Abstract
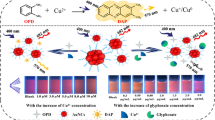
The interaction between psoralen (PSA) and cucurbit[8]uril (Q[8]) was studied by UV spectroscopy, fluorescence spectroscopy, infrared spectroscopy, 1H NMR and X-ray crystal diffraction. The results showed that PSA and Q[8] can form a 2:1 host–guest inclusion complex with a binding constant of 1.83 × 106 M−2. PSA and PSA2@Q[8] can be used as fluorescent probes to selectively recognize Fe3+. The recognition mechanism is due to the coordination effect of Fe3+, the electron transfer in PSA and PSA2@Q[8] reduces fluorescence intensity and leads to fluorescence quenching. The fluorescence intensity of PSA and PSA2@Q[8] showed a good linear correlation with the concentration of Fe3+ at 3.0 × 10–5–2.4 × 10–4 M and 6.0 × 10–6–4.2 × 10–5 M, and the limit of detection was 1.06 × 10–7 M and 1.05 × 10–8 M, respectively, which can quantitatively detect trace Fe3+ in aqueous solution.














Similar content being viewed by others
References
Kagit, R., Yildirim, M., Ozay, O., Yesilot, S., Ozay, H.: Phosphazene based multicentered naked-eye fluorescent sensor with high selectivity for Fe3+ ions. Inorg. Chem. 53, 2144–2151 (2014). https://doi.org/10.1021/ic402783x
Brugnara, C.: Iron deficiency and erythropoiesis: new diagnostic approaches. Clin. Chem. 49, 1573–1578 (2003). https://doi.org/10.1373/49.10.1573
Zhang, X.-B., Cheng, G., Zhang, W.-J., Shen, G.-L., Yu, R.-Q.: A fluorescent chemical sensor for Fe3+ based on blocking of intramolecular proton transfer of a quinazolinone derivative. Talanta 71, 171–177 (2007). https://doi.org/10.1016/j.talanta.2006.03.036
Cabon, J.Y., Giamarchi, P., Le Bihan, A.: Determination of iron in seawater by electrothermal atomic absorption spectrometry and atomic fluorescence spectrometry: a comparative study. Anal. Chim. Acta 664, 114–120 (2010). https://doi.org/10.1016/j.aca.2010.02.014
Saracoglu, S., Soylak, M., Peker, D.S.K., Elci, L., dos Santos, W.N.L., Lemos, V.A., Ferreira, S.L.C.: A pre-concentration procedure using coprecipitation for determination of lead and iron in several samples using flame atomic absorption spectrometry. Anal. Chim. Acta 575, 133–137 (2006). https://doi.org/10.1016/j.aca.2006.05.055
Bakkaus, E., Collins, R.N., Morel, J.-L., Gouget, B.: Anion exchange liquid chromatography–inductively coupled plasma-mass spectrometry detection of the Co2+, Cu2+, Fe3+ and Ni2+ complexes of mugineic and deoxymugineic acid. J. Chromatogr. A 1129, 208–215 (2006). https://doi.org/10.1016/j.chroma.2006.07.004
Pomazal, K., Prohaska, C., Steffan, I., Reich, G., Huber, J.F.K.: Determination of Cu, Fe, Mn, and Zn in blood fractions by SEC-HPLC-ICP-AES coupling. Analyst 124, 657–663 (1999). https://doi.org/10.1039/A809688K
Bobrowski, A., Nowak, K., Zarębski, J.: Application of a bismuth film electrode to the voltammetric determination of trace iron using a Fe(III)–TEA–BrO3−catalytic system. Anal. Bioanal. Chem. 382, 1691–1697 (2005). https://doi.org/10.1007/s00216-005-3313-2
Zhang, J.F., Zhou, Y., Yoon, J., Kim, J.S.: Recent progress in fluorescent and colorimetric chemosensors for detection of precious metal ions (silver, gold and platinum ions). Chem. Soc. Rev. 40, 3416–3429 (2011). https://doi.org/10.1039/C1CS15028F
