Abstract
Barium strontium titanate (BST), with varying Sr do** levels (x = 0, 0.05, 0.075, 0.1, 0.15, 0.3), was successfully synthesized using the solid-state reaction technique. The aim was to investigate the microstructural, dielectric, and impedance properties as Sr do** increases. X-ray diffraction analysis revealed a tetragonal phase structure for these materials, belonging to the P4mm space group, confirmed via Rietveld refinement using the Fullprof suite. SEM analysis indicated the decrement in grain sizes ranging from 0.198 to 0.0582 μm as do** concentration increases. The temperature and frequency dependencies of the dielectric constant were examined, with the Curie temperature observed in the range of 295 to 351 K with decreasing trend with substitution of strontium in pure barium titanate, showing an increase in dielectric constant with rising temperatures and non-relaxor behavior. P–E loops of BST samples illustrated bulk ferroelectric behavior, with maximum values of retentivity and coercivity reaching 1.56 and 13.97, respectively, in the highly doped BST sample. Various analytical techniques, including Nyquist plots, real and imaginary components of impedance, conductivity measurements, modulus formalism, and determination of charge carrier activation energy, were employed to elucidate the relationships between microstructure and electrical properties. Temperature-dependent resistivity demonstrated the negative temperature coefficient of resistance (NTCR) behavior in Sr-doped barium titanate. Impedance studies revealed semicircular arcs in Nyquist plots, indicating contributions from both grains and grain boundaries. The formation of well-defined grains in the BST samples was further confirmed through modulus spectroscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Advanced materials attract tremendous attention of researchers for further advancement in their versatile applicability [1,2,3,4]. Ferroic materials have been studied extensively since a long period due to its potential in a variety of applications of material science and technology [5,6,7,8,9,27,28,29,30,31,32,33].
Barium strontium titanate (BST) holds promise for energy storage devices due to its high dielectric constant and tunable properties. BST-based capacitors can store electrical energy efficiently and are used in various applications such as dynamic random-access memory (DRAM) and energy storage capacitors for pulse power systems. By adjusting the composition and processing parameters, BST can exhibit enhanced energy storage capabilities, making it a valuable material for the development of high-performance energy storage devices [34]. The study investigates the synthesis and characterization of polycrystalline bulk composite samples comprising (BaFe11.9Al0.1O19)1−x–(BaTiO3)x, revealing the coexistence of Ba-hexaferrite (BaM) and Ba-titanate (BTO) phases without chemical interactions or impurities. Notably, the ferromagnetic Curie temperature remains stable with varying concentration, while coercivity decreases due to microstructure’s influence on magnetic anisotropy. Electric properties, particularly the real part of permittivity ε′(T), exhibit strong dependence on microstructure, with BTO (x = 1) and BaM (x = 0) showing single peaks indicative of phase transitions. Composite samples (0.25 ≤ x ≤ 0.75) reveal two broad diffusion peaks in ε′(T), suggesting two electrical phase transitions influenced by microstructure and relaxation, warranting further investigation, particularly regarding magnetoelectric effects [35].
Moreover, the incorporation of BT-BLN nanofillers, prepared via mechanical ball milling and sieving, enhances the dielectric properties and energy storage density of P(VDF-HFP)-based nanocomposite films. The uniform size and dispersibility of these nanofillers alleviate field intensification, promoting microscopically dense and homogeneous structures. These nanocomposites exhibit high dielectric homogeneity, resulting in improved breakdown strength and achieving a maximum electric displacement of ≈ 9.02 μC cm−2. With a 3 vol% filler content, the nanocomposite films reach a maximum breakdown strength (Eb) of 497 MV m−1 and discharged energy density (Ud) of 14.2 J cm−3, offering a cost-effective strategy for industrial applications [36,37,38]. Furthermore, a novel core–shell BaTiO3@MgO (BT@MO) nanostructure is introduced, enhancing breakdown strength and electric displacement in high electric fields. BT@MO/P(VDF-HFP) composites demonstrate a large electric displacement (D ≈ 9.8 μC cm−2 at 571.4 MV m−1) and high discharge energy density (Ud ≈ 19.0 J cm−3), 187% higher than P(VDF-HFP) films with 3 wt% filler content. The highly insulating MgO shell improves breakdown strength by preventing charge injection, confirmed by leakage current measurements and finite element simulations. This comprehensive review highlights the significant advancements and potential applications of these innovative materials in dielectric energy storage capacitors [36, 37, 39,40,41,42,43,44].
After extensive survey it is found that the BST material as a ferroelectric material is not extensively studied or reported. This work describes the synthesis and characterization of Ba1−xSrxTiO3where x varies from 0 to 0.3 ferroelectric ceramics. In this paper, the BST material is prepared by conventional solid-state route and characterize it through different techniques such as XRD for phase confirmation, dielectric studies, impedance spectroscopy and P–E to confirm its property for storage applications.
2 Materials and methods
The solid-state reaction technique stands out for its simplicity, as it involves mixing solid reactants and initiating desired chemical reactions through heating. This simplicity contributes to its cost-effectiveness, as fewer processing steps and equipment are typically required compared to other synthesis methods, reducing production costs. Moreover, the absence of solvents or other reaction media ensures high-purity products, minimizing the risk of impurities being introduced during synthesis. Its versatility allows for the synthesis of a wide range of materials, including ceramics, alloys, and composites, making it applicable across various industries. Additionally, solid-state reactions offer scalability for large-scale production and enable precise control over parameters such as temperature, time, and stoichiometry to tailor the properties of the final product to specific requirements. This compatibility with a variety of precursor materials further enhances flexibility in material selection and synthesis conditions. Overall, the solid-state reaction technique presents a convenient, cost-effective, and versatile approach for synthesizing materials with customized properties to meet diverse industrial needs [45,46,47].
