxygen toxicity in biology. J Biol Chem 265:13589–13594. https://doi.org/10.1016/S0021-9258(18)77389-4
Enami S, Sakamoto Y, Colussi AJ (2014) Fenton chemistry at aqueous interfaces. Proc Natl AcadSci USA 111:623–628. https://doi.org/10.1073/pnas.1314885111
Li Y, Pan W, Zheng C, Pu S (2020) A diarylethene derived Fe3+ fluorescent chemosensor and its application in wastewater analysis. J Photochem Photobiol A 389:112282. https://doi.org/10.1016/j.jphotochem.2019.112282
Faculty of Applied Sciences, UniversitiTeknologi MARA, Hasan S, Zakaria S, Adnan SNAM (2017) Fluorescent chemosensor bearing amine and benzenyl functionality for Fe3+ ions detection in aqueous solution. IJCEA 8:28–32. https://doi.org/10.18178/ijcea.2017.8.1.626
He Y, Yin J, Wang G (2018) New selective “on-off” fluorescence chemosensor based on carbazole Schiff base for Fe3+ detection. Chem Heterocycl Comp 54:146–152. https://doi.org/10.1007/s10593-018-2246-6
Khan SA, Asiri AM (2017) Physicochemical properties of novel methyl 2-{(E)-[(2-hydroxynaphthalen-1-yl)methylidene] amino}-4,5,6,7-tetrahydro-1-benzothiophene-3-carboxylate as turn-off fluorometric chemosensor for detection Fe3+ ion. J Mol Liq 243:85–90. https://doi.org/10.1016/j.molliq.2017.07.054
Harris HH, Pickering IJ, George GN (2003) The chemical form of mercury in fish. Science 301:1203–1203. https://doi.org/10.1126/science.1085941
Nolan EM, Lippard SJ (2008) Tools and tactics for the optical detection of mercuric ion. Chem Rev 108:3443–3480. https://doi.org/10.1021/cr068000q
Bernhoft RA (2012) Mercury toxicity and treatment: A review of the literature. J Environ Public Health 2012:1–10. https://doi.org/10.1155/2012/460508
Carter KP, Young AM, Palmer AE (2014) Fluorescent sensors for measuring metal ions in living systems. Chem Rev 114:4564–4601. https://doi.org/10.1021/cr400546e
Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D (2003) Review: Environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol 18:149–175. https://doi.org/10.1002/tox.10116
Bhaskar R, Sarveswari S (2020) Thiocarbohydrazide based schiff base as a selective colorimetric and fluorescent chemosensor for Hg 2+ with “turn-off” fluorescence responses. ChemistrySelect 5:4050–4057. https://doi.org/10.1002/slct.202000652
Jiao Y, Zhou L, He H et al (2017) A new fluorescent chemosensor for recognition of Hg2+ ions based on a coumarin derivative. Talanta 162:403–407. https://doi.org/10.1016/j.talanta.2016.10.004
Jiao Y, Liu X, Zhou L et al (2017) A Schiff-base dual emission ratiometric fluorescent chemosensor for Hg2+ ions and its application in cellular imaging. Sens Actuators B Chem 247:950–956. https://doi.org/10.1016/j.snb.2017.01.124
Su Q, Niu Q, Sun T, Li T (2016) A simple fluorescence turn-on chemosensor based on Schiff-base for Hg2+-selective detection. Tetrahedron Lett 57:4297–4301. https://doi.org/10.1016/j.tetlet.2016.08.031
Wang A, Fan R, Dong Y et al (2017) Novel hydrogen-bonding cross-linking aggregation-induced emission: Water as a fluorescent “ribbon” detected in a wide range. ACS Appl Mater Interfaces 9:15744–15757. https://doi.org/10.1021/acsami.7b01254
Wang A, Fan R, Wang P et al (2017) Research on the mechanism of aggregation-induced emission through supramolecular metal-organic frameworks with mechanoluminescent properties and application in press-jet printing. Inorg Chem 56:12881–12892. https://doi.org/10.1021/acs.inorgchem.7b01687
Srivastava SK, Gupta VK, Jain S (1995) Determination of lead using a poly(vinyl chloride)-based crown ether membrane. Analyst 120:495. https://doi.org/10.1039/an9952000495
Chai F, Wang C, Wang T et al (2010) Colorimetric detection of Pb2+ using glutathione functionalized gold nanoparticles. ACS Appl Mater Interfaces 2:1466–1470. https://doi.org/10.1021/am100107k
Karachi N, Azadi O, Razavi R et al (2018) Combinatorial experimental and DFT theoretical evaluation of a nano novel thio-dicarboxaldehyde based Schiff base supported on a thin polymer film as a chemosensor for Pb2+ detection. J Photochem Photobiol A 360:152–165. https://doi.org/10.1016/j.jphotochem.2018.04.039
