Abstract
This article aims to study the modification in the structural, optical and electrical properties of indium oxide thin film after gamma irradiation and estimation of sensitivity for gamma sensing applications. The thin film of indium oxide was deposited on a 450 °C preheated glass substrate using the spray pyrolysis technique. The deposited thin film of molar concentration 0.15 M and thickness of around 600 nm was irradiated with different gamma doses (100 Gy, 200 Gy, 300 Gy and 400 Gy). The optical properties of the irradiated film are studied using UV–Visible spectroscopy. Transmittance increased after irradiation up to 200 Gy and beyond that, it decreased. Indium oxide is an n-type semiconductor which exhibits both direct and indirect transitions. Both direct and indirect bandgap energy are calculated using Tauc’s plot. Extinction coefficient and refractive index variation with irradiation were also estimated. Photoluminescence study confirmed the gamma-induced defect formation and annihilation for an irradiation dose of 400 Gy and 200 Gy, respectively. Resistivity also decreased up to 200 Gy and beyond that, it increased. The sensitivity of the deposited film was estimated from the electrical measurements, and it lies between 10.7 and 53.4 mA/cm2/Gy.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cobalt-60 is a radioactive isotope which decays by releasing high-energy gamma rays with energies between 1.17 and 1.33 MeV, or an average of 1.25 MeV if the source is a monochromatic [1]. Gamma radiation has a wide variety of applications that are used in medical equipment, food sterilisation, research as well as in radiopharmaceutical production. It also finds its application in industrial applications for the modification of material properties [2]. It is a well-established fact that gamma rays can be harmful to living tissue and the ionisation by the radiation can damage cells and even DNA [2]. However, if the radiation is monitored properly, the benefits of gamma radiation can outweigh the risks. Hence, it is important to detect and monitor gamma radiation for the benefit of human beings.
Thin films can be used for manufacturing gamma sensors as they can be easily patterned and integrated into a variety of device architectures. The linear response of its electrical or optical properties with gamma dose can be exploited for sensor applications. Here we have used indium oxide (INO) thin film since its sensitivity, conductivity and stability are relatively high [3]. Moreover, INO possesses wide bandgap energy due to which, it can be used in optoelectronic devices [4].
The thin film deposition can be done using different deposition techniques which include thermal evaporation, spin coating, sputtering, spray pyrolysis, etc. [2]. Among these methods, we have chosen spray pyrolysis as the film deposition technique as it is the most suitable method for depositing metal oxides. Moreover, the film produced using this technique is expected to be of high purity and uniformity. Additionally, this method is simple and inexpensive compared to other deposition techniques and also doesn’t require a vacuum at any of the stages [5].
This study mainly focuses on the change in electrical and optical properties of INO thin film under different doses of gamma exposure. Only a few studies, to the best of our knowledge, were reported on gamma sensor with indium oxide thin film. The sensitivity estimation of INO thin films is also the least focused in the literature.
Experimental details
Synthesis of INO thin film
For the synthesis of INO thin film, we have utilised the spray pyrolysis technique. 0.15 M precursor solution was prepared using indium chloride tetrahydrate and double distilled water with a constant stirring of the solution for half an hour. The carrier gas (compressed air) is released into the nozzle containing a homogenous precursor solution. The aqueous precursor solution is atomized and carried to a heated glass substrate from where the decomposition of the compound and solvent evaporation takes place [6]. The decomposition of the precursor at a high temperature helps to form the desired material. The deposited films were removed from the spraying chamber after being allowed to cool to ambient temperature. Before deposition, the glass substrate was cleaned by conventional cleaning methods using acetone and isopropyl alcohol (IPA).
Gamma irradiation
The thin film thus synthesised using spray pyrolysis is irradiated with different doses of Co-60 gamma radiation. The chosen gamma doses are 100, 200, 300 and 400 Gy. Gamma Chamber 5000 with a dose rate of 9.5 KGy/hr is used to irradiate the samples.
Characterisation
The thin film thickness is estimated using the BRUKER stylus profilometer. The structural changes of the sample after an exposure of 200 Gy were studied using the Rigaku Miniflex-600 X-ray diffractometer in a 2θ range of 20°–70° and with a step size of 0.02°. For optical properties, Micro Raman spectra were acquired with a compact Raman spectrometer and a 532 nm laser was used for excitation. UV–Visible transmission and absorption spectra were recorded in the spectral range of 300–1100 using a SHIMADZU UV 1800 spectrophotometer. The defect levels created after irradiation were studied using the Jasco FP-8300 fluorescence spectrometer with an excitation wavelength of 325 nm. I-V characteristics were studied using two probe methods and hall measurements using the Vander Pauw method with Keithley 2450 as the source metre.
Results and discussion
Structural properties
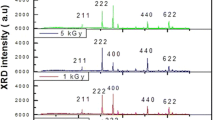
The conduction mechanism and dielectric properties of a material are highly dependent on the structural evolution of the irradiated film [7]. Hence, the role of phase identification and degree of crystallisation is crucial for a device. The structural properties and phase change with gamma irradiation are studied using the XRD spectrum. A typical X-ray diffractogram of the thin film exposed with a radiation dose of 200 Gy is shown in Fig. 1a. It is compared with unirradiated XRD data published elsewhere [8]. The 2θ scan between 20° and 70° in the XRD analysis indicates the polycrystallinity of the film and cubic structure retainment after gamma irradiation. The peaks seen in spectra are properly complemented with ICDD data and correspond to (222), (400), (444) and (622) planes that match with the In2O3 cubic phase [4]. The (400) peak shows the highest intensity indicating the preferential orientation in that plane as in the unirradiated INO. When the energy of gamma radiation is large enough, the orientation of the atoms along the predominant plane is highly dependent on the surface energy [9].
