Abstract
This article presents the results of a study on laser ablation of selected thermoplastic polymers using an economical fiber laser with a scanning galvanometer system and an IR laser with a wavelength of λ = 1064 nm. The study aimed to develop comparative characteristics of the ablation processes of commonly used constructional thermoplastics, namely acrylonitrile butadiene styrene terpolymer (ABS), polystyrene (PS), poly(ethylene terephthalate) (PET), polylactide (PLA), and polyamide 6 (PA6). The ablation characteristics of the tested materials were determined, and the ablation depth and surface structures were calculated and presented. A comparative analysis of the contact angle, surface free energy, and strength of adhesive connections of materials modified with laser radiation and unmodified was performed. The study showed that the dynamics of laser ablation were much higher in ABS, PS, PET, and PC than in PLA and PA6. Although modification of the surface layer of the polymeric material did not have a significant impact on the surface free energy and wettability of the sample surface, it increased the strength of adhesive connections even several times (in ABS, PS, PET) with the lowest energy consumption. The results also demonstrated that some polymers, such as ABS or PS, can be effectively treated with near-infrared laser radiation of this wavelength, which is particularly important for surface layer modification and marking of details.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laser ablation (LA) is the process of removing the surface layer (SL) of a material by focusing on a laser beam. It only occurs when a material has absorbed enough energy to break up some of that material’s chemical bonds. This process may be of a different physicochemical nature. In general, ablation involves photochemical or photothermal reactions; however, the nature of the process is often complex. Many models describe the course of the laser ablation process. The scientific community is not homogeneous in determining the mechanisms of this process. Laser ablation has seemed to be a complex process. Initially, it was thought that ablation is a purely chemical process in which only photochemical reactions take place. There was also a different perspective among researchers considering ablation mainly as photothermal in nature [1]. Currently, it is assumed that in the ablation of polymers, both photochemical and photothermal ablation are involved [2]. A model of ablation that combines both chemical and thermal reactions can be described by the Beer-Lambert law (photochemical ablation) and by the Arrhenius law (photothermal ablation) [3]. In real conditions, these components are difficult to separate because these processes take place altogether [4]. The research about the ablation of polymers focused on such parameters of laser radiation as wavelength, beam power, frequency of pulses, and duration. Studies on the light absorption (UV, Vis, NIR) of polymers have been carried out [5,6,7]. Results indicate that the best absorption range is in the UV range below 300 nm, regardless of the pulse width. The results of these studies allowed a gradual understanding of the LA process. Laser ablation has found wide application in the processing of metals, ceramics, glass, and polymers. It is polymers have gained a lot of interest in the industry in recent years due to their unique features: low density, corrosion resistance, resistance to tribological wear [8], and the possibility of making decorations using electroless/current metallization [9]. The first information on polymer ablation appeared in the seventies of the twentieth century. Since then, there has been a rapid increase in the use of polymeric materials, and research of LA in polymer processing [10].
So far, there have not been many publications describing the ablation of thermoplastic polymers treated with 1064 nm wavelength rays. Most of the previous work has focused on wavelengths below 300 nm. Other lasers, particularly those emitting at 1064 nm, were deemed ineffective. Therefore, this article presents a novelty in the field. It reports the results of tests conducted on six popular thermoplastics commonly used in the industry, where they were subjected to NIR radiation. The tests aimed to determine their susceptibility to ablation, effective radiation absorption coefficients, surface energy, wettability, and the strength of adhesive connections. Surface morphology studies were performed. The test results were compared with one another to identify the most suitable material for laser modification. The novelty of the idea also lies in the fact that have not used an expensive laboratory laser. Instead, the Nd:YAG laser was used, a relatively simple and inexpensive device that can be easily controlled using a galvanic system to scan the beam. The information gathered from this study sheds new light on the use of 1064 nm lasers in the processing of thermoplastic polymers, particularly in modifying the surface layer or marking details.
Methodology
Materials and reparation of samples
Selected polymer matrices widely used in polymer processing were used in the research. Their names and basic physical properties have listed in Table 1.
Samples to test were made using a TRX 80 ECO 60 injection molding machine (Tederic Machinery Manufacture Co. LTD, Taiwan). The temperatures of the zones (I, II, and III) of the cylinder, head (H), and injection mold are summarized in Table 2. The granules used for injection were previously dried at a temperature of 50 °C per 24 h. The final result of the injection of the polymer materials was plates with dimensions of 60 × 60 mm and a thickness of 1 mm (Fig. 1).
