Abstract
Since 20 years, titanium oxide materials and in particular, lithium titanate spinel Li4Ti5O12 (LTO) were considered as promising negative electrode materials for lithium-ion cells. In the case of the TiNb2O7 compound (TNO), which has a larger theoretical lithiation capacity of 388 mAh g−1, few studies have focused on the outgassing of the material. In this work, we quantified the volume produced as a function of different cycling parameters and then characterized the produced species and show a prevalence of H2 gas. The use of carbon coating as a mitigation strategy and its limitation is also discussed.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rechargeable lithium-ion batteries (LiBs) have been widely used as power sources for various applications, including portable electronic devices, electrical vehicles and large-scale energy storage [24].
Quickly becoming the most used technology, LiBs cells mainly use graphite as negative electrode material because of its low cost and good theoretical capacity (372 mAh g−1) [4]. However, this electrode suffer from stability issues, such as thermal runaway [19, 20] that is usually the final consequence of uncontrolled SEI growth, lithium plating, and dendrite formation after cycling in hard conditions.
Alternatively negative electrodes using titanates such as lithium titanate spinel \(\text{Li}_{4}\text{Ti}_{5}\text{O}_{12}\) (LTO) have been extensively studied. Its high operating voltage preventing lithium plating as well as its long cycle life (> 20 000 cycles) and its ultra-low volume change during the lithium insertion [7] made this technology a good solution to substitute graphite electrode. However, a low specific capacity (175 mAh g−1) [28] as well as a gassing phenomenon drastically hinder the deployment of this material [3].
With this drawback in mind, Goodenough et al. showed in 2011 that TiNb2O7 (TNO) can be used as a negative electrode material [11], kee** LTO’s advantages (e.g., high power ability, long life, and ultra-safe performance in abusive conditions) but offering a higher theoretical capacity of 388 mAh g−1. This value is almost twice the one of LTO due to five exchangeable electrons (Ti4+/Ti3+, Nb5+/Nb3+) and comparable to the one of graphite [6].
Widely studied for LTO [12, 14, 16], titanates materials are well known for their gassing behavior. However, only few studies have been carried out on this specific point for TNO [5, 21].
Buannic et al. [5] highlighted a strong dependence of generated gases volume to the specific surface of the active material. They have also reported that gassing phenomenon could be more intense in TNO cells than in LTO’s ones, pointing out the presence of niobium cation at the surface of the material.
More recently, Parikh et al. [21] reported the first operando gas analysis on TNO-NMC cell, confirming the hypothesis that the gas generated is the result of the decomposition of the solvent in the electrolyte during the formation of a pseudo-SEI on the surface of the active material grains. The formation of SEI has also been observed using various characterization techniques [X-ray photoelectron spectroscopy (XPS), Fourier transform infrared (FTIR), scanning electron microscopy (SEM), and high-resolution transmission electron microscopy (HRTEM)] by Wu et al. [27]. They also show that using a carbon coating could lead to a drastic reduction of the generated volume.
In this study, we chose to characterize the volume of gas produced by the technique of Archimedes weighing on pouch cell as well as the chemical species produced during cycling by online electrochemical mass spectrometry operando.
2 Methods
2.1 Material synthesis
The mixed oxide TiNb2O7 was prepared via a simple one-step solid-state synthesis.
Titanium oxide (TiO2 anatase, 99.0%, Huntsman) and niobium pentoxide (\(\text{Nb}_{2}\text{O}_{5}\), 99.9%, Aldrich) powders have been used as precursors and introduced in stoichiometric amounts. The mix was ball milled (PM 100 CM, Retsch) 4 h at 400 rpm using agate bowl and balls. Finally, the product was calcined at 1100 °C, for 16 h in a muffle furnace under air.
The coated material was obtained by mixing polyvinyl alcohol (PVA, Solvay), used as a carbonaceous precursor, with TNO in a mortar and then decomposing the mixture in a tubular furnace under inert atmosphere (Argon) at 800 °C for 8 h. Final product has been characterized in TGA under air and its carbon content evaluated to 0.85 wt%.
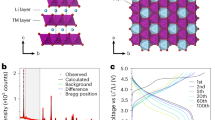
After cooling, the powder was crushed manually with a mortar and pestle to reduce its granulometry. To ensure the purity of the synthesized phase, X-ray powder diffraction (XRD) was carried out on a Brüker D8 Advance using a Cu anticathode. This reveals a pure TiNb2O7 material as shown in Fig 1.
The particle size distribution (PSD) was obtained on a Malvern Mastersizer MS-S.
2.2 Electrochemical characterization
Electrodes are made from an organic formulation. Firstly, our active material was mixed with an electronic conductor (Super P C65 from Imeris) in cyclohexane. After total evaporation of the latter, the binder was added with a solution of PVDF Solef 1530 (Arkema) at 10 wt% in N-methyl-2-pyrrolidone (NMP, Sigma Aldrich) until a formulation of 80% active material, 10% electronic conductor 10% binder, and a dry extract of 30% was reached.