It is already been reported that indium oxide is highly resistant to highly energetic gamma radiation [4]. Here also INO thin film retained the cubic phase after irradiation suggesting its stability. The intensity variation after irradiation can be due to defects and disorders formed in the material to the gamma interaction [4]. The increase in peak intensity of the 200 Gy sample can be correlated to the degree of crystallinity [10]. Even though the comparison of the XRD pattern of irradiated samples with that of unirradiated doesn’t provide any evidence for phase transformation or other crystallographic changes, a slight shift of the peaks to a lower angle was observed for the 200 Gy irradiated sample. This can be due to radiation heating or changes occurred due to atomic displacements. The line shifting can also attribute to macro stress induced in the film after the irradiation [11]. In short, the XRD studies indicate the retainment of the In2O3 crystalline phase, even though it possesses adequate energy for the displacement of individual atoms from their respective lattice sites [4, 12].
The crystallite size of the material was calculated using the Debye Scherrer formula as well as the size–strain plot (SSP). The Debye formula for crystallite size calculation is given below.
where k is the shape factor and for spherical grain, it is taken as 0.9 λ is the incident X-ray wavelength, β indicates the FWHM (full-width half maxima) of the predominant plane and θ gives the diffraction peak position [13].
Figure 1b shows the size–strain plot (SSP) for indium oxide thin film irradiated with 200 Gy. The slope of the linear fit of (dβcosθ)2 versus (d2βcosθ) plot yields the grain size [13]. The crystallite size increased from 28.2 nm for pristine to 35.0 for the 200 Gy sample. The grain boundary collapse occurring in the film due to the gamma irradiation induces the coalescence of smaller crystallites resulting in an enhanced crystallite size [10]. Along with atomic displacement and ionisation, irradiation can also produce lattice vibrations which can generate local heating in the material. The increase in D value can be also attributed to this local heating [14]. The enhancement in the INO film crystallinity exposed to gamma radiations was also previously reported [15]. Hence, we can conclude that gamma irradiation has an influence on the structural properties of the thin film.
Strain in the film can be estimated using the following relation [3],
The dislocation density (δ), as well as the number of crystallites per unit area (Nc) of the sample irradiated with 200 Gy, was determined using the relations given below [4].
where t denotes the thickness of the irradiated film. The variation of those parameters for the 200 Gy irradiated sample is tabulated in Table 1.
Both dislocation density and the number of crystallites per unit area (Nc) decreased after the gamma irradiation of 200 Gy. Nefzi et al. [16] have published similar dislocation density and Nc decrement suggesting the improvement in structural properties after irradiation. The authors explained the improvement by the occupancy vacancy sites by the oxygen atoms displaced from interstitial sites due to gamma radiation.
It is a prevailing fact that oxygen vacancies create the majority of the defects found in the material after gamma irradiation and that too beyond a certain limit leads to stoichiometric degradation in thin films [17]. The structural changes observed in the irradiated films can be attributed to defects like colour centres/oxygen vacancies and impurities. Gamma ray can either enhance or degrade the structural properties. Even though we observed an enhanced structural property for the sample irradiated at 200 Gy, gamma rays can also degrade the structural properties as reported elsewhere [4].
The interactions of gamma with materials are highly dependent on the atomic arrangement of the substance, absorbed gamma dose and also thickness of the film [4]. The amount of deposited energy is determined by the target’s atomic number and the energy of the incident radiation [15].
It is a well-established fact that gamma irradiation on a material induces several defects and dislocations. This atomic motion in turn results in the production of new defects like vacancies and self-interstitial [15, 18]. As in indium oxide, the oxygen atom is lighter than the indium, the oxygen atom located in the lattice position will get easily displaced to other sites by the energetic radiation. The defects thus produced are termed colour centres [4]. However, the predominant mechanism of energetic gamma photon interactions with matter is ionisation which results in the generation of free electron–hole pairs [15]. If the radiation possesses a sufficient amount of energy, incomplete gamma photon absorption can also generate secondary electron–hole pairs [15]. Electrons that have previously migrated to the conduction band may become trapped in crystal lattice defects (grain boundaries, impurities) after gamma irradiation is ceased [15]. As our focus is optical properties and sensitivity measurement, an in detail study of the structural properties with irradiation dose is not done.
Raman spectroscopy
The vibrational modes and phase purity of the sample after irradiation are explored using non-destructive Raman spectroscopy. Figure 2 shows the Raman spectroscopy of gamma-irradiated INO thin film displaying five peaks with two strong peaks at 131 cm−1 and 306 cm−1 [19]. Other weaker peaks are found around 106 cm−1,365 cm−1 and 495 cm−1 and all these peaks correspond to cubic-structured INO [12]. A new vibrational mode emerged at 365 cm−1 for all the irradiated film that also corresponds to bcc structured INO [16, 20]. The obtained peak positions are tabulated in Table 2. The bcc structure of INO belongs to the Ia3 space group having a space group number of 206. This type of structure is expected to possess a total of 32 Raman active optical modes including Ag, Eg and Tg modes and also infrared active Tu mode [21].