Barium strontium titanate was made utilizing the solid-state reaction approach employing a mixture of carbonates and oxides. Aldrich’s barium carbonate (BaCO3), strontium carbonate (SrCO3), and titanium oxide (TiO2) was taken as starting materials with purity more than 99.9% and were mixed in a stoichiometric proportion for 4 to 6 h. The resulting powder was then subjected to calcination at 1100 °C in a high-temperature furnace to initiate phase formation. After calcination, the powder was mixed with a binder, such as polyvinyl alcohol (PVA), and compacted into pellets using a hydraulic press under high pressure. These pellets were subsequently sintered in a furnace at 1200 °C for 2 h to promote densification and further phase development. This multi-step process ensures the formation of high-quality BST ceramics suitable for various applications. The chemical equation for prepared sample is
On a Philips X-ray diffractometer with CuKα radiation (λ = 1.540 Å) and a scanning rate of 0.02° s−1 in the 20° to 70° range, an X-ray diffractogram of the sintered material was captured. The Cambridge Stereo Scan 360 microscope were used to capture a scanning electron micrograph (SEM) of the sample. For dielectric studies, to be used as electrodes, after sintering the pellets were polished to a 1 mm thickness and then both the sides were coated using silver paste and cured at 300 °C for 15 min. The dielectric parameter was measured on an Agilent 4284A LCR metre at a 1-V oscillation amplitude from 100 Hz to 1 MHz. Electrical measurements were conducted from 25 to 250 °C at a heating rate of 1 °C per minute using a Keithley 6517A high precision electrometer. The impedance measurements were performed using an Agilent 4284A precision LCR meter in the frequency range 100 Hz–1 MHz in the temperature range 300–500 °C at a 50 °C interval.
3 Result and discussion
3.1 Structural property
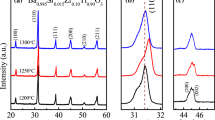
The indexed XRD pattern of synthesized BST sample is shown in Fig. 1 where the 2θ ranges from 20° to 70° with a 0.05 step size. Figure 1 of BST material showed prominent peaks at approximately 32°, 46°, and 57°, respectively, which correspond to the diffraction from the BST (101), (200), and (211) crystal planes matched with PDF card numbers 96-151-2121 and 00-044-0093. Additionally, it can be shown that the prepared sample’s strongest BST peaks occurred at about 32°, which supports the presence of perovskite structure. The XRD pattern of calcined powder up to do** concentration x = 0.3, exhibits a tetragonal phase with space group P4mm. But it is clear that when do** concentration increases, the splitting of (200) and (002) diminishes, indicating that if strontium is doped with x > 0.3 concentration, the phase will convert to pseudo cubic. The calculated lattice parameters are tabulated in Table 1. Due to the lesser ionic radii of Sr2+ (1.12 Å) than Ba2+ (1.34 Å), there is a slight peak shift and change in intensities, which causes variations in lattice parameters, crystallite size, and strain as do** levels have increased. After removing the contributions from instrumental broadening and strain, which were both confirmed by SEM, the average crystallographic size was derived from X-ray peak broadening using the Scherer formula (Table 1). The computed tolerance factor is tabulated in Table 1 and according to published research, the perovskite structure is even within the tolerance factor range 0.880 < t < 1.090. It implies that a stable perovskite structure is forming and the tetragonal phase is prominent. The assumed value of t for a cubic symmetry is 1.0. So, it is confirmed that if further increase in strontium content i.e., x > 0.3 will lead to change in structure from tetragonal to cubic. We could see the increase in strain as Sr content increases and the values of density indicating well densified samples (Fig. 1b) [21].
The relative density (ρr) is defined as the quotient of the observed density (ρm = m/v) and the theoretical density (ρt = ZM/V0NA), where m, v, M, V0, and NA represent the mass of the pellet, volume of the pellet, molar mass, volume of the unit cell, and Avogadro’s constant, respectively. The density values of BST ceramics are shown in Fig. 1b. The relative densities for these ceramics are 86 to 99%, suggesting proper densification for all samples.
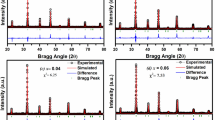
Figure 2 displays the structural refinement made using the FullProf software. It is clear that the data represented by the black line that was produced from the original crystallographic parameters and agrees well with the data represented by the red pointed data. The alteration curve (Yobs ~ Ycalc) is thereafter suitably reduced. All of these contribute to a synthetization of the sample and XRD technique characterization. Table 2 lists the reliability factors and Goodness of Fit (χ2) that were determined by the fitting of the data [28, 48].
The following formula is used to calculate different refinement parameters.
Figure 3 illustrates the SEM images studied sample for different x = 0, 0.05, 0.075, 0.1, 0.15 and 0.3. For x = 0, 0.05, 0.075, 0.1, 0.15 and 0.3, the grain size changes to 0.198, 0.167, 0.132, 0.093, 0.078 and 0.0582 μm, respectively. Although the morphology is unchanged, a little reduction in grain size is seen when the Sr ion concentration rises because Sr ions have lower ionic radii than Ba ions.
3.2 Dielectric property
A lot of research has been done on BaTiO3 for ferroelectric and dielectric applications. The material has a very high dielectric permittivity and minimal loss. In accordance to this, the bulk pellets produced in the current work had their dielectric properties thoroughly investigated. Complex impedance spectroscopy is a non-destructive method for figuring out how dielectric characteristics, structure, and microstructure relate to one another [49,50,51,52].