Research techniques
In this study, it was proposed to determine ablation indirectly by measuring the mass loss after each irradiation of the sample. Mass loss testing is widely used in materials science, e.g. tribology [11, 12]. It is an indirect method of assessing the energy state of the surface layer. This loss can be a quantitative parameter characterizing the chemical activity of the material. This activity depends on the type of surface treatment used [13]. Laser ablation causes the formation of plastic deformations that cause the accumulation of certain amounts of energy in the structure of the surface layer. This can initiate depolymerization, destruction, and degradation processes. Destruction of polymer chains leads to the loss of molecular weight of polymers [14, 15]. Destruction and degradation processes can be caused by factors such as heat, light, and high-energy radiation. Destruction consists of the decomposition of polymer chains with the release of low-molecular compounds other than the monomer, while degradation is a partial decomposition of the polymer into fragments with high molecular weights, but smaller than the starting polymer. When the polymer is exposed to laser radiation, both destruction and degradation occur [16, 17]. The result of irradiating the surface of the sample is its mass loss.
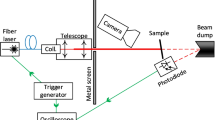
Nd:YAG FIBER TS-20W laser (TechSol, Poland) with an average power of 20 W and a wavelength of 1064 nm was used for laser irradiation. It is a pulsed laser with a pulse width (at 20 kHz) of 80—140 ns and a single pulse energy of 0.8—1.0 mJ. The minimum line width is 15 µm. This device allows the scanning of the material with a laser beam with various speeds and power. The laser beam is moved by the optical system. The entire surface of the sample was irradiated from one edge to the other, line by line (Fig. 1). After each irradiation with a laser beam samples were cleared with compressed air, and the mass was noticed using a laboratory balance WAS 160 / x, (Radwag, Poland).
The laser beam was moving at a speed of 2 m/s. The laser power was increased with a resolution of 2W. To present the test results, the samples were named, as shown in Table 3.
Measurements of each type of material were repeated 10 times (n = 10 samples), and then the expected value of the measurement was estimated as the arithmetic mean. The uncertainty of a single measurement was determined as the standard deviation.
Based on these measurements, the absolute and relative weight loss was determined as:
where: Δm—an absolute mass loss of the sample, m%—relative mass loss of the sample, m1—the weight of the sample after irradiation, mi—sample weight measured after the next laser irradiation.
To better show the depth of changes in the structure of SL, this mass loss was converted to the depth (ΔH) of the removed polymer layer as:
where: ρ—material density, S—sample surface area, m%—relative mass loss of the sample.
The Q200 Thermogravimetric Analyzer (TA Instruments, USA) was used in this study to investigate the effect of temperature on the thermal degradation of the polymeric material. The tests were carried out by changing the temperature in the range from 20 °C to 700 °C at a rate of 10 °C/min. surrounded by a neutral gas—nitrogen. Also, differential thermogravimetric analysis (DTG) was performed by determining the first derivative of the TG versus the temperature curve.
A gas pycnometer MVP-D160E (Quantachrome Instruments, USA) was used to test the density of the polymeric material. The study used probing gas—helium, which allows it to reach the smallest pores with a diameter of less than one nanometer.
Wettability tests were made using a DSA 100 goniometer (Kruss GmbH, Germany). The device has a system for automatic dosing of the measuring liquid, a light source with a shutter, adjustment of the sample position in three perpendicular directions, and a CCD camera. Two types of measuring liquid were used in the study: water and diiodomethane, which differ significantly in the values of the polar and dispersion components of the surface free energy. A drop of 7 µl of water was dispensed at a rate of 50 µl / min, while a drop of 3 µl of diodomethane was dispensed at a rate of 100 µl / min. The calculations used Young's equation describing the relationship between the free surface energy of a liquid (γL), the free surface energy of the material (γS), and the free surface energy of the interfacial phase (γSL) as below [18, 19]:
where: θ—contact angle (the angle between the tangent to the surface of the liquid drop and the surface of the tested material).
In calculations of surface energy (SE), the Owens–Wendt method was used, in which SE is divided into two components: polar and dispersion:
where: \({\gamma }_{S}^{d}\)—dispersion component of SE, \({\gamma }_{S}^{p}\)—polar component of SE.