The Raman intensity increased for all doses after irradiation. The Raman spectra of irradiated samples themselves show an increase in the intensity with dose but the relative increase in intensity is small. The high intensity of the peaks confirms the phase purity of the sample. The 200 Gy sample shows the least intensity and the highest for the 400 Gy sample. This change can be ascribed to the structural modification that occurred with the irradiation [4]. The high intensity of the 400 Gy sample can be also due to the high defect concentration [12]. Abhirami et al. [19] attributed the intensity variation and change in FWHM to the dampened indium oxide vibrational modes generated by the gamma-induced chemical or structural disorder. Saeed et al. [21] attributed the intensity and width enhancement of the 365 cm−1 peak to the defects like oxygen vacancies and In interstitials. The FWHM of the high-intensity peak (at 131 cm−1) is highest for the 400 Gy sample and least for the 200 Gy sample. The decreased FWHM of 200 Gy indicates reduced dislocation density due to decreased intergranular volume fraction. This leads to an increment in the crystallite size [22].
The InO6 bending vibration is represented by the 306 cm−1 peak and is sensitive to the oxygen vacancies [21]. Hence, the Raman data confirms that the 200 Gy irradiated sample has the least concentration of oxygen vacancies and is in correspondence with PL. Also, the 400 Gy sample having higher defect concentration shows high Raman intensity. The other modes at 365 and 495 cm−1 can be ascribed to the stretching vibrations of In-O-In and InO6 octahedra, respectively. The modes at 106 and 131 cm−1 are due to the In-O vibrations in the InO6 units [21].
The blue shift of all Raman bands except that found at 106 cm−1 after gamma irradiation is ascribed to the generation of oxygen vacancies. The shifting of modes can arouse due to defects like oxygen vacancies, grain boundaries and interstitials as these defects can generate stress in the material [21]. Hazem et al. [23] associated the blue shifting of peaks with compressive stress generated in the material under the gamma irradiation.
Optical properties
An in-depth study of optical characteristics is necessary for the appropriate comprehension of the material and optimization of the material’s performance for device application. The fundamental facts about a substance, such as its band gap, electronic transition, localised states and trap** levels, can be inferred from its optical characteristics [24]. The transmittance and absorbance of the irradiated samples were measured using UV–Visible spectroscopy.
Figure 3 shows the transmittance spectra of INO thin layers exposed to different gamma exposures. Higher transmittance with better interference fringes was observed for the 200 Gy sample whereas the 400 Gy sample on the contrary shows lesser transmittance. At a wavelength of 550 nm, the transmission value got increased rapidly from 56 to 67% after gamma irradiation at a dose of 200 Gy. The pronounced transmittance at 200 Gy sample is due to better crystallinity, less scattering, low defect density, a decrease in lattice strain [9] or structural homogeneity of the film [12]. Ramaiah et al. [25] ascribed the transmittance change to the thin film composition. According to Sarker et al. [26] defects created in nanomaterial with gamma ray interaction can act as colour centres and shorten the transmittance. They concluded that the transmittance falls in the visible region of the deposited film due to the presence of hole centres. Analysis of SnO thin film by Abhirami et al. [19] also concurs with the similar conclusion. The study carried out by Tripathi et al. [27] concluded that a rise in oxygen content can be attributable to the lowering of defect level which further enhances the transparency. Crystallinity is also expected to increase after defect level annealing which is also confirmed by the XRD study at 200 Gy. Hence, the filling up of oxygen vacancies and defect level annealing could be the reason for the increased transparency of the 200 Gy sample and this is supported by the PL results. Additionally, grain size variation, grain boundaries with charged impurities, trap** centres, and other imperfections affect the film’s transparency [15, 28].
The pronounced fringes observed for the samples confirm the uniformity in thickness of the thin layers and homogeneity of the thin film surface [2]. The number of interference fringes reduced for the 400 Gy sample may indicate thickness variation of the layers or refractive index variation after the gamma exposure [29]. The redshift and blue shift of the absorption edge after irradiation indicate the decrement and increment of bandgap energy compared to the unirradiated [4]. The bond angle distortion enhanced by gamma radiation also can cause the shifting of the absorption edge to a higher wavelength. Also, the absorption edge variation with wavelength seems to follow the empirical Urbach rule [30]
Many reports are available suggesting that radiation exposure can change the bandgap energy of numerous compound semiconductor materials [31]. An analysis of the relationship between the absorption coefficient (α) and the photon energy (hν) revealed the kind of optical transition and the energy band gap of In2O3. Using Tauc’s relation it is possible to investigate whether the optical transition between parabolic bands occurs directly or indirectly [32]. Mott and Davis’s model was used in estimating the optical bandgap of thin films irradiated with different gamma radiation doses. This model predicts the equation for direct allowed transition as
where the incident photon energy is denoted by hν and B is a constant [33]. The plot of (αhν)2 versus hν was plotted following the equation for the direct allowed transition of gamma-irradiated thin films (Fig. 4). The x-intercept will provide the direct band gap value. The direct band gap values estimated from Tauc’s plot are comparable with the values quoted in the literature [2, 30]. We have observed indirect allowed transition also in the material. Reports confirm the dual transition nature in indium oxide with indirect transitions having a lower bandgap value [34]. The obtained values of direct and indirect bandgap energy are tabulated in Table 3 with the 200 Gy irradiated sample showing an increased bandgap of 3.69 eV and the lowest value was obtained for the 400 Gy sample. The decreased bandgap energy at 400 Gy can be attributed to the increased band tail width of the sample [12]. The density of state model explains the optical band gap decreases with an increase in the disorder [35]. El-Nahass et al. [36] attributed the bandgap energy increase to the microstructure variations, increase in crystallite size and decreased structural disorder after irradiation. The energy bandgap variation after irradiation is clearly indicated by the absorption edge shift as observed in the transmission spectra [16].