Real and imaginary part can be used to express the complex permittivity ε* as
And the dielectric loss is expressed as tanδ and can be determined by the given equation
Figure 4a demonstrates the dielectric permittivity (ε′) of the pellets at room temperature in a broad frequency range between 100 Hz and 5 MHz. Within the experimental frequency range, all of the samples exhibit excellent dielectric permittivity frequency independence. Higher values of the relative permittivity are present in the low frequency region and is observed to decrease mildly as frequency increases, which is likely caused by the dipole relaxation mechanism. This behavior points to the presence of charged species that cannot follow the fluctuation of the applied alternating electric field for high frequencies such as grain boundary effect, oxygen vacancies, etc. In general terms, when frequency rises the net polarization of the sample decreases as each polarization mechanism stops contributing and as a response its dielectric constant declines. But occasionally, increases at extremely low frequencies appear to be caused by electrode issues. The dielectric loss (tan δ) is shown in Fig. 4b for BST samples. The plot undeniably demonstrates the decrease in loss with frequency caused by the space charge polarization effect being minimized as the do** concentration increases in the studied sample [17, 53,54,55].
The analysis of the dielectric permittivity with the temperature at 1 MHz frequency is plotted. According to Fig. 5a, the Curie temperature for barium titanate is approximately 351 K and is reduced to 295 K by strontium do**. The rise in dielectric constant below the Tc is caused by an increase in polarization brought on by the ease with which dipoles can be oriented, conductivity. In general terms, the vibrations of the lattices induced the atoms or molecules nearby to vibrate, which in turn excited the atoms. As a result, the conductivities rise with increasing frequency. The increase in thermal oscillation of molecules and the degree of randomness in the dipoles above the transition temperature leads to the decrement of dielectric constant. As the transition temperature (Tc) rises, the porosity of the sample reduces, and vice versa as it falls. So, the strontium do** increases the porosity of the sample as the Curie temperature shows negative values for higher concentration. Figure 5b depicts the effect of temperature on dielectric loss. The dielectric loss with temperature is showing decreasing trend which is in good agreement for various applications [56].
3.3 Ferroelectric property
At room temperature, P–E hysteresis loops have been carried out. According to Fig. 6, the maximum polarization steadily declines and coercivity increases as the amount of Sr2+ increases. The maximum and minimum values are tabulated in Table 3. The presence of ferroelectric structure was shown by the creation of appropriate loops which must be due to strongly sup-pressed leakage of dielectric loss. These results demonstrate that the significant increase of the dielectric displacement is somehow related to the broadening of the loops as referring to Fig. 4. The charge storage energy was identified by the following equation
where Pm is the extreme or saturated polarization.
The discharge energy was calculated from the formula
And dividing the charge storage density by the discharge density, the efficiency is determined, expressed as
The value of charge storage density, discharge density and the efficiency were observed from the P–E hysteresis loops which shows the decreasing trend with increasing concentration of Strontium up to x = 0.1 then sudden increase. This gives the clarity of do** Sr in BT for x ≥ 0.1, storage density will increase. The lower value of η in BST indicated large hysteresis loses. If we compare BT and BST on the basis storage application the former is more appropriate due to the low remnant polarization with slim hysteresis than later.
Figure 7 shows how the resistivity of doped Ba1−xSrxTiO3 samples varies with temperature. At room temperature, all of the sample’s resistivities are moderate and with increase in temperature it slightly increases. Sr content affects how swiftly the ferroelectric/paraelectric phase transition occurs. The increased mobility of Sr2+ ions due to their smaller ionic radius and the lower degree of crystallinity shown in the XRD analysis, are most likely responsible for the decrease in room temperature resistivity that occurs as the Sr2+ content increases in the low do** level. This decrease reaches its minimum value at x = 0.3 substitution [27, 28].
3.4 Impedance spectroscopy
Generally, the complex impedance study is the study to acquire the information about grain, grain boundaries and electrode on the resistive, inductive and capacitive features of the material [57].
The complex impedance equation is described as:
where Z′ is the real component and Z″ is the imaginary component of impedance in kΩ. Figure 8 shows how the real part of impedance of BST ceramics changes with temperature between 240 and 500 °C for entire compositions. With rise in frequency, the plot shows a diminishing trend that eventually reaches zero. At lower frequencies, different types of polarization, such as electronic, ionic, interfacial, and orientational polarization, contribute. However, at higher frequencies, orientational and interfacial polarization contribute less, resulting in a constant value of impedance. With an increase in temperature, the charge carriers that have collected at the level of grain boundaries have enough energy to overcome the barrier, underscoring a rise in conductivity. Additionally, when Z′ proved to be frequency independent, no peak was seen and a movement near the high frequency zone with respect to temperature was seen. The negative temperature coefficient of resistance (NTCR) behavior is indicated by the decrease in Z′ with rising temperature at low frequencies. This graph is appropriate for assessing the relaxation frequency of the majority of resistive components.
The imaginary component of the impedance is analyzed through a certain frequency range at numerous temperatures shown in Fig. 9. For all samples, there is a peak that can be seen in the Z″ plots between 320 and 495 °C. It has been noted that when temperature rises, peaks broaden and peak maximum values of Z″ move to higher frequency regions. This can be attributed to the samples’ shorter relaxation times as temperature rises. At low temperatures, the relaxation frequency fmax, which corresponds to Z″max, is minimal. This indicates that charge carriers and oxygen vacancies move slowly from one location to another. Also, the amplitude of peak Z″max increases as Sr content increases and this increase confirms the good agreement of contribution of grains and grain boundary resistance. This nature enhances the electrical behavior of the sample as Sr content increases [58, 59].