The tendency to wet a solid with a liquid is related to the works of cohesion and adhesion. The work of adhesion is characterized by the necessary work that must be performed to separate the liquid from a unit surface while creating new surfaces of the same size. The value of this work determines the relationship [20]:
A Q200 differential scanning calorimeter (TA Instruments, USA) was used to determine the specific heat of the materials. The heat-flux DSC method was used, consisting in measuring the temperature difference between the reference sample and the test sample. Both samples were placed in the identical measuring tool, symmetrically placed in a common furnace. The samples were heated to the temperature of 50 °C, then cooled to the temperature of 10 °C, and reheated to the temperature of 50 °C. The heating/cooling rate (β) was 5 °C/min. The specific heat of a given material was determined for a sample temperature of 25ºC.
Morphological tests of SL were made using the DMS 300 digital optical microscope (Leica Microsystems, Germany) with a variable focal length of 8: 1 connected to a digital camera. It allows observing objects in real-time in high definition (HD) quality with a refresh rate of 30 frames per second. A high-performance electron microscope (SEM) JSM 6480LV (Jeol, Japan) with a resolution of 3.0 nm was also used. This device is equipped with a five-axis eucentric table with rotation and comocentric tilt. It has standard functions: autofocus/auto stigmator, auto gun (saturation adjustment and equalization), and automatic contrast and brightness. The scans were made using the primary secondary electron beam interaction (SEI) method, with an accelerating voltage value of 1 kV.
Strength tests of adhesive connections were carried out according to PN-EN ISO 4624: 2003. The expansion test was performed with the use of the PostiTest AT device (DeFelsko, USA) and measuring stamps with a diameter of 20 mm. The stamps were glued to the tested surface. Araldite 2000 (Huntsman Advanced Materials, Switzerland) glue was used. Stamps were pressed to the surface with a force of about 6 N and left for 48 h (curing time) at room temperature.
Results and discussion
There are many methods for determining the depth of ablation. It is worth mentioning the most commonly used ones: measurements of crater diameters for different values of laser radiation energy density [21], the acoustic method used to determine the degree of surface cleaning by laser ablation [22], determination of the laser radiation absorption coefficient [23], measurements of weight loss due to the interaction of laser radiation [24]. In this study, the method of determining the mass loss of the tested sample was used as a measure of the intensity of laser ablation. Loss of weight was converted to loss of thickness using Eq. (3). Figure 2 shows the ablation charts of the tested materials. Curves present the loss of thickness of the sample as a function of laser power. The graph shows the point (threshold) from which the asymptotic cut-off of the ablation curve takes place. The power value at this point is defined as the ablation threshold (\({P}_{\mathrm{th}}\)) [25]. This is the lowest value of the laser beam power for which mass/layer thickness loss is observed. The ablation threshold values were determined by the orthogonal projection of these points onto the x-axis. These values are shown in Table 4
By transforming the Lambert–Beer equation, we obtain the formula for the ablation depth:
where: H—ablation depth measured from the sample surface, Pj—the power of the incident laser radiation, \({P}_{th}\)- the value of the power needed to initiate ablation (ablation threshold), αeff—effective radiation absorption coefficient.
The linear least-squares fit (linear regression method) [26, 27] was applied to Eq. (7). Using Eqs. (3) and (7), the effective absorption coefficient (αeff) was determined for each tested material. The effective absorption coefficient provides information about additional factors affecting ablation. During the laser pulse, there is a sudden increase in the temperature of the material. There is a dynamic change in the optical properties of the polymer, resulting in a stronger absorption of laser radiation [28]. These data are presented in Table 4.
Based on the data presented in Table 4, it can be concluded that the most dynamic process of ablation occurred in PET, ABS, PS, and PC because their αeff values were the highest. On the other hand, the ablation threshold for PC occurred only after exceeding 12W of laser power, which was almost twice as much as in the case of ABS or PS. The most energy needed to modify the material was for PA6 (almost 13W laser power) and PLA (about 16W laser power). ABS and PS laser ablation started at relatively low laser power (around 7.5W). The absorption coefficients of these materials also had a similar value.