The indirect bandgap energy decreased from 2.75 to 2.58 eV for the gamma radiation dose of 400 Gy. Clearly, the rate of band gap variation shows a weak dependence on radiation dose. Maity et al. [33] also reported such a weak dependence of bandgap with radiation dose. However, the wide bandgap energy obtained for the thin films assures the membership of INO in the TCO family.
Both defect creation and annihilation can occur when the material interacts with gamma ray and these two processes can even coexist [33]. Accordingly, the formation of localised states and change in fermi level are to be expected after irradiation. The high concentration of these localised states could be a possible reason for the least value of the optical band gap at 400 Gy [24]. The development of a new energy state has been influenced by several radiation-induced events, such as ionisation and the displacement of atoms within the lattice, which have an impact on the structural, optical and morphological behaviour of INO. According to El-Nahass et al. [36] gamma radiation can reorganise the chemical bonds in a substance. Whereas Waikar et al. [37] attributed the decreased bandgap to the charge trap** of radiolytic electrons and holes. There can be many other factors which can contribute to bandgap modification and the appearance of new energy levels which include grain size, preferred orientation, surface morphology, atomic displacement and rearrangement, etc. [15]. The optical bandgap energy is expected to change in thin films as the particle size is comparable to the Bohr excitonic radius [38]. The shift in optical bandgap is minimal with irradiation, which suggests that the fundamental crystal structure of INO has not changed [27].
For the design of semiconductor devices, the refractive index value plays a major role. The refractive index depends on the optical absorption of thin films, impurities in the thin films and also the defects present in the crystal lattice [16]. It determines the optical and electrical properties of the thin film. The spectral dependence of the refractive index of gamma-irradiated thin films is represented in Fig. 5a. The refractive index variation with wavelength follows Kronig–Krammer’s relation [39]. The refractive index variation after irradiation can be attributed to the gamma-induced defects and resulting atomic rearrangement [2]. At 550 nm, the refractive index is higher for 400 Gy. Whereas the refractive index of the 200 Gy irradiated sample reduced from 2.16 to 1.96 and hence proves the enhancement of optical transmittance at that particular dose. Defect annihilation at a dose of 200 Gy can be the reason for the reduced defect density which in turn increases the optical bandgap.
Sudha et al. [2] also observed such a trend in bandgap and refractive index variation due to the phenomenon of defect annihilation but for higher doses. The gamma ray-induced change in the structure of the film could also be a reason for this change. Hadia et al. [32] attributed the lower refractive index value to the low packing density of grains. Abu EL-Fadl [40] attributed the refractive index variation with irradiation to the change in the structure of the material and chemical bond distribution. The refractive index value can also be correlated to sample density. Such a density variation of approximately 2% after irradiation was reported elsewhere [41]. There exists a general fact that a high refractive index value indicates a high density of film whereas a low extinction coefficient indicates higher transparency of the film [40].
The extinction coefficient quantifies the light absorbed by the film. The formula used to find the extinction coefficient is given by
where α denotes the absorption coefficient and λ is the incident wavelength [16]. The spectral dependence of k is presented in Fig. 5b. With wavelength, the k value decreases as reported elsewhere [4]. It can be observed from the extinction coefficient plot that the k value is maximum near the absorption edge and a rapid decline afterwards. It attained its minimum at a wavelength near bandgap energy. Beyond this wavelength, minimal change in k is observed. The light interaction with free carriers explains the emergence of a high k value in the near-infrared region [32]. With gamma irradiation dose k value shows marginal variation and the minimum value of k is exhibited by the 200 Gy sample indicating the high optical transparency of the sample. Similar observations have been also reported for Ag10Te90 thin films [40]. Compared to other reports we observed a lesser value of extinction coefficient which can be ascribed to fewer crystallographic defects like grain boundaries or voids and minimum imperfections. The extinction coefficient value also exhibits a correlation with the absorption coefficient which in turn depends on the band tail width [42].
Defect formation can be explained using the exponential dependence of the absorption coefficient with the photon energy [16]. Strong ionic bonding, temperature-induced disorder, static disorder and average phonon energies affect the exponential absorption tails, commonly referred to as Urbach’s energy [31]. This Urbach region is created by the extension of the valence band and/or conduction band into the energy band gap [30]. With induced defects, the density of localised states increases which in turn increases the energy width of band tails of localised states [33].
The ln α versus hν plots for the irradiated films are considered for the Urbach energy estimation (Fig. 6) and the values are tabulated in Table 3. The slopes of straight-line portions of these plots give Urbach energy [33]. The tabulated value of Urbach energy summarises that the band tail width of localised states is maximum for an irradiation dose of 400 Gy supporting the increased defect formation due to gamma exposure. The band tail width of localised states is minimum in INO for a radiation dose of 200 Gy. The decreased width of localised states of the 200 Gy sample proves the reduced defect present in the material. The optical bandgap and Urbach energy show an inverse trend.
Hence, from the optical studies we can conclude that the optical properties of the 200 Gy irradiated sample enhanced significantly. It is previously reported that defect annihilation dominates over defect creation at low doses [9]. Here also, at a low dose of 200 Gy, we can observe the dominating defect annihilation rather than creation which is also confirmed by the PL analysis. Dewan et al. [29] attributed the optical property change to the electronic band structure distortion that emerged due to the displacement of oxygen from the lattice. Hence, gamma ray displaced oxygen has an influence on the optical properties and also it correlates the oxygen-related defects with changes in the structural arrangement [29]. Baydogan et al. [1] also attributed the decreased transmittance, bandgap energy and grain size to the gamma radiation-created colour centre.