Figure 10 (Nyquist plots) illustrates how the real and imaginary portions of impedance vary. Impedance spectrum was found to have excellent semicircles. A semicircle with its center on the real axis indicates the response of a desirable parallel circuit with resistance R and capacitance C. While C is derived from the frequency of semicircle maxima, R is derived from the semicircle’s diameter. Impedance spectroscopy is the most effective method for analyzing the electrical response of dielectric materials in relation to frequency. The contributions of the three primary elements of a dielectric ceramic—grains, grain boundaries, and the electrode/sample interface—can be taken into account in the picture using a complex impedance spectrum. Semicircles tend to emerge at lower frequencies, which may indicate the presence of the grain boundary effect, whereas semicircles appear at higher frequencies, which may indicate the presence of the grain or bulk properties effect in the sample. The Cole–Cole kind of distribution centered around a two-layer model, according to which the resulting complex impedance is made up of two overlap** semicircles, is how the phenomena is frequently described.
The complex impedance of a system at an applied frequency represented in Eq. 13 in which Z′ and Z″ parts of the impedance are as follows
where ωg and ωgb are the two frequencies at the highest points of the semicircles for the grain and grain boundary, respectively, and Rg and Cg signify the resistance and capacitance of the grain, respectively, while Rgb and Cgb signify the analogous terms for the grain boundary. It is a successful approach for separating the constituent parts of complex materials whose characteristics and purposes rely on the careful control of their structure, composition, ceramic texture, dopants, and dopant distribution. It works by taking advantage of the various frequency obligations of constituent components. However, the data in the series connection of two cells, which were related to the effects of grains and grain borders, respectively, were revealed by the modeling of the experimental data using the appropriate equivalent electrical circuit (ZViewprogramme). The values of the grain resistance and capacitance (Rg and Cg) and grain boundary resistance and capacitance (Rgb and Cgb) were determined by fitting. A material with a single relaxation period produces an ideal semicircle centred on the real axis, according to Debye’s model. Two semicircles, one at lower frequencies and one at higher frequencies, are associated to transport phenomena at grain boundaries and the first semicircle (at higher frequencies) is attributed to transport phenomena in the bulk [60]. The values for grain resistance Rg (kΩ), grain boundary resistance Rgb (kΩ) and capacitance Cg and Cgb for grain and grain boundary respectively (Table 4). The variation of Z′/Z″ with frequency also plotted and the variation is shown in Fig. 11.
Since Z′ = Z is the highest height in each semicircle corresponds to the capacitance and intercept arc corresponds to the resistance. Utilizing relations (13) and (14), the following formulas may be computed for capacitance:
The relaxation times for grain and grain boundary were determined via relations mentioned above, represented by:
The activation energies corresponding to grain and grain boundary resistance werefound by linear fitting (Fig. 12) and the observed values are shown in Fig. 12b. The observed activation energy Ea = 0.09–0.15 eV shows that the doubly ionized oxygen vacancies produced by the oxygen loss from the crystal lattice distortion. The short rage hop** remains similar as oxygen vacancies being produced which is related to dielectric relaxation. The lower activation energy that was discovered has verified that grains have higher conductivity than grain boundaries. An interruption in the migration of charge carriers at the grain boundary, which is largely seen in polycrystalline ceramics, is typically the main cause of the drop in electrical conductivity. So, the grain boundary inhibits long-range motion, which causes conduction to decrease as Sr content rises.
3.5 Modulus spectroscopy
The modulus of the sample is calculated by taking the dielectric permittivity into consideration. The equation for calculating the real and imaginary part of modulus is given below:
where ε′ is the real part of dielectric permittivity and ε″ is the imaginary part of dielectric permittivity.
Figure 13 depicts the frequency-dependent change of the real part of the modulus. The modulus is almost zero in the lower frequency region, and as the frequency increases, it increases until it reaches saturation values. As the temperature rises, this temperature-dependent frequency causes the amplitude of M′ to saturate at a higher value. The conduction mechanism caused by short-range mobility charge carriers, where M′ becomes frequency independent, may be the cause of the continuous dispersion. The dispersive region’s shift to the higher frequency side may be caused by charge carriers with long-range mobility. Also, the M′ (ω) behavior demonstrates that the value of M′ decreases with increasing temperature in the observed frequency range, supporting the theory that conduction in BST ceramics may result from charge carriers’ short-range mobility. The Fig. 13 shows M″ with frequency which indicates energy loss in the influence of electric field. The graphs show a relaxation-related peak that is centered at the real component of the electric modulus dispersion area. Therefore, a peak in the higher frequency region can be seen in the data collected M (ω) loss spectra. The region of long-range mobile charge carriers, where these carriers migrate towards nearby sites via hop** conduction, is where the loss peak maximum in the M (ω) against frequency spectra corresponds. This region is in the lower frequency range. In addition to giving a clear indicator of the change from long to short range mobility in the direction of rising frequency, the frequency of the peak position at the x-axis also raises the possibility of a temperature-dependent electrical hop** conduction mechanism within the BST system. Therefore, the short-range localized charge carriers are constrained to potential wells at the higher frequency of peak maximum (Fig. 14). It is more evident that the electrode effect does not exist in the BST system since the M″ has a low value at all temperatures (573–648 K) between 1 Hz and 10 kHz. This low value is most likely brought on by a high degree of capacitance in this region. The theoretical Kohlrausch–William–Watts (KWW) function (Bergman modified) fit to the experimental data produces the values of M″max and fmax, which are the maximum point of modulus M″ and angular frequency.
where, M″max is the maximum value of M″ at maximum frequency fmax and β is the stretched exponent parameter. This model appears to have good agreement with the experimental results. The fitted data showed the stretched parameter β < 1 for all compositions, confirms the non-Debye type relaxations [61,62,63,64,65,66].