Wu, D., Chen, L., Lee, W., Ko, G., Yin, J., Yoon, J.: Recent progress in the development of organic dye based near-infrared fluorescence probes for metal ions. Coord. Chem. Rev. 354, 74–97 (2018). https://doi.org/10.1016/j.ccr.2017.06.011
Sedgwick, A.C., Wu, L., Han, H.-H., Bull, S.D., He, X.-P., James, T.D., Sessler, J.L., Tang, B.Z., Tian, H., Yoon, J.: Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 47, 8842–8880 (2018). https://doi.org/10.1039/c8cs00185e
Park, S.-H., Kwon, N., Lee, J.-H., Yoon, J., Shin, I.: Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 49, 143–179 (2020). https://doi.org/10.1039/c9cs00243j
Marshall, M.E., Conley, D., Hollingsworth, P., Brown, S., Thompson, J.S.: Effects of coumarin (1,2-benzopyrone) on lymphocyte, natural killer cell, and monocyte functions in vitro. J. Biol. Response Modif. 8, 70–85 (1989). https://doi.org/10.1016/S0090-8258(89)80046-0
Wang, X., Cheng, K., Han, Y., Zhang, G., Dong, J., Cui, Y., Yang, Z.: Effects of psoralen as an anti-tumor agent in human breast cancer MCF-7/ADR cells. Biol. Pharm. Bull. 39, 815–822 (2016). https://doi.org/10.1248/bpb.b15-00957
Ng, T.B., Liu, F., Wang, Z.T.: Antioxidative activity of natural products from plants. Life Sci. 66, 709–723 (2000). https://doi.org/10.1016/S0024-3205(99)00642-6
Ren, Y., Song, X., Tan, L., Guo, C., Wang, M., Liu, H., Cao, Z., Li, Y., Peng, C.: A review of the pharmacological properties of psoralen. Front. Pharmacol. (2020). https://doi.org/10.3389/fphar.2020.571535
Tian, G., Zhang, Z., Li, H., Li, D., Wang, X., Qin, C.: Design, synthesis and application in analytical chemistry of photo-sensitive probes based on coumarin. Crit. Rev. Anal. Chem. 51, 565–581 (2021). https://doi.org/10.1080/10408347.2020.1753163
Barrow, S.J., Kasera, S., Rowland, M.J., del Barrio, J., Scherman, O.A.: Cucurbituril-based molecular recognition. Chem. Rev. 115, 12320–12406 (2015). https://doi.org/10.1021/acs.chemrev.5b00341
Assaf, K.I., Nau, W.M.: Cucurbiturils: from synthesis to high-affinity binding and catalysis. Chem. Soc. Rev. 44, 394–418 (2015). https://doi.org/10.1039/c4cs00273c
Masson, E., Ling, X.X., Joseph, R., Kyeremeh-Mensah, L., Lu, X.Y.: Cucurbituril chemistry: a tale of supramolecular success. Rsc. Adv. 2, 1213–1247 (2012). https://doi.org/10.1039/c1ra00768h
Shetty, D., Khedkar, J.K., Park, K.M., Kim, K.: Can we beat the biotin-avidin pair?: Cucurbit 7 uril-based ultrahigh affinity host-guest complexes and their applications. Chem. Soc. Rev. 44, 8747–8761 (2015). https://doi.org/10.1039/c5cs00631g
Samanta, S.K., Moncelet, D., Briken, V., Isaacs, L.: Metal-organic polyhedron capped with cucurbit 8 uril delivers doxorubicin to cancer cells. J. Am. Chem. Soc. 138, 14488–14496 (2016). https://doi.org/10.1021/jacs.6b09504
Lazar, A.I., Biedermann, F., Mustafina, K.R., Assaf, K.I., Hennig, A., Nau, W.M.: Nanomolar binding of steroids to cucurbit n urils: selectivity and applications. J. Am. Chem. Soc. 138, 13022–13029 (2016). https://doi.org/10.1021/jacs.6b07655