Photoluminescence spectroscopy
Utilising the non-destructive PL spectroscopy, the localised defects and impurity states may be examined [16]. The photoluminescence (PL) spectrum of gamma-irradiated INO thin film along with unirradiated at room temperature is reported in Fig. 7. Figure 7 makes it obvious that gamma radiation had an impact on the PL characteristic. INO stoichiometrically behaves as an insulator and does not emit light [27]. Hence, the PL emission confirms the stoichiometric deviation of INO. Defects that can be present in nonstoichiometric samples like singly ionised oxygen vacancies, oxygen vacancies and interstitial, antisite oxygen, indium vacancies and interstitials contribute to the PL [27]. Excitation is carried out at a wavelength of 325 nm as the energy corresponding to this is greater than bandgap energy of the material.
The oxygen vacancies will act as donors for n-type INO semiconductors. Numerous oxygen vacancies are inherent in In2O3 and this is due to the partial oxidation of the metallic indium [32]. Due to its low formation energy in comparison to the indium interstitials, oxygen vacancy creates shallow donor levels. During the defect creation process by gamma irradiation, electronic states are introduced to the material by electronic excitation. The relaxation process of electrons from this level to the ground state induces emission, i.e. gamma radiation generates oxygen vacancies and other solid defects like point defects, interstitial, trap** centres, etc. in the material. The radiative transition rate rises as defect concentration increases which leads to increase in the luminescence intensity [27].
The deconvoluted PL spectra show 5 peaks and the obtained peaks are tabulated in Table 4. Another category of PL emission includes deep-level defect emission and it mainly arises from intrinsic or extrinsic defects [27]. Possible causes of the deep-level emission peaks in the blue region are structural defects such as oxygen vacancies [12]. Souli et al. considered the defect level variation which emerged due to atomic rearrangement may be the reason for blue–green emission. Also, they considered that defect levels will act as recombination centres so that with the variation of defect levels those recombination centres also get moved [15]. The obtained spectra show a decreased peak intensity at 200 Gy and possess a high intensity at 400 Gy. The defect density in the sample is reflected in the area beneath the peak, i.e. the rise in PL peak intensity means a larger area under the peak, which in turn denotes a higher defect concentration [24]. Therefore, the PL spectra show a high defect concentration in the 400 Gy sample. The PL intensity of the 400 Gy sample almost doubled compared to 200 Gy indicating the extensive incorporation of structural defects. For 200 Gy oxygen vacancies might have to get filled up by interstitial oxygen and observes a systematic suppression of PL emission. A slight increase in crystallinity of the 200 Gy sample also reveals the defect annihilation at that dose. Waikar [37] attributed the decreased PL intensity to the localised heating occurring in the material after the irradiation.
After irradiation, the NBE peak shifted to a higher wavelength for all irradiation doses. We also observed the disappearance of peaks (375 and 381 nm) and the emergence of a new peak (480 nm). Similar missing and appearance of peaks have been reported in our previous work [43]. The emerging new peak also corresponds to the generation of oxygen vacancies [4]. Nefzi et al. [16] attributed the decrease in the number of observed PL peaks after irradiation to the decrease in lattice defects. In short, as discussed in the earlier section, the interaction of gamma radiation can create defects, or it can annihilate the defects. It has been concluded from the previous studies that for low doses defect annihilation will be the dominant mechanism and for higher doses defect creation dominates.
Electrical properties
It is well known that resistivity, a crucial component in the design of electrical circuits and devices, is sensitive to ionising radiation. Ionising radiation has been widely used to modify the characteristics and performance of polymers, bulk materials and thin films [31]. Applied dose, the type of radiation and even the composition of oxide have an impact on the electrical resistivity of the film [1]. The resistivity, mobility and carrier concentration after irradiation at each particular dose are calculated using hall measurements and are summarised in Table 5. The sign of Hall effect measurements validates the n-type semi-conductivity of INO. The resistivity variation of gamma-irradiated thin films is shown in Fig. 8. The resistivity decreased after irradiation for all doses except for 400 Gy. The resistivity decreases from 0.18 to 0.04 Ω cm for unirradiated and 200 Gy irradiated samples. It increased to 0.29 Ω cm when irradiated with a dose of 400 Gy. The low resistivity and higher mobility were observed for 200 Gy suggesting the improved electrical properties of that sample. The increased conductivity after irradiation can be attributed to the electron–hole pair formation due to the absorption of kinetic energy from the irradiated radiation [2]. Abhirami et al. [19] reported that the interaction of gamma radiation with the thin film will transfer energy and hence the carrier will get transferred from the valence band to the conduction band creating electron–hole pairs, thereby improving the electrical conductivity. Grain boundaries can also affect net electron flow in the material which in turn controls the conductivity of the material [2]. In fact, film deposition always contributes to intrinsic defects. The material’s resistivity may be decreased by this defect recombination [33]. Moreover, irradiation results in an increased number of oxygen vacancies. Oxygen vacancies, which is a major radiation defect have the capability to degrade the thin film stoichiometry. The higher resistivity of the 400 Gy sample may be due to oxygen vacancies induced by the gamma radiation [2]. Abhirami et al. [19] reported a decrease in resistivity and attributed not only to oxygen vacancies but also to broken bonds. Due to irradiation bond breakage occurs and oxygen will capture the holes. The radiolytic electrons resulting from the irradiation are free to move inside the structure and thus contribute to the resistivity decrement [1].