The complex modulus spectrum depicting M″ versus M′ for BST (x = 0–0.3) exhibits two distinct half circles, as depicted in Fig. 15. The first semicircle observed at lower frequencies corresponds to the influence of grains, whereas the second semicircle observed at higher frequencies is associated with the grain boundary effect. Additionally, a minor semicircle is noticeable at higher frequencies, which is attributed to the electrode effect.
4 Conclusion
The Ba1−xSrxTiO3 (x from 0 to 0.3) material was synthesized using the solid-state reaction approach. To learn about the structural, electrical and impedance studies on the barium titanate with increasing Sr do**, the sample was characterized under XRD, SEM, dielectric and impedance spectroscopy. The microstructural studies reveal that tetragonal phase (space group—P4mm) with uniform perovskite structure. The grain size was confirmed by SEM images in the range 0.198–0.0582 μm. The dielectric studies show the decreasing trend with frequency and Curie temperature around 350–295 K which contribute to diffusive nature. Impedance studies were carried out and the semicircular arc was revealed by the plots which confirms the contribution of grain and grain boundaries by the Nyquist plots at different temperatures. When temperatures are high, grain capacitance and resistance play a considerable role, as seen by the frequency-dependent imaginary portion of impedance and electrical modulus. Two relaxation processes caused by grain and grain border capacitance were clearly visible in the electric modulus. According to the activation energy and characteristic conductivity time values, the relaxation is mostly brought on by the Maxwell–Wagner effect.
Data availability
Data will be made available on request.
References
D. Wang, X. Wang, M.L. **, P. He, S. Zhang, Molecular level manipulation of charge density for solid-liquid TENG system by proton irradiation. Nano Energy 103, 107819 (2022)
S.E. Shirsath, C. Cazorla, T. Lu, L. Zhang, Y.Y. Tay, X. Lou, Y. Liu, S. Li, D. Wang, Interface-charge induced giant electrocaloric effect in lead free ferroelectric thin-film bilayers. Nano Lett. 20, 1262–1271 (2020)
Y. Yan, K. Zhang, G. Qin, B. Gao, T. Zhang, X. Huang, Y. Zhou, Phase engineering on MoS2 to realize dielectric gene engineering for enhancing microwave absorbing performance. Adv. Funct. Mater. (2024). https://doi.org/10.1002/adfm.202316338
Q. Zhu, J. Chen, G. Gou, H. Chen, P. Li, Ameliorated longitudinal critically refracted—attenuation velocity method for welding residual stress measurement. J. Mater. Process. Technol. 246, 267–275 (2017)
Y. Slimani, R. Sivakumar, S.S. Meena, R. Vignesh, G. Yasin, E. Hannachi, M.A. Almessiere, Z. Trabelsi, K.M. Batoo, A. Baykal, N. Sfina, S. Brini, S.E. Shirsath, I. Ercan, B. Özçelik, BaTiO3/(Co0.8Ni0.1Mn0.1Fe1.9Ce0.1O4)x composites: analysis of the effect of Co0.8Ni0.1Mn0.1Fe1.9Ce0.1O4 do** at different concentrations on the structural, morphological, optical, magnetic, and magnetoelectric coupling properties of BaTiO3. Ceram. Int. 48, 30499–30509 (2022)
T.M. Alfareed, Y. Slimani, M.A. Almessiere, S.E. Shirsath, M. Hassan, M. Nawaz, F.A. Khan, E.A. Al-Suhaimi, A. Baykal, Structure, magnetoelectric, and anticancer activities of core-shell Co0.8Mn0.2R0.02Fe1.98O4@BaTiO3 nanocomposites (R = Ce, Eu, Tb, Tm, or Gd). Ceram. Int. 48, 14640–14651 (2022)
Y. Slimani, S.E. Shirsath, E. Hannachi, M.A. Almessiere, M.M. Aouna, N.E. Aldossary, G. Yasin, A. Baykal, B. Ozçelik, I. Ercan, (BaTiO3)1-x+(Co0.5Ni0.5Nb0.06Fe1.94O4)x nanocomposites: structure, morphology, magnetic and dielectric properties. J. Am. Ceram. Soc. 104, 5648–5658 (2021)
J. Zhang, X. Wang, L. Zhou, G. Liu, D.T. Adroja, I. Da Silva et al., A ferrotoroidic candidate with well-separated spin chains. Adv. Mater. (Weinheim) 34(12), e2106728 (2022)
A.S. Gaikwad, R.H. Kadam, S.E. Shirsath, S.R. Wadgane, J. Shah, R.K. Kotnala, A.B. Kadam, Surprisingly high magneto-electric coupling in cubic Co0.7Fe2.3O4-SrTiO3 nano-composites. J. Alloys Compd. 773, 564–570 (2019)
Y. Wang, R. **ao, N. **ao, Z. Wang, L. Chen, Y. Wen, P. Li, Wireless multiferroic memristor with coupled giant impedance and artificial synapse application. Adv. Electron. Mater. 8(10), 2200370 (2022)
A.S. Gaikwad, S.E. Shirsath, S.R. Wadgane, R.H. Kadam, J. Shah, R.K. Kotnala, A.B. Kadam, Magneto-electric coupling and improved dielectric constant of BaTiO3 and Fe-rich (Co0.7Fe2.3O4) ferrite nano-composites. J. Magn. Magn. Mater. 465, 508–514 (2018)