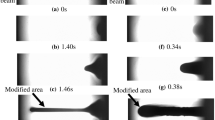
After each irradiation with a laser beam, the morphology of the surface was observed. Tests were made using an optical microscope. Figure 3 compares the surface views of laser-irradiated samples. The surfaces of samples after laser irradiation with power below the power value at the ablation threshold and power above the ablation threshold are shown. Each tested polymer material had a different ablation threshold value. These values are summarized in Table 4.
On the surface of ABS, PS, PC, and PET, after exceeding some value of the laser beam power, a significant impact of radiation was visible. In the case of the PA6.16 sample, exposure to radiation with a power of 16 W caused few changes on the surface of the sample. However, for the sample made of PLA, no significant changes were noticed in the SL. Most of the laser pulse energy was absorbed by the lower layers of the material. There was some kind of discoloration and cracks of an internal nature. To thoroughly examine the surface condition of the samples, the SEM test was made.
Figure 4 shows (analogous to Fig. 3) a comparison of the surfaces of the tested samples. SEM imaging has shown a minimal modification of SL in PLA and PA6 materials. The images of PLA samples obtained from the optical microscope have shown some discoloration. There were probably cracks in the structure of the material. Most of the pulse energy was absorbed by lower layers of material. It can therefore be assumed with a high probability that changes in PLA took place inside the material. When using a 1064-nm-wavelength laser to process PLA and PA6, higher laser beam powers are required to initiate surface modification of this type of polymer.
For ABS, PC, PS, and PET materials irradiated with a laser power value below the ablation threshold, only slight changes in the surface structure were noticed. After exceeding the ablation threshold, surface cracks, torn pieces of material, and bubbles (possibly with gas) inside the material were observed in these materials. The holes visible in the SL may indicate gas eruption under the influence of laser irradiation. The locally dark discoloration was noticed in samples from PC and PET irradiated with laser beams. It can be assumed that it was related to the charring process of SL. The appearance of pyrolysis products suggests that the laser beam raised the temperature to point above the degradation temperature of the material.
The laser ablation of PC and PET, apart from changes in the SL of the other polymers, also induced thermal processes inside the material. This is evidenced by holes in the sample presented in Fig. 5 through which the gas was released. Traces of hot and then solidified paths arranged in the direction of the laser beam were noticed. The laser radiation-induced thermal processes locally increase the temperature of the material above the melting point of the polymer.
In the PC.20 sample (laser beam with maximum power), the entire irradiated area was melted down. Many holes were observed through which gas escaped during ablation. The PET.20 sample also observed the melting of the SL material. Moreover, many cracks and detachments of the material were noticed at the border of the irradiated area. The reason for this may be the temperature difference between the irradiated and non-irradiated areas. Thermal stresses resulting from changes in the temperature of the material under the influence of laser radiation could cause its crack or plastic deformation. Temperature gradients, thermal expansion, contraction, and thermal shocks may have been factors that led to thermal stresses. The higher the temperature difference, the higher the level of mechanical stress will be. When the material is heated or cooled rapidly, the surface temperature and the internal temperature will be different. Rapid heating of the material causes local thermal expansion while cooling causes shrinkage, resulting in thermal stresses. The stress largely depends on the material's thermal expansion coefficient. The material expands or contracts as appropriate for a given coefficient of thermal expansion [29, 30].
The analysis of the SEM and optical microscopy results for PC and PET showed that when the laser power exceeds 14W, it causes PC to melt. The PET melting process took place earlier after exceeding 10W of the laser power. Many cracks and detachments of material fragments were observed in the SL of PET. This may indicate extensive polymer (PET) degradation. Observation of the PC showed no cracks in the SL (Fig. 4). The point effect of the laser beam was noticed. In the place of irradiation of the laser beam, changes such as discoloration (pyrolysis), and blisters with gas accumulating, were noticed. PET had a higher value than the PC of this effective absorption coefficient. A comparison of the results of PC and PET laser ablation showed that PET is a material more susceptible to laser radiation than PC.
In the analysis of the surface morphology of PS and ABS samples, many depressions, pores, and cavities in the material structure were noticed. In the case of PS, traces of hot tracks of the polymeric material in the direction of scanning with the laser beam were noticed. In ABS samples, after the ablation threshold was exceeded, the fragments of the polymeric material were rapidly detached.