Mobility will depend on lattice deficiencies like interstitials and oxygen vacancies [4]. The mobility for the 200 Gy sample exhibits the highest value, which can be attributed to a reduction in structural disorder and oxygen vacancies [30]. Depending on the charge states, the defects created after irradiation, like In and O interstitials and/or vacancies, establish deep and shallow-level states inside the band gap. With a rise in irradiation dose, deep-level and shallow-level defect concentrations enhance. Furthermore, above a certain point, there is a higher likelihood that a shallow defect level will occur, which will in turn increase mobility. This is a result of defects and impurities rearranging themselves at ambient temperature during irradiation [19]. Grain boundary and impurity scattering have a great influence on the mobility of carriers inside the material [1]. The decreased charge carrier concentration of the 400 Gy sample can be ascribed to vacancy generation and charge capture at the defects [35].
Sensitivity estimation
The current–voltage characteristics were done on a thin film sample irradiated with different doses using two probe methods. The current density for the sensitivity estimation was calculated from the I-V plot. The current density versus gamma dose plot is shown in Fig. 9 and the applied voltage was chosen from 0.2 to 1 V with an increment of 0.2 V to monitor the current density change with gamma irradiation dose. The graph depicts a linear variation of current density with an increase in dose up to a level of 200 Gy. Beyond 200 Gy current density reached a plateau as radiation increased up to 300 Gy. A similar kind of dose response was expected for TL materials which contain a linear region, supralinear region and saturation region. The energy of the incident gamma ray and the kind of radiation are known to affect the supra linearity [44]. Reports are available on TL materials that don’t exhibit a supra linearity [45]. The variation in material qualities provides a plausible explanation for the variations in the supralinear region’s dependency on the radiation dose [44]. The saturation region is the area where the radiation dose has almost no impact on the current density value. A high degree of structural disorder could be the cause of the saturation region’s low sensitivity. This damage creates a large concentration of defect levels and thereby decreases the sensitivity parameters [35]. Radiation dosimetry, however, prefers the region of linear response. Moreover, the dose response of the material depends on instrumental factors, radiation field and also material properties [44].
The change in current density per unit change in gamma radiation exposure is represented by the sensitivity. The sensitivity of In2O3 thin films of a thickness of about 600 nm was found to be in the range of 10.6–53.3 mA/cm2/Gy for different applied voltages ranging from 0.2 V to 1 V (Table 6). We have previously reported a much higher sensitivity of 101 mA/cm2/Gy at 1 V for INO thin film with a lower concentration of 0.1 M but for lower doses [43]. Hence, it can be concluded that the molar concentration of the sample also plays a major role in determining the sensitivity of thin film. In Fig. 8 we can observe that the film sensitivity rises with the voltage that has been applied. Increased sensitivity with voltage can be associated with the fact that the semiconductor properties of the material ensure current augmentation with voltage [46]. For the prepared thin films, the linear response of the current density with the gamma dose up to the dose of 200 Gy is considered to be the working region. However, the current density decreased drastically beyond 300 Gy.
It has been reported that the sensitivity of thin film towards gamma radiation will depend on thickness. Maity et al. [33] reported that the critical dose will be higher for thicker films. This fact is reassured by our previous study as a 0.2 M sample at the same deposition temperature possesses a higher thickness and hence a higher critical dose [47]. Radiation-induced recombination of inherent defects and ordering of disordered phases contributes to the healing process. Most of the time, healing occurs at low dosages, which is why the mechanism is known as the “small dose effect” [31]. As discussed in PL analysis, the increase in current up to 200 Gy could be ascribed to the healing effect. The healing effect also explains the dependence of critical dose on the thickness of the film. Since thinner films recover more quickly than thicker ones, the latter requires a higher radiation dose [33].
Gamma radiation takes more complicated trajectories inside the target substance, and the energy deposits are widely dispersed. Radiation-induced oxide traps are responsible for the large mutation in the properties of the thin film. These irradiation-activated oxide traps deteriorate device performance. The dosimetric performance of the material may be affected by the device shape, sample thickness and material composition [48]. Defects like broken indium and oxygen bonds also worsen the electrical properties of the thin film [17].
Hence, one can conclude that the indium oxide thin film exhibit good sensitivity and its sensitivity can be tuned according to our requirements by varying thickness and other deposition parameters. Moreover, modifications in the properties of gamma-irradiated INO thin films confirms the viability of the materials for a variety of applications.
Conclusion
The structural, optical and electrical properties of gamma-irradiated indium oxide thin film were studied in this article. The structural changes observed in the irradiated films can be attributed to defects like colour centres/oxygen vacancies and impurities. The transmittance variation observed after gamma irradiation is due to the defects generated in the material after gamma ray interaction. The band gap variation shows a weak dependence on radiation dose. PL data validates the dominancy of defect creation at 400 Gy and defect annihilation at 200 Gy. This is in accordance with the optical data and follows the small dose effect. The noticeable variation in the electrical and optical properties of the sample irradiated at 400 Gy could be due to the material degradation and increased structural disorder of that sample. The 200 Gy may be considered as critical dosage as the current density rises linearly up to this dose. The sensitivity of the In2O3 thin film of thickness around 600 nm was found to be in the range 10.6–53.3 mA/cm2/Gy for different applied voltages ranging from 0.2 V to 1 V. Hence, it can be concluded that indium oxide thin film exhibits good sensitivity to gamma radiation.
Data availability
Not applicable.