A. Von Hippel, Ferroelectricity, domain structure, and phase transitions of barium titanate. Rev. Mod. Phys. 22(3), 221 (1950)
M.M. Vijatović, J.D. Bobić, B.D. Stojanović, History and challenges of barium titanate: part I. Sci. Sinter. 40(2), 155–165 (2008)
K. Tewatia, A. Sharma, M. Sharma, A. Kumar, Factors affecting morphological and electrical properties of barium titanate: a brief review. Mater. Today: Proc. 44, 4548–4556 (2021)
F.A. Ismail, R.A.M. Osman, M.S. Idris, Review on dielectric properties of rare earth doped barium titanate, in AIP Conference Proceedings, vol. 1756, no. 1 (AIP Publishing, 2016)
E.N. Bunting, G.R. Shelton, A.S. Creamer, Properties of barium-strontium titanate dielectrics. J. Am. Ceram. Soc. 30(4), 114–125 (1947)
S.B. Herner, F.A. Selmi, V.V. Varadan, V.K. Varadan, The effect of various dopants on the dielectric properties of barium strontium titanate. Mater. Lett. 15(5–6), 317–324 (1993)
S. Ghezelbash, S. Mesdaghi, M. Kargarrazi, Organic dye degradation by barium strontium titanate/kaolinite nanocomposites. Optik 262, 169293 (2022)
K.G. Kelele, A. Tadesse, T. Desalegn, S. Ghotekar, R. Balachandran, H.C.A. Murthy, Synthesis and characterizations of metal ions doped barium strontium titanate (BST) nanomaterials for photocatalytic and electrical applications: a mini review. Int. J. Mater. Res. 112(8), 665–677 (2021)
S.E. Shirsath, M.H.N. Assadi, J. Zhang, N. Kumar, A.S. Gaikwad, J. Yang, H.E. Maynard-Casely, Y.Y. Tay, J. Du, H. Wang, Y. Yao, Z. Chen, J. Zhang, S. Zhang, S. Li, D. Wang, Interface-driven multiferroicity in cubic BaTiO3-SrTiO3 nanocomposites. ACS Nano 16, 15413–15424 (2022)
A. Ioachim, M.I. Toacsan, M.G. Banciu, L. Nedelcu, F. Vasiliu, H.V. Alexandru, G. Stoica, Barium strontium titanate-based perovskite materials for microwave applications. Prog. Solid State Chem. 35(2–4), 513–520 (2007)
S.G. Lu, X.H. Zhu, C.L. Mak, K.H. Wong, H.L.W. Chan, C.L. Choy, High tunability in compositionally graded epitaxial barium strontium titanate thin films by pulsed-laser deposition. Appl. Phys. Lett. 82(17), 2877–2879 (2003)
J.W. Liou, B.S. Chiou, Effect of direct-current biasing on the dielectric properties of barium strontium titanate. J. Am. Ceram. Soc. 80(12), 3093–3099 (1997)
A. Ioachim, M.I. Toacsan, M.G. Banciu, L. Nedelcu, A. Dutu, S. Antohe et al., Transitions of barium strontium titanate ferroelectric ceramics for different strontium content. Thin Solid Films 515(16), 6289–6293 (2007)
K.A. Razak, A. Asadov, J. Yoo, E. Haemmerle, W. Gao, Structural and dielectric properties of barium strontium titanate produced by high temperature hydrothermal method. J. Alloys Compd. 449(1–2), 19–23 (2008)
M. Viviani, M. Leoni, M.T. Buscaglia, V. Buscaglia, P. Nanni, Positive temperature coefficient of electrical resistivity below 150 K in barium strontium titanate. J. Am. Ceram. Soc. 87(4), 756–758 (2004)
G. Dai, S. Wang, G. Huang, G. Chen, B. Lu, D. Li et al., Direct and indirect measurement of large electrocaloric effect in barium strontium titanate ceramics. Int. J. Appl. Ceram. Technol. 17(3), 1354–1361 (2020)
T. Zaman, M.K. Islam, M.A. Rahman, A. Hussain, M.A. Matin, M.S. Rahman, Mono and co-substitution of Sr2+ and Ca2+ on the structural, electrical and optical properties of barium titanate ceramics. Ceram. Int. 45(8), 10154–10162 (2019)
S. Chaudhary, S. Devi, S. **dal, Comparison of structural and dielectric properties of doped (M, R and A) barium strontium titanate. Mater. Today: Proc. (2023). https://doi.org/10.1016/j.matpr.2023.01.264
S. Devi, A.K. Jha, Structural, dielectric and ferroelectric studies of tungsten substituted barium strontium titanate. Ferroelectrics 402(1), 168–174 (2010)
A.K. Jha, S. Devi, Microstructural and electrical characterization of w substituted barium strontium titanate ferroelectric ceramics. Ferroelectrics 420(1), 101–109 (2011)
S. Chaudhary, M. Chaudhary, S. Devi, S. **dal, Dopant and milling time effect on impedance and electrical properties of perovskite ceramics. J. Theor. Appl. Phys. (2023). https://doi.org/10.57647/J.JTAP.2023.1702.22
S. Devi, P. Ganguly, S. Jain, A.K. Jha, Effect of W substitution on structural, dielectric and electrical properties of BaTiO3 ferroelectric ceramics. Ferroelectrics 381(1), 120–129 (2009)