The slurries were then coated onto an aluminum foil. Two different coating processes were used. Lab-scale electrode was produced using a laboratory coating table (Elcometer 4330) and pilot-scale electrode was produced on an Upscale Coating Machine built by Ingecal.
Both kinds of electrodes were then calendered and dried. Their typical loading is 1.8 mg cm−2 corresponding to a theoretical surface capacity of 0.7 mAh cm−2. With a density of 1.35, the electrodes have a porosity of 60% after calendering.
To allow a comparison of the gassing of the two materials, \(\text{Li}_{4}\text{Ti}_{5}\text{O}_{12}\) electrodes were made in the same way from active materials supplied by PRAYON.
For electrochemical measurements, we used a full cell set-up. Counter electrodes were made following the same process, using \(\text{LiFePO}_{4}\) (LFP further), industrial grade supplied by PRAYON, displaying a loading of 0.82 mAh cm−2 and achieving a balancing of 120% vs. negative electrodes ensuring full use of the negative electrode. We choose this compound rather than a NMC material type because of its great stability and its flat voltage plateau.
Pouch cells were assembled under controlled atmosphere (− 20 °C dew point), by stacking 2 single-side-coated TNO electrodes (\(3.5\times 3.5\) cm, 24.5 cm2) and a double-side-coated LFP electrode (\(3.75\times 3.75,\) 28.2 cm2), each side separated by a polypropylene separator (CG2500, Celgard). 800 μL of a solution of ethylene carbonate (EC), propylene carbonate (PC), dimethyl carbonate (DMC) (1:1:3 vol), and Lithium hexafluorophosphate \(\text{LiPF}_{6}\) (1 M) (LP100, UBE Industries) were used to activate the cell.
2.3 Gases studies
2.3.1 Qualitative studies
Operando gas analysis required the use of specially designed cells, ECC-Air cell provided by El-Cell®, allowing argon circulation. Circular single-side electrodes of LFP and TNO with a diameter of 16 mm were assembled with polypropylene separator (CG2500, Celgard) in an Argon-filled glovebox ([\(\text{H}_{2}\text{O}\)] and [O2] < 0.1 ppm) and activated with 150 μL of EC, PC (1:1) with 2% vinylene carbonate (VC) and Lithium hexafluorophosphate \(\text{LiPF}_{6}\) (1 M) (UBE Industries). This electrolyte is less volatile due to the absence of DMC and limits the risk of saturation of the mass spectrometer sensor. Pure argon flow of 2.2 mL min−1 was used through the El cell to push the gases formed during the electrochemical reactions to a mass spectrometer. Pfeiffer Omnistar GSD320 mass spectrometer was used for the gas analysis.
Galvanostatic discharges and charges were performed on a Bio-logic VMP laboratory battery tester at C/10 rate (assuming C = 388 mAh g−1) in a potential range within 2.6–0.8 V vs. Li+/Li.
2.3.2 Quantitative studies
The measurement of the produced gas volumes, either following a calendar aging or during cycling, was carried out using the Archimedes density method. Initially introduced by Aiken et al. [1], this technique is based on Archimedes’ principle 1.
with \(F_{\rm Archimedes}\) is the Archimedes’ principle, \(\rho \) is the fluid density, V is the submerged volume in m3, and g is the acceleration of gravity in m s−2,
Pouch cells were weighed with a precision scale, in air and immersed in an ethanol solution. The apparent weight \(P_{\rm App}\) can be written as in the relation 2:
with \(P_{\rm App}\) the apparent weight and \(P_{\rm Real}\) obtained from the mass.
Archimedes’ principle can be described with the following relation 3:
with \(P_{\rm Fluid}\), the weight of displaced liquid, \(\rho_{\rm Fuid}\) the fluid density, and \(V_{\rm Fluid}\) the fluid volume.
By defining \(\Delta P\) as \(\Delta P= P_{\rm Real} - P_{\rm App}\), we obtain the relation 4 allowing to know the volume of the cell \(V_{\rm Cell}\):
The measurement of the temperature of the fluid, in our case absolute ethanol (CARLO ERBA Reagents), makes it possible to determine its exact density via the relation 5 [8].
Measurements have been carried out either after periods of calendar storage of the cells at room temperature (22 °C) or after cycling. In all cases, for each considered series of samples, the same cell was weighed and the plotted volume mean the measured volume of gas at the time mentioned (number of cycles or storage time).
For relative comparison, even if the electrode loadings were designed to be nearly the same, every measured gases volume was normalized by the negative (either TNO or LTO) active material mass.