References
Baydogan N, Ozdemir O, Cimenoglu H (2013) The improvement in the electrical properties of nanospherical ZnO:Al thin film exposed to irradiation using a Co-60 radioisotope. Radiat Phys Chem 89:20–27
Sudha A, Sharma SL, Gupta AN (2019) Achieving sensitive and stable indium oxide thin films for gamma radiation monitoring. Sens Actuators A Phys 285:378–385
Yahia A, Attaf A, Saidi H, Dahnoun M, Khelifi C, Bouhdjer A, Saadi A, Ezzaouia H (2019) Structural, optical, morphological and electrical properties of indium oxide thin films prepared by sol gel spin coating process. Surf Interfaces 14:158–165
Nefzi C, Souli M, Beji N, Mejri A, Kamoun-Turki N (2017) Enhancement by high gamma radiations of optical and electrical properties of indium oxide thin films for solar devices. J Mater Sci 52:336–345
Narmada A, Kathirvel P, Lakshmi M, Saravanakumar S, Marnadu R, Chandrasekaran J (2020) Jet nebuliser spray pyrolysed indium oxide and nickel doped indium oxide thin films for photodiode application. Optik (Stuttg) 202:163701
Jung DS, Bin Park S, Kang YC (2010) Design of particles by spray pyrolysis and recent progress in its application. Korean J Chem Eng 27:1621–1645
Kaya S, Yıldız I, Lok R, Yılmaz E (2018) Co-60 gamma irradiation influences on physical, chemical and electrical characteristics of HfO2/Si thin films. Radiat Phys Chem 150:64–70
Aparna C, Shetty PK, Mahesha MG (2022) Structural optimization of indium oxide thin film for gamma dosimetry applications. Mater Sci Semicond Process 150:106931
Sudha A, Sharma SL, Sharma SD (2017) Study of structural, optical and electrical properties of gamma irradiated In2O3 thin films for device applications. J Mater Sci Mater Electron 28:4619–4624
Sudha A, Maity TK, Sharma SL (2016) Effects of gamma irradiations on structural and electrical properties of indium oxide thin films prepared by thermal evaporation. Mater Lett 164:372–375
Kaya S, Yilmaz E (2018) Modifications of structural, chemical, and electrical characteristics of Er2O3/Si interface under Co-60 gamma irradiation. Nucl Instrum Methods Phys Res Sect B Beam Interact with Mater Atoms 418:74–79
Nefzi C, Beji N, Souli M, Mejri A, Alleg S, Kamoun-Turki N (2019) Effect of gamma-irradiation on optical, structural and electrical properties of In2O3: F thin films for photocatalysis application. Opt Laser Technol 112:85–92
Dey PC, Sarkar S, Das R (2020) X-ray diffraction study of the elastic properties of jagged spherical CdS nanocrystals. Mater Sci Pol 38:271–278
Gurer U, Yilmaz O, Karacali H, Kaya S, Yilmaz E (2020) Co-60 gamma radiation influences on the electrochemical, physical and electrical characteristics rare-earth dysprosium oxide (Dy2O3). Radiat Phys Chem 171:108684
Souli M, Reghima M, Secu M, Bartha C, Enculescu M, Mejri A, Kamoun-Turki N, Badica P (2019) Physical properties investigation of samarium doped calcium sulfate thin films under high gamma irradiations for space photovoltaic and dosimetric applications. Superlattices Microstruct 126:103–119
Nefzi C, Souli M, Beji N, Mejri A, Kamoun-Turki N (2019) Improvement of structural, optical and electrical properties of iron doped indium oxide thin films by high gamma radiations for photocatalysis applications. Mater Sci Semicond Process 90:32–40
Arshak K, Korostynska O (2006) Response of metal oxide thin film structures to radiation. Mater Sci Eng B Solid-State Mater Adv Technol 133:1–7
Arshak K, Korostynska O, Henry J (2005) Structural modifications in thin films caused by gamma radiation. Mater Sci Forum 480–481:13–20
Abhirami KM, Sathyamoorthy R, Asokan K (2013) Structural, optical and electrical properties of gamma irradiated SnO thin films. Radiat Phys Chem 91:35–39
Beji N, Souli M, Azzaza S, Alleg S, Kamoun Turki N (2016) Study on the zinc do** and annealing effects of sprayed In2O3 thin films. J Mater Sci Mater Electron 5:4849–4860
Saeed F, Farooq A, Ali A, Mehmood S, Cepek C, Bhardwaj S, Ul-Hamid A, Bhatti AS (2020) Anomalous optical behavior in pyramid-like indium oxide (In2O3) nanostructures. Mater Sci Eng B Solid-State Mater Adv Technol 262:114781
Dasari SG, Nagaraju P, Yelsani V, Tirumala S, Ramana Reddy MV (2021) Nanostructured indium oxide thin films as a room temperature toluene sensor. ACS Omega 6:17442–17454
Hazem R, Izerrouken M, Cheraitia A, Djehlane A (2019) Raman study of ion beam irradiation damage on nanostructured TiO 2 thin film. Nucl Instru Methods Phys Res Sect B Beam Interact with Mater Atoms 444:62–67
Choudhary R, Chauhan RP (2016) Gamma irradiation induced modifications in spin coated CdSe thin films. J Mater Sci Mater Electron 27:11674–11681
Ramaiah KS, Raja VS, Bhatnagar AK, Juang FS, Chang SJ, Su YK (2000) Effect of annealing and γ-irradiation on the properties of CuInSe2 thin films. Mater Lett 45:251–261
Sarker P, Sen SK, Mia MNH et al (2021) Effect of gamma irradiation on structural, morphological and optical properties of thermal spray pyrolysis deposited CuO thin film. Ceram Int 47:3626–3633