D.V. Karpinsky, M.V. Silibin, S.V. Trukhanov, A.V. Trukhanov, A.L. Zhaludkevich, S.I. Latushka et al., Peculiarities of the crystal structure evolution of BiFeO3–BaTiO3 ceramics across structural phase transitions. Nanomaterials 10(4), 801 (2020)
S.V. Trukhanov, A.V. Trukhanov, M.M. Salem, E.L. Trukhanova, L.V. Panina, V.G. Kostishyn et al., Preparation and investigation of structure, magnetic and dielectric properties of (BaFe11.9Al0.1O19)1−x–(BaTiO3)x bicomponent ceramics. Ceram. Int. 44(17), 21295–21302 (2018)
P.J. Wang, D. Zhou, J. Li, L.X. Pang, W.F. Liu, J.Z. Su et al., Significantly enhanced electrostatic energy storage performance of P(VDF-HFP)/BaTiO3-Bi(Li0.5Nb0.5)O3 nanocomposites. Nano Energy 78, 105247 (2020)
A.V. Trukhanov, V.O. Turchenko, I.A. Bobrikov, S.V. Trukhanov, I.S. Kazakevich, A.M. Balagurov, Crystal structure and magnetic properties of the BaFe12−xAlxO19 (x = 0.1–1.2) solid solutions. J. Magn. Magn. Mater. 393, 253–259 (2015)
A.V. Trukhanov, V.G. Kostishyn, L.V. Panina, V.V. Korovushkin, V.A. Turchenko, P. Thakur et al., Control of electromagnetic properties in substituted M-type hexagonal ferrites. J. Alloys Compd. 754, 247–256 (2018)
P.J. Wang, D. Zhou, H.H. Guo, W.F. Liu, J.Z. Su, M.S. Fu et al., Ultrahigh enhancement rate of the energy density of flexible polymer nanocomposites using core–shell BaTiO3@MgO structures as the filler. J. Mater. Chem. A 8(22), 11124–11132 (2020)
S.V. Trukhanov, A.V. Trukhanov, V.A. Turchenko, A.V. Trukhanov, E.L. Trukhanova, D.I. Tishkevich et al., Polarization origin and iron positions in indium doped barium hexaferrites. Ceram. Int. 44(1), 290–300 (2018)
T. Dippong, E.A. Levei, I.G. Deac, I. Petean, G. Borodi, O. Cadar, Sol-gel synthesis, structure, morphology and magnetic properties of Ni0.6Mn0.4Fe2O4 nanoparticles embedded in SiO2 matrix. Nanomaterials 11(12), 3455 (2021)
M. Ştefănescu, T. Dippong, M. Stoia, O. Ştefănescu, Study on the obtaining of cobalt oxides by thermal decomposition of some complex combinations, undispersed and dispersed in SiO2 matrix. J. Therm. Anal. Calorim. 94(2), 389–393 (2008)
M. Stoia, M. Stefanescu, T. Dippong, O. Stefanescu, P. Barvinschi, Low temperature synthesis of Co2SiO4/SiO2 nanocomposite using a modified sol–gel method. J. Sol-Gel. Sci. Technol. 54, 49–56 (2010)
T. Dippong, E.A. Levei, C. Leostean, O. Cadar, Impact of annealing temperature and ferrite content embedded in SiO2 matrix on the structure, morphology and magnetic characteristics of (Co0.4Mn0.6Fe2O4)δ (SiO2)100-δ nanocomposites. J. Alloys Compd. 868, 159203 (2021)
T. Dippong, E.A. Levei, F. Goga, O. Cadar, Influence of Mn2+ substitution with Co2+ on structural, morphological and coloristic properties of MnFe2O4/SiO2 nanocomposites. Mater. Charact. 172, 110835 (2021)
T. Dippong, E.A. Levei, D. Toloman, L. Barbu-Tudoran, O. Cadar, Investigation on the formation, structural and photocatalytic properties of mixed Mn-Zn ferrites nanoparticles embedded in SiO2 matrix. J. Anal. Appl. Pyrol. 158, 105281 (2021)
T. Dippong, D. Toloman, M. Dan, E.A. Levei, O. Cadar, Structural, morphological and photocatalytic properties of Ni-Mn ferrites: influence of the Ni:Mn ratio. J. Alloys Compd. 913, 165129 (2022)
E. Yustanti, M.A.E. Hafizah, A. Manaf, Exploring the effect of particle concentration and irradiation time in the synthesis of barium strontium titanate (BST) Ba(1–x)SrxTiO3 (x: 0–1) nanoparticles by high power ultrasonic irradiation. Int. J. Technol. 7, 1016 (2016)
M. Hashim, S.J. Salih, M.M. Ismail, A. Ahmed, S. Singh Meena, A.A. Gaikwad, R.B. Jotania, S. Kumar, D. Ravinder, R. Kumar, A. Imran, K.M. Batoo, S.E. Shirsath, Effect of lightly substituted samarium ions on the structural, optical, magnetic and dielectric properties of the sonochemically synthesized M-type Sr-hexaferrite nanoparticles. Physica B: Condens. Matter 681, 415840 (2024)
Y. Slimani, S.S. Meena, S.E. Shirsath, E. Hannachi, M.A. Almessiere, A. Baykal, R. Sivakumar, K.M. Batoo, A. Thakur, I. Ercan, B. Özçelik, Impact of magnetic spinel ferrite content on the structure, morphology, optical, and magneto-dielectric properties of BaTiO3 materials. Z. Phys. Chem. 237(11), 1753–1774 (2023)
S.S. Kadam, V.D. More, P.K. Gaikwad, K.M. Batoo, S. Hussain, R.H. Kadam, S.E. Shirsath, A.B. Kadam, Structural characterization and enhanced magnetic and dielectric properties of Ce3+ substituted Co–Cr–Fe–O nano-ferrites synthesized using sol–gel method. Appl. Phys. A 129(10), 730 (2023)