Using near-infrared laser ablation in polymers, no significant chemical changes occur, but only changes in the geometrical structure of the sample SL. Irradiation of polymers laser with 1064 nm wavelength corresponds to energies less than chemical bond strengths. The energy of the photon is incapable of directly forcing the molecule to undergo an electronic transition such that a bond is broken [31]. Thus it seems that the photon energy excites vibrations within the molecules (i.e. heat) [32]. This energy in the form of heat is stored in the lower layers of the material. It causes swelling, melting, and cracking as a result of successive impulses of the same places of the material. Subsequent irradiation of the same surface takes place in different conditions than the previous one. The geometric and chemical structure and transparency change. The material seems to be more susceptible to laser radiation. This phenomenon is described as the incubation effect. It is related to the lowering of the ablation threshold as a result of irradiation of the same surface with successive laser pulses. When the incubation factor S = 1, the ablation threshold remains independent of the number of pulses. However, when the damage accumulation mechanism reduces the ablation threshold, the incubation factor decreases. The physical mechanisms underlying incubation are still debated in the scientific community [33,34,35,36,37]. On this basis, it can be assumed with a high probability that in examined polymers, the dominant effect of laser radiation on the sample is photothermal.
The influence of temperature on the thermal degradation of the polymer material was investigated. A thermogravimetric test was made, and DTG curves are shown in Fig. 6.
The maxima of DTG curves visible for each polymer material indicate the highest dynamics of mass loss—thermal degradation of the material [38,39,40,41].
Therefore, it is worth comparing these maxima with the theoretical/model increase in temperature that can be caused by the laser beam. Using laser parameters, the unit laser energy was determined per unit area as:
where: E—the energy of a single laser pulse, SL—cross-sectional area of the laser beam
The unit energy at the ablation threshold was determined:
where: \({P}_{max}\)—maximum value of laser power.
The temperature increase of the polymeric material as a result of the absorption of laser radiation was also determined, according to the model described in detail in the work [42]:
where ρ—density of the polymer, cP—specific heat of the polymer
It should be mentioned here that the estimation of the value of ΔT is theoretical and not factual, and the purposefulness of this action may serve to evaluate the model.
The specific heat value is described in the following equation:
where \({\Phi }_{c}\)—heat flux, cP—specific heat of the polymer, β—speed of temperature change.
Based on the calculation results (Table 5), it was found that in ABS, PS, PET, and PC, the theoretical/model temperature increase is higher than the degradation temperature of this material resulting from calorimetric measurements (TG/DTG). That is why in these materials the ablation process was dynamic. In the case of PLA and PA6, the laser beam in these samples is not able to generate heat, the value of which exceeds the temperature at which the maximum degradation occurs (Fig. 6), hence slight changes in the structure of PA6 material samples and almost zero for PLA material (Fig. 3).
To directly investigate the influence of SL laser modification on adhesive strength, the strength of adhesive connections was measured. The tear strength was determined as follows:
where: F—force, A—the cross-section surface area of the stamp
It is generally known that with the increase in surface roughness of the connected materials, the strength of adhesive connections also increases [43]. The purpose of testing the strength of adhesive connections was to test this theory in relation to the laser-modified SL of the material. The results of measurements and calculations are presented in Fig. 7, where: A—unmodified polymer samples, B—laser-modified polymer samples by the power of laser below the ablation threshold, C—laser-modified polymer samples by the power of laser above the ablation threshold.
Based on the analysis of the obtained test results, it can be shown that modifying SL increases the adhesion between the sample surface and the stamp. In materials where laser ablation took place dynamically, the bond strength of the adhesive connection increased significantly. It is particularly worth noting the increase in the adhesion strength of ABS materials (increase by approx. 390%), PET (increase by approx. 270%), and PS (increase by approx. 230%). On the other hand, for materials with less tendency to laser ablation, the increase in adhesion strength was small.
Laser modification of polymeric materials is often used to shape the adhesive properties of polymeric materials. Laser ablation can lead to oxidizing the SL and changing its geometric structure. It leads to an increase in free surface energy and better adhesive properties. The wettability was investigated, and the SE of the tested polymers was calculated. This was done indirectly by determining the contact angle. The value of the work of adhesion was also calculated. The results of the contact angle and surface free energy tests are summarized in Table 6
Knowledge of SE is of great importance in the processing of polymeric materials. This value is used for a preliminary evaluation of the adhesive properties, e.g. for printing, gluing, laminating, decorating, or metalizing. Using the determined values of adhesive forces and the values of surface free energies, a comparative characteristic of the tested materials before and after laser ablation was plotted (Fig. 8). The analysis of the results presented in Table 6, and Fig. 8 shows a slight influence of the SL state on the value of the adhesion work between the polymer and the measuring liquid.