Sun T, Niu Q, Guo Z, Li T (2017) A simple highly sensitive and selective turn-on fluorescent chemosensor for the recognition of Pb2+. Tetrahedron Lett 58:252–256. https://doi.org/10.1016/j.tetlet.2016.12.022
Hariharan PS, Anthony SP (2014) Selective turn-on fluorescence for Zn2+ and Zn2+Cd2+ metal ions by single Schiff base chemosensor. Anal Chim Acta 848:74–79. https://doi.org/10.1016/j.aca.2014.07.042
Chandra R, Manna AK, Sahu M et al (2020) Simple salicylaldimine-functionalized dipodal bis schiff base chromogenic and fluorogenic chemosensors for selective and sensitive detection of Al3+ and Cr3+. Inorganica Chim Acta 499:119192. https://doi.org/10.1016/j.ica.2019.119192
Zhu W, Yang L, Fang M et al (2015) New carbazole-based schiff base: Colorimetric chemosensor for Fe3+ and fluorescent turn-on chemosensor for Fe3+ and Cr3+. J Lumin 158:38–43. https://doi.org/10.1016/j.jlumin.2014.09.020
Yin P, Niu Q, Liu J et al (2021) A new AIEE-active carbazole based colorimetric/fluorimetric chemosensor for ultra-rapid and nano-level determination of Hg2+ and Al3+ in food/environmental samples and living cells. Sens Actuators B Chem 331:129418. https://doi.org/10.1016/j.snb.2020.129418
Zhang S, Wu X, Niu Q et al (2017) Highly selective and sensitive colorimetric and fluorescent chemosensor for rapid detection of Ag+, Cu2+ and Hg2+ based on a simple schiff base. J Fluoresc 27:729–737. https://doi.org/10.1007/s10895-016-2005-y
Zhang S, Niu Q, Lan L, Li T (2017) Novel oligothiophene-phenylamine based Schiff base as a fluorescent chemosensor for the dual-channel detection of Hg2+ and Cu2+with high sensitivity and selectivity. Sens Actuators B Chem 240:793–800. https://doi.org/10.1016/j.snb.2016.09.054
Dong G, Duan K, Zhang Q, Liu Z (2019) A new colorimetric and fluorescent chemosensor based on schiff base-phenyl-crown ether for selective detection of Al3+ and Fe3+. Inorganica Chim Acta 487:322–330. https://doi.org/10.1016/j.ica.2018.12.036
Dong Y, Fan R, Chen W et al (2017) A simple quinolone Schiff-base containing CHEF based fluorescence ‘turn-on’ chemosensor for distinguishing Zn2+ and Hg2+ with high sensitivity, selectivity and reversibility. Dalton Trans 46:6769–6775. https://doi.org/10.1039/C7DT00956A
Gao W, Zhang Y, Li H, Pu S (2018) A multi-controllable selective fluorescent turn-on chemosensor for Al3+ and Zn2+ based on a new diarylethene with a 3-(4-methylphenyl)-1H-pyrazol-5-amine Schiff base group. Tetrahedron 74:6299–6309. https://doi.org/10.1016/j.tet.2018.09.017
Horak E, Vianello R, Hranjec M, Murković Steinberg I (2018) Colourimetric and fluorimetric metal ion chemosensor based on a benzimidazole functionalised Schiff base. Supramol Chem 30:891–900. https://doi.org/10.1080/10610278.2018.1436708
İnal EK (2020) A fluorescent chemosensor based on schiff base for the determination of Zn2+, Cd2+and Hg2+. J Fluoresc 30:891–900. https://doi.org/10.1007/s10895-020-02563-6
Iyappan M, Dhineshkumar E, Anbuselvan C (2020) Novel schiff base of E-2-(((4-aminophenyl)imino)methyl)-5-(difluoromethoxy)phenol fluorescence chemosensor for detection of Al3+, Fe2+, Cu2+ ions and its application towards live cell imaging. Asian J Chem 32:739–745. https://doi.org/10.14233/ajchem.2020.22394
Purkait R, Dey S, Sinha C (2018) A multi-analyte responsive chemosensor vanilinyl Schiff base: fluorogenic sensing of Zn(ii), Cd(ii) and I −. New J Chem 42:16653–16665. https://doi.org/10.1039/C8NJ03165G
Roy N, Dutta A, Mondal P et al (2017) Coumarin based fluorescent probe for colorimetric detection of Fe3+ and fluorescence turn on-off response of Zn2+ and Cu2+. J Fluoresc 27:1307–1321. https://doi.org/10.1007/s10895-017-2065-7
Tajbakhsh M, Chalmardi GB, Bekhradnia A et al (2018) A new fluorene-based schiff-base as fluorescent chemosensor for selective detection of Cr3+ and Al3+. Spectrochim Acta Part A Mol Biomol Spectrosc 189:22–31. https://doi.org/10.1016/j.saa.2017.08.007
Mandal J, Ghorai P, Pal K et al (2019) 2-hydroxy-5-methylisophthalaldehyde based fluorescent-colorimetric chemosensor for dual detection of Zn2+ and Cu2+ with high sensitivity and application in live cell imaging. J Lumin 205:14–22. https://doi.org/10.1016/j.jlumin.2018.08.080