M. Hashim, D. Ravvinder Nayak, A. Ahmed, S. Kumar, S.E. Shirsath, M.M. Ismail, S.K. Sharma, M.A. Alayasreh, R. Kumar, D. Ravinder, R.B. Jotania, S. Singh Meena, K.M. Batoo, Structural, dielectric, electric and magnetic properties of magnesium substituted lithium nanoferrites. Ceram. Int. 49, 31114–31123 (2023)
T.M. Shaw, Z. Suo, M. Huang, E. Liniger, R.B. Laibowitz, J.D. Baniecki, The effect of stress on the dielectric properties of barium strontium titanate thin films. Appl. Phys. Lett. 75(14), 2129–2131 (1999)
H.A. Gatea, H. Abbas, I.S. Naaji, Effect of Sr+ ion concentration on microstructure and dielectric properties of barium strontium titanate ceramics. Int. J. Thin Film Sci. Technol. 11(2), 239–244 (2022)
J.H. Jeon, Y.D. Hahn, H.D. Kim, Microstructure and dielectric properties of barium–strontium titanate with a functionally graded structure. J. Eur. Ceram. Soc. 21(10–11), 1653–1656 (2001)
M. Hashim, M. Raghasudha, J. Shah, S.E. Shirsath, D. Ravinder, S. Kumar, S. Singh Meena, P. Bhatt, Alimuddin, R. Kumar, R.K. Kotnala, High temperature dielectric studies of indium substituted NiCuZn nano ferrites. J. Phys. Chem. Solids 112, 29–36 (2018)
M. Hashim, Alimuddin, S. Kumar, S.E. Shirsath, R.K. Kotnala, J. Shah, R. Kumar, Synthesis and characterizations of Ni2+ substituted cobalt ferrite nanoparticles. Mater. Chem. Phys. 139, 364–374 (2013)
R. Jacob, H.G. Nair, J. Isac, Impedance spectroscopy and dielectric studies of nanocrystalline iron doped barium strontium titanate ceramics. Process. Appl. Ceram. 9(2), 73–79 (2015)
M. Hashim, Alimuddin, S. Kumar, S.E. Shirsath, R.K. Kotnala, J. Shah, R. Kumar, Influence of Cr3+ ion on the structural, ac conductivity and magnetic properties of nanocrystalline Ni-Mg ferrite. Ceram. Int. 39, 1807–1819 (2013)
S. Sen, R.N.P. Choudhary, Impedance studies of Sr modified BaZr0.05Ti0.95O3 ceramics. Mater. Chem. Phys. 87(2–3), 256–263 (2004)
K. Verma, S. Sharma, Impedance spectroscopy and dielectric behavior in barium strontium titanate–nickel zinc ferrite composites. Physica Status Solidi (b) 249(1), 209–216 (2012)
A. Kaur, L. Singh, K. Asokan, Electrical relaxation and conduction mechanisms in iron doped barium strontium titanate. Ceram. Int. 44(4), 3751–3759 (2018)
O.P. Thakur, C. Prakash, Dielectric properties of samarium substituted barium strontium titanate. Phase Transit. Multinatl. J. 76(6), 567–574 (2003)
Y. Slimani, M.A. Almessiere, S.E. Shirsath, E. Hannachi, A. Baykal, N. Alwadai, M.S. Alshatwi, F.N. Almutairi, M. Shariq, K.M. Batoo, A. Thakur, P. Thakur, I. Ercan, Impact of CoFe1.98Nb0.02O4 phase on the structural, morphological, and dielectric properties of barium titanate material. Inorg. Chem. Commun. 153, 110753 (2023)
M. A. Hashim, S. Kumar, B.H. Koo, S. E. Shirsath, E.M. Mohammed, J. Shah, R.K. Kotnala, H.K. Choi, H. Chung, Ravi Kumar. Structural electrical and magnetic properties of Co–Cu ferrite nanoparticles. J. Alloys Compd. 518, 11–18 (2012). https://doi.org/10.1016/j.jallcom.2011.12.017
S. E. Shirsath, R. H. Kadam, K. M. Batoo, D. Wang, S. Li, Co–Al-substituted strontium hexaferrite for rare earth free permanent magnet and microwave absorber application. Journal of Physics D: Applied Physics 54(2) 024001-10.1088/1361-6463/abb9d5
Acknowledgements
Author M. Hadi extends his appreciation to the Deanship of Scientific Research and graduate studies at King Khalid Univrsity for funding this work through Large Research Project under Grant Number RGP2/393/45.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Open Access funding enabled and organized by CAUL and its Member Institutions. King Khalid University through Large Research Project under Grant Number RGP2/393/45.
Author information
Authors and Affiliations
Contributions
Sheela Devi: writing—original draft, methodology, investigation, supervision. Shristi Chaudhary: writing—original draft, methodology, investigation. Mohd. Hashim, Muhammad Hadi: methodology, investigation. Khalid Mujasam Batoo, Sagar E. Shirsath: writing—review & editing, supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Devi, S., Chaudhary, S., Hashim, M. et al. Co-relation between Rietveld analysis, dielectric studies and impedance spectroscopy of the Ba1−xSrxTiO3 ceramics. J Mater Sci: Mater Electron 35, 1077 (2024). https://doi.org/10.1007/s10854-024-12788-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12788-x