In almost all cases, the value of the work of adhesion was higher than zero. This fact indicates that the measuring liquid does not completely wet the surface of the modified polymer. There was only one exception—modified PS, which was tested to a wetting test with diiodomethane. According to the commonly accepted definition [18], a decrease in the value of the contact angle is accompanied by an increase in the surface free energy of the tested material, and thus—an increase in the wettability of the material surface. Laser ablation of the tested materials did not significantly change the wettability of the tested materials. Although the SE of the tested material did not change significantly after laser ablation, the adhesive forces did change. This can be explained by the oxidative degradation of the polymer. It takes place at an increased temperature under the influence of oxygen. The laser beam initiates the degradation process due to the local temperature increase of the material. Thermal oxidation can occur throughout the entire volume, not just on the surface of the sample. The value of the accumulated energy exceeds the energy necessary to break the chemical bond. This results in a change in the chemical structure of the material, a decrease in molecular weight, and the formation of cracks and swelling. Physicochemical changes of the SL may take a significant role in the strength of the adhesive bond [44, 45]. In polymers with a lower value of SE, an increase in adhesive connection strength was noticed.
Conclusions
PLA and PA6 were not suitable for laser ablation as they absorbed too little energy to initiate the process. Even at the maximum available power of 20W, the laser beam was unable to raise the sample material's temperature above the polymer degradation temperature. Laser irradiation had no significant effect on the change of surface energy or wettability of the samples, nor did it affect adhesion. To modify PLA and PA6, a higher average laser power is required. In contrast, PC and PET were more dynamic in the laser ablation process, with the NIR laser beam raising the sample material's temperature above the polymer degradation temperature. PET wettability slightly increased, while PC wettability remained similar. SL modification significantly increased adhesion to the surface for PET by 270% and PC by 155%. Laser beam absorption caused fragmentation of the polymer and carbonization of the sample surface. ABS and PS were the most susceptible to laser ablation among the tested materials, with the lowest ablation threshold values. The ablation process resulted in cracks, holes, pores, and cavities in the SL structure. Laser ablation slightly decreased the surface energy and wettability of ABS and PS, but adhesion increased significantly by 390% for ABS and 230% for PS. Laser ablation of ABS and PS required the lowest average laser power among the tested materials, making SL modification of these materials the most energy-efficient.
Despite the poor absorption of NIR laser radiation by most polymers, research shows that it is still possible to modify polymers. The accumulation of photon energy in the incubation process and the physic-chemical changes in the surface layer enable the effective marking of polymers and increase the strength of adhesive bonds.
References
Kuper S, Stuke M (1987) Alzglied physics physics B. laser femtosecond uv excimer laser Ablation. Appl Phys B 44:199–204
Srinivasan V, Smrtic MA, Babu SV (1986) Excimer laser etching of polymers. Cit J Appl Phys 59:3861. https://doi.org/10.1063/1.336728
Vogel A, Venugopalan V (2003) Mechanisms of pulsed laser ablation of biological tissues. Chem Rev 103:577–644
Feng Y, Liu ZQ, Yi X-S (2000) Co-occurrence of photochemical and thermal effects during laser polymer ablation via a 248-nm excimer laser. Appl Surf Sci 156:177–182
Kaino T (2014) Optical absorption of polymers. Encycl Polym Nanomater, pp 1–14. https://doi.org/10.1007/978-3-642-36199-9_118-1
Allen NS (1981) A study of the light absorption properties of polymer films using UV-visible derivative spectroscopy. Polym Photochem 1:43–55. https://doi.org/10.1016/0144-2880(81)90014-2