Liao, P.-Q., Huang, N.-Y., Zhang, W.-X., Zhang, J.-P., Chen, X.-M.: Controlling guest conformation for efficient purification of butadiene. Science 356, 1193–1196 (2017). https://doi.org/10.1126/science.aam7232
Wu, M.-X., Yang, Y.-W.: Metal-organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 29, 1606134 (2017). https://doi.org/10.1002/adma.201606134
Karcher, S., Kornmüller, A., Jekel, M.: Effects of alkali and alkaline-earth cations on the removal of reactive dyes with cucurbituril. Acta Hydrochim. et Hydrobiol. 27, 38–42 (1999). https://doi.org/10.1002/(SICI)1521-401X(199901)27:1%3C38::AID-AHEH38%3E3.0.CO;2-U
Karcher, S., Kornmüller, A., Jekel, M.: Removal of reactive dyes by sorption/complexation with cucurbituril. Water Sci. Technol. 40, 425–433 (1999). https://doi.org/10.1016/S0273-1223(99)00526-0
Shinde, M.N., Choudhury, S.D., Barooah, N., Pal, H., Bhasikuttan, A.C., Mohanty, J.: Metal-ion-mediated assemblies of thiazole orange with cucurbit 7 uril: a photophysical study. J. Phys. Chem. B 119, 3815–3823 (2015). https://doi.org/10.1021/jp512802u
Xu, Y., Panzner, M.J., Li, X., Youngs, W.J., Pang, Y.: Host–guest assembly of squaraine dye in cucurbit[8]uril: its implication in fluorescent probe for mercury ions. Chem. Commun. 46, 4073–4075 (2010). https://doi.org/10.1039/C002219P
Zeng, Z.-S., Zhang, Y.-Q., Zhang, X.-D., Luo, G.-Y., **e, J., Tao, Z., Zhang, Q.-J.: Selective detection of Zn2+ and Cd2+ ions in water using a host-guest complex between chromone and Q[7]. Chin. Chem. Lett. 32, 2572–2576 (2021). https://doi.org/10.1016/j.cclet.2021.03.071
Thordarson, P.: Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 40, 1305–1323 (2011). https://doi.org/10.1039/C0CS00062K
Ding, S.-Y., Dong, M., Wang, Y.-W., Chen, Y.-T., Wang, H.-Z., Su, C.-Y., Wang, W.: Thioether-based fluorescent covalent organic framework for selective detection and facile removal of mercury(II). J. Am. Chem. Soc. 138, 3031–3037 (2016). https://doi.org/10.1021/jacs.5b10754
Zhao, B., Liu, T., Fang, Y., Wang, L., Song, B., Deng, Q.: Two ‘turn-off’ Schiff base fluorescence sensors based on phenanthro[9,10-d]imidazole-coumarin derivatives for Fe3+ in aqueous solution. Tetrahedron Lett. 57, 4417–4423 (2016). https://doi.org/10.1016/j.tetlet.2016.08.064
Kargar, H., Fallah-Mehrjardi, M., Behjatmanesh-Ardakani, R., Munawar, K.S., Ashfaq, M., Tahir, M.N.: Diverse coordination of isoniazid hydrazone Schiff base ligand towards iron(III): synthesis, characterization, SC-XRD, HSA, QTAIM, MEP, NCI, NBO and DFT study. J. Mol. Struct. 1250, 131691 (2022). https://doi.org/10.1016/j.molstruc.2021.131691
Funding
The Science and Technology Support Plan of Guizhou Province [GuiZhou Science and Technology Cooperation Support (2020)4Y218] is acknowledged.
Author information
Authors and Affiliations
Contributions
Xuanxun Wang and Qianjun Zhang wrote the main manuscript text and Guangyan Luo prepared Fig. 9. Other authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Luo, G., Zhang, L. et al. Study on the recognition of psoralen and psoralen@cucurbit[8]uril fluorescent probe for Fe3+ ions. J Incl Phenom Macrocycl Chem 102, 893–903 (2022). https://doi.org/10.1007/s10847-022-01169-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-022-01169-8