Tripathi N, Rath S (2014) Effect of thermal annealing and swift heavy ion irradiation on the optical properties of indium oxide thin films. ECS J Solid State Sci Technol 3:P21–P25
Beena D, Lethy KJ, Vinodkumar R, Pillai VPM, Ganesan V, Phase DM, Sudheer SK (2009) Effect of substrate temperature on structural, optical and electrical properties of pulsed laser ablated nanostructured indium oxide films. Appl Surf Sci 255:8334–8342
Dewan N, Sreenivas K, Gupta V (2008) Comparative study on TeO2 and TeO3 thin film for γ-ray sensor application. Sens Actuators A Phys 147:115–120
Girtan M, Folcher G (2003) Structural and optical properties of indium oxide thin films prepared by an ultrasonic spray CVD process. Surf Coat Technol 172:242–250
Arshak K, Korostynska O (2002) Gamma radiation dosimetry using Tellurium dioxide thin film structures. Sensors 2:347–355
Hadia NMA, Mohamed HA (2013) Synthesis, structure and optical properties of single-crystalline In 2O3 nanowires. J Alloys Compd 547:63–67
Maity TK, Sharma SL (2008) Effect of gamma radiation on optical and electrical properties of tellurium dioxide thin films. Bull Mater Sci 31:841–846
Erhart P, Klein A, Egdell RG, Albe K (2007) Band structure of indium oxide: indirect versus direct band gap. Phys Rev B Condens Matter Mater 75:153205
Arshak K, Korostynska O (2004) Preliminary studies of properties of oxide thin/thick films for gamma radiation dosimetry. Mater Sci Eng B Solid-State Mater Adv Technol 107:224–232
El-Nahass MM, Darwish AAA, El-Zaidia EFM, Bekheet AE (2013) Gamma irradiation effect on the structural and optical properties of nanostructured InSe thin films. J Non Cryst Solids 382:74–78
Waikar MR, Raste PM, Sonker RK, Gupta V, Tomar M, Shirsat MD, Sonkawade RG (2020) Enhancement in NH3 sensing performance of ZnO thin-film via gamma-irradiation. J Alloys Compd 830:154641
Göde F, Güneri E, Emen FM, Emir Kafadar V, Ünlü S (2014) Synthesis, structural, optical, electrical and thermoluminescence properties of chemically deposited PbS thin films. J Lumin 147:41–48
Prathap P, Subbaiah YPV, Devika M, Reddy KTR (2006) Optical properties of In2O3 films prepared by spray pyrolysis. Mater chem phys 100:375–379
Abu EL-Fadl A, Hafiz MM, Wakaad MM, Aashour AS (2007) Influence of γ-radiation on the optical parameters of Ag10Te90 thin films. Radiat Phys Chem 76:61–66
Craciun D, Socol G, Caër SL, Trinca LM, Galca AC, Pantelica D, Ionescu P, Craciun V (2016) Gamma irradiation effects on the properties of indium zinc oxide thin films. Thin Solid Films 614:2–6
Salim SR (2014) The Effect of irradiation on structural and optical characteristics of indium oxide thin films. Int J Sci Res 3:2004–2014
Aparna C, Kumara P, Mahesha MG, Karunakara N, Yashodhara I (2022) Structural, optical and sensitivity study of optimized indium oxide thin film for gamma sensing applications. Sens Actuators A Phys 345:113785
Arshak K, Korostynska O, Henry J (2004) Thick film PN-junctions based on mixed oxides of indium and silicon as gamma radiation sensors. Microelectron Int 21:19–27
Horowitz Y, Olko P (2004) The effects of ionisation density on the thermoluminescence response (efficiency) of LiF:Mg, Ti and LiF:Mg, Cu. P Radiat Prot Dosim 109:331–348
Maity TK, Sharma SL (2011) Effects of gamma irradiation on electrical, optical and structural properties of tellurium dioxide thin films. Indian J Pure Appl Phys 49:606–612
Aparna C, Shetty PK, Mahesha MG, Karunakara N (2022) Temperature dependence of optical and electrical properties of gamma-irradiated indium oxide thin film. Optik (Stuttg) 269:169881
Arshak K, Corcoran J, Korostynska O (2005) Gamma radiation sensing properties of TiO2, ZnO, CuO and CdO thick film pn-junctions. Sensors Actuators A Phys 123–124:194–198
Acknowledgements
The authors are grateful to NITK, Surathkal for the Raman facility; CARRT Mangalore University, Mangalagangothri, India for the gamma irradiation facility. Ms Aparna would like to thank the Manipal Academy of Higher Education (MAHE) for providing the scholarship for research work and instrumentation facilities.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Contributions
CA is presently working as a research scholar at the Department of Physics, Manipal Institute of Technology, MAHE, Manipal, India. She played role in data curation, formal analysis, investigation and writing—original draft. PKS played role in resources, software, supervision, validation, and writing—review and editing. MGM played role in resources, software, supervision, validation, and writing—review and editing. IY contributed to experimentation and investigation. NK contributed to experimentation and investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Additional information
Handling Editor: Megumi Kawasaki.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aparna, C., Shetty, P.K., Mahesha, M.G. et al. Sensitivity estimation of indium oxide thin film for gamma sensing. J Mater Sci 58, 11374–11391 (2023). https://doi.org/10.1007/s10853-023-08720-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08720-z