Manjunatha MG, Adhikari AV, Hegde PK et al (2009) Optical characterization of a new Donor-acceptor type conjugated polymer derived from 3,4-diphenylthiophene. J Mater Sci 44:6069–6077. https://doi.org/10.1007/S10853-009-3838-4
Becker H, Gärtner C (2000) Polymer microfabrication methods for microfluidic analytical applications. Electrophoresis 21:12–26
Augustyn P, Rytlewski P, Moraczewski K, Mazurkiewicz A (2021) A review on the direct electroplating of polymeric materials. J Mater Sci 56:14881–14899. https://doi.org/10.1007/S10853-021-06246-w
Yeh JTC (1986) Laser ablation of polymers. J Vac Sci Technol A Vacuum, Surfaces, Film 4:653–658. https://doi.org/10.1116/1.573823
Matuszewski M (2010) Zużycie masowe elementów pary ciernej z powierzchniami anizotropowymi. Tribologia 41:93–102
Dobrzański LA, Dobrzańska A. (2011) Zmiany struktury i własności powierzchni materiałów inżynierskich w wyniku eksploatacji. In: Obróbka powierzchni materiałów inżynierskich. Open Access Library Volume 5 2011, pp 368–416
Wojciechowski Ł (2009) The evaluation methods of surface layer energy conditions. Tribologia 6:137–154
Porejko S, Zakrzewski L (1974) Chemia związków wielkocząsteczkowych. WNT, Warszawa
Sikorski T (1985) Podstawy chemii i technologii polimerów. PWN, Warszawa
Stępak B, Antończak AJ, Abramski KM (2016) Optimization of femtosecond laser cutting of biodegradable polymer for medical devices manufacturing. Photonics Lett Pol 8:116–118. https://doi.org/10.4302/plp.2016.4.09
Hendricks F, Patel R, Matylitsky V V. (2015) Micromachining of bio-absorbable stents with ultra-short pulse lasers. Front Ultrafast Opt Biomed Sci Ind Appl XV 9355:935502. https://doi.org/10.1117/12.2079014
Żenkiewicz M, Karasiewicz T, Moraczewski K, et al (2012) Metody badań i oceny niektórych właściwości tworzyw polimerowych i metali. Wydaw. Uniwersytetu Kazimierza Wielkiego, Bydgoszcz
Firlik S, Borycki J, Molenda J (2010) Porównanie metod wyznaczania swobodnej energii powierzchniowej polimerowych powłok orientujących ciekle kryształy. Chemik 64:238–245
Wojciechowski Ł (2009) The contemplation on the utilisation of free surface energy related to the characteristics of energy conditions of surface layers. Tribologia 1:177–196
Czyż K, Strzelec M, Marczak J et al (2016) Bezpośrednia laserowa litografia interferencyjna w periodycznej obróbce biomateriałów. Bull Mil Univ Technol 65:31–53. https://doi.org/10.5604/12345865.1228618
Marczak J (2008) Metoda akustyczna i kolorymetryczna do określania stopnia oczyszczenia obiektów kamiennych czyszczonych ablacją laserową - Prace Instytutu Elektrotechniki - Tom Z. 234 (2008). PAK 53:364–367
Ravi-Kumar S, Lies B, Zhang X et al (2019) Laser ablation of polymers: a review. Polym Int 68:1391–1401
Mele A, Giardini Guidoni A, Kelly R et al (1997) Laser ablation of metals: Analysis of surface-heating and plume-expansion experiments. Appl Surf Sci 109–110:584–590. https://doi.org/10.1016/S0169-4332(96)00742-8
Phipps CR (2007) Laser Ablation and its Applications. Springer Nature, Switzerland, https://books.google.pl/books?hl=pl&lr=&id=iQjSBwAAQBAJ&oi=fnd&pg=PA1&dq=laser+ablation+threshold+papers&ots=wE-NCGIL97&sig=JOVNPHfRi9QebMfK2h1moOdBtDo&redir_esc=y#v=onepage&q=laser%20ablation%20threshold%20papers&f=false. Accesed 2022–09–20
Ludbrook J Linear regression analysis for comparing two measurers or methods of measurement: But which regression? https://doi.org/10.1111/j.1440-1681.2010.05376.x
Schneider A, Hommel G, Blettner M (2010) Lineare regressionsanalyse - Teil 14 der serie zur bewertung wissenschaftlicher publikationen. Dtsch Arztebl 107:776–782
Lippert T (2010) UV Laser ablation of polymers: from structuring to thin film deposition. Springer Ser Mater Sci 130:141–175. https://doi.org/10.1007/978-3-642-03307-0_7/COVER
Gerle A, Wałęga-Chwastek H, Kalarus A (2011) Badania porównawcze odporności na wstrząsy cieplne materiału magnezytowego, Praca badawcza, Instytut Ceramiki i Materiałów Budowlanych w Warszawie, Oddział Materiałów Ogniotrwałych w Gliwicach, Gliwice.