Wang L, Li W, Zhi W et al (2018) A new coumarin Schiff based fluorescent-colorimetric chemosensor for dual monitoring of Zn2+ and Fe3+ in different solutions: An application to bio-imaging. Sens Actuators B Chem 260:243–254. https://doi.org/10.1016/j.snb.2017.12.200
Kaur N, Kaur B (2020) Colorimetric and fluorescent multi-ion recognition by Anthracene appended di-Schiff base chemosensor. Inorg Chem Commun 121:108239. https://doi.org/10.1016/j.inoche.2020.108239
Iyappan M, Dhineshkumar E, Anbuselvan C (2020) Schiff base of 4E,10E–4-(2-(4-nitrophenyl)-N-((1H-indol-3-yl)methylene) benzenamine-based “turn-on” fluorescence chemosensor for highly selective detection of Ni2+, Fe3+ and Mg2+ ions. Chem Pap 74:4213–4226. https://doi.org/10.1007/s11696-020-01236-9
Manna AK, Rout K, Chowdhury S, Patra GK (2019) A dual-mode highly selective and sensitive schiff base chemosensor for fluorescent colorimetric detection of Ni2+ and colorimetric detection of Cu2+. Photochem Photobiol Sci 18:1512–1525. https://doi.org/10.1039/C9PP00114J
Rout K, Manna AK, Sahu M et al (2019) Triazole-based novel bis Schiff base colorimetric and fluorescent turn-on dual chemosensor for Cu2+ and Pb2+: application to living cell imaging and molecular logic gates. RSC Adv 9:25919–25931. https://doi.org/10.1039/C9RA03341F
Kaczmarek MT, Zabiszak M, Nowak M, Jastrzab R (2018) Lanthanides: Schiff base complexes, applications in cancer diagnosis, therapy, and antibacterial activity. Coord Chem Rev 370:42–54. https://doi.org/10.1016/j.ccr.2018.05.012
Kundu BK, Mandal P, Mukhopadhyay BG et al (2019) Substituent dependent sensing behavior of Schiff base chemosensors in detecting Zn2+and Al3+ ions: Drug sample analysis and living cell imaging. Sens Actuators B Chem 282:347–358. https://doi.org/10.1016/j.snb.2018.11.076
Singh B, Moudgil L, Singh G, Kaura A (2018) The growth of ZnO nanostructures using Arginine. Bikaner, India, p 030234
Mahal A, Goshisht MK, Khullar P et al (2014) Protein mixtures of environmentally friendly zein to understand protein–protein interactions through biomaterials synthesis, hemolysis, and their antimicrobial activities. Phys Chem Chem Phys 16:14257–14270. https://doi.org/10.1039/C4CP01457J
Cui M, **n Y, Song R et al (2020) Fluorescence sensor for bovine serum albumin detection based on the aggregation and release of CdS QDs within CMC. Cellulose 27:1621–1633. https://doi.org/10.1007/s10570-019-02865-4
Zhu B, Zhang X, Jia H et al (2010) A highly selective ratiometric fluorescent probe for 1,4-dithiothreitol (DTT) detection. Org Biomol Chem 8:1650. https://doi.org/10.1039/b923754b
Gupta KC, Sutar AK (2008) Catalytic activities of Schiff base transition metal complexes. Coord Chem Rev 252:1420–1450. https://doi.org/10.1016/j.ccr.2007.09.005
Ma C, Xu B, **e G et al (2014) An AIE-active luminophore with tunable and remarkable fluorescence switching based on the piezo and protonation–deprotonation control. Chem Commun 50:7374–7377. https://doi.org/10.1039/C4CC01012D
Yoon B, Lee J, Park IS et al (2013) Recent functional material based approaches to prevent and detect counterfeiting. J Mater Chem C 1:2388. https://doi.org/10.1039/c3tc00818e
Ling P-X, Fang S-L, Yin X-S et al (2015) Palladium-catalyzed arylation of unactivated γ-Methylene C(sp3)-H and δ-C-H bonds with an oxazoline-carboxylate auxiliary. Chem Eur J 21:17503–17507. https://doi.org/10.1002/chem.201502621
Acknowledgements
One of the authors(NK) is thankful for the financial assistance from MHRD, India.
Author information
Authors and Affiliations
Contributions
Neha Kumari: Studies, data collection, compilation, and draft preparation; Shalini Singh: Draft preparation, critical revision; MinatiBaral: Conceptualisation, supervision, editing, and correction; B K Kanungo: Editing, formatting, and correction.
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies involving animals performed by any authors.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumari, N., Singh, S., Baral, M. et al. Schiff Bases: A Versatile Fluorescence Probe in Sensing Cations. J Fluoresc 33, 859–893 (2023). https://doi.org/10.1007/s10895-022-03135-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-03135-6