Orłoś Z, (1991) Naprężenia cieplne PWN, Warszawa.
Yalukova O, Sárady I (2006) Investigation of interaction mechanisms in laser drilling of thermoplastic and thermoset polymers using different wavelengths. Compos Sci Technol 66:1289–1296. https://doi.org/10.1016/j.compscitech.2005.11.002
Garrison B, Srinivasan R (1985) Laser ablation of organic polymers: Microscopic models for photochemical and thermal processes. J Appl Phys 57:2909–2914
Gómez D, Goenaga I (2006) On the incubation effect on two thermoplastics when irradiated with ultrashort laser pulses: broadening effects when machining microchannels. Appl Surf Sci 253:2230–2236
Račiukaitis G, Brikas M, Gečys P et al (2009) Use of high repetition rate and high power lasers in microfabrication: How to keep the efficiency high? J Laser Micro Nanoeng 4:186–191. https://doi.org/10.2961/jlmn.2009.03.0008
Mannion PT, Magee J, Coyne E et al (2004) The effect of damage accumulation behaviour on ablation thresholds and damage morphology in ultrafast laser micro-machining of common metals in air. Appl Surf Sci 233:275–287. https://doi.org/10.1016/j.apsusc.2004.03.229
Raciukaitis G, Brikas M, Gecys P, Gedvilas M (2008) Accumulation effects in laser ablation of metals with high-repetition-rate lasers. In: High-Power Laser Ablation VII. SPIE, p 70052L
McCann R, Bagga K, Groarke R et al (2016) Microchannel fabrication on cyclic olefin polymer substrates via 1064 nm Nd:YAG laser ablation. Appl Surf Sci 387:603–608. https://doi.org/10.1016/j.apsusc.2016.06.059
Menczel JD, Prime RB (2008) Thermal Analysis of Polymers: Fundamentals and Applications. In: Thermogravimetric analysis (TGA). John Wiley & Sons, Ltd, Hoboken
Szumera M (2012) Charakterystyka wybranych metod termicznych. Cz. 1. LAB Laboratoria, Apararatura, Badania R17, 6:28–34
Porowski R (2017) Wprowadzenie do analizy termicznej polimerów. Zeszyty Naukowe SGSP/Szkoła Główna Służby Pożarniczej 64(4):67–87
Kosmalska D, Kaczmarek H, Malinowski R, Bajer K (2019) Advances in studies of thermal degradation of polymeric materials Part II. The influence of various factors on the thermal degradation of polymeric materials during their processing. Polimery/Polymers 64:317–326. https://doi.org/10.14314/polimery.2019.5.1
Rytlewski P (2015) Studium laserowego i plazmowego modyfikowania warstwy wierzchniej materiałów polimerowych. Wydawnictwo Uniwersytetu Kazimierza Wielkiego, Bydgoszcz
Żenkiewicz M (2002) Adhezja i modyfikowanie warstwy wierzchniej tworzyw. WNT, Warszawa
M. Rojek (2011) Metodologia badań diagnostycznych warstwowych materiałów kompozytowych o osnowie polimerowej. Open Access Libr 2:11–31 moz-extension://6321e781–5c50–47bc-b0a2–919206b714b4/enhanced-reader.html?openApp&pdf=http%3A%2F%2F. www.openaccesslibrary.com%2Fvol02%2Fvol02_2.pdf. Accesed 2023–02–20
Chmielewski AG, Zimek Z (2017) Wykorzystanie promieniowania jonizującego w przemyśle. Instytut Chemii i Techniki Jądrowej, Warszawa
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they are not affiliated with any organization or anyone who has a direct financial contribution to the subject of research or materials studied in a given work
(e.g. employment, consulting, shareholding, fees).
Reprint permissions for illustrations
I declare that the illustrations and graphics were made by me, and I have all rights to them
Additional information
Handling Editor: Maude Jimenez.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Augustyn, P., Rytlewski, P., Moraczewski, K. et al. Ablation of selected thermoplastic polymers using an Nd:YAG laser. J Mater Sci 58, 9073–9086 (2023). https://doi.org/10.1007/s10853-023-08566-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08566-5