3 Results and discussion
3.1 Volume of gas produced in LFP/TNO cells
Figure 2 displays both discharge capacity and gas volume produced during cycling in different setups. It can be noted that, despite a doubled specific discharge capacity compared to the LTO cell, the TNOs based cells also generated almost twice the gas volume, regardless of the electrode processing method used. For lab-scale electrodes, the gas volume produced upon cycling is almost three times higher than for the LTO electrode.
Comparison of the volume of produced gas (triangle, right scale) and electrochemical performances (square, left scale) for the TNO according to the type of coating (TiNb2O7- Upscale Coating Machine, Black series and Laboratory Coated, Red series) and the LTO (\(\text{Li}_{4}\text{Ti}_{5}\text{O}_{12}\) Laboratory Coated, Blue series). The small jumps in capacity observed around 25, 50, and 85 cycles are linked to isolated trouble of thermal regulation in the cycling chamber. (Color figure online)
It seems that the physicochemical reaction, leading to the gas formation does not follow the same reaction path: in case of LTO, we note a very quick stabilization of the produced volume, without any evolution after 10 cycles, whereas for both TNO cases, the gas seems to be continuously evolved during the whole experiment.
We can suppose that the passivation layer formed on the surface of the electrodes is not stable and fractures at each cycle, following the important expansion of the particles observed in in situ X-ray diffraction [10], leading to a constant gas formation [2].
We have also noticed that the coating method used to produce the electrodes plays a very important role for the electrochemical performances as well as for the gas production during cycling. Both TNO electrodes were designed to have similar characteristics (loading, porosity, etc.) but the use of pilot-scale coating bench leads to a better homogeneity allowing a more efficient percolation for the electronic network. In consequence, we can notice a better electrochemical performance as well as a higher mechanical resistance of the electrode during cycling, which probably leads to an improved accommodation of the volume expansion of the material and limits the constant reformation of the passivation layer.
Following these observations, we also considered the use of a carbon coating on the material to improve its electrochemical behavior and try to reduce the gassing phenomenon. This solution have been widely studied for different kinds of active materials in lithium-ion battery field [15] and in the LTO case in particular by He et al., showing a higher stability of the passivation layer and a lower gassing production inside the cell [13].
Figure 3 shows the result obtained for both carbon-coated and raw TNO. Carbon-coated material has been synthesized while targeting a final formulation with 0.8 wt% of C. This formulation had previously demonstrated interesting performance in rigid casing.
It can be noticed that the insertion capacity is initially much higher than the one of the uncoated TiNb2O7 material. However, this trend is reversed after 40 cycles when the insertion capacities of the coated sample fell below those of the untreated compound. In terms of gas volume, the TiNb2O7/C composite produces about 10% less gas, which proves a positive effect of the treatment.
It seems that the carbon coating limits the formation of gas during cycling. It is possible that coating the TNO particles with a thin layer of carbon creates an interface between the surface of the active material and the electrolyte, thus preventing/limiting the decomposition of the electrolyte on the surface of the TNO grains.
However, by improving the insertion capacities on the first slow cycles through an optimization of the contacts between the active particles and the network of carbonaceous electronic conductors, it is likely that the internal degradation of the material is also accelerated.
If we consider the volume of gas generated during the storage at room temperature, i.e., without imposed electrochemical activity for two identical material samples, as it is shown in Fig. 4, the carbon coating leads to a 60% gas volume reduction compared to the untreated compound.
It can also be noted that, for both measured samples, the production of gas seems to take place almost immediately. The volume of gas produced hardly changes after 7 days and the maximum value is reached after 14 days, showing an internal electrochemical process, leading to the formation of passivation layer onto the grains, mainly composed of a degraded electrolyte [2].
Gas production versus times of calendar stored cells for two different negative lab-coated electrodes: TiNb2O7 (TNO, black solid square series) and TiNb2O7 carbon-coated (TNO/C, green solid square series). For comparison, the volume of gas generated during cycling (C/10) as a function of time has also been added: TiNb2O7 (TNO, black solid circle series) and TiNb2O7 carbon-coated (TNO/C, green solid circle series). (Color figure online)
The comparison between the amounts of gas formed with and without cycling as a function of a duration of the measurements brings new elements to the discussion. It appears that the volume of gas generated in the case of calendar storage reaches its maximum value more quickly before staying a plateau, generating even more gas during the first 7 days than in the case of cycling, whatever the studied material is. This fact supports our hypothesis that a contact of TNO with the electrolyte generates an interfacial layer resulting from the electrolyte degradation and this process forms a certain volume of gas. However, in the case of cycling, the significant evolution of the particle size will lead to a continuous regeneration of this layer and consequently of the gas.
Therefore, the carbon coating appears to be a limited solution. The electrode formulation and the carbon coating ratio still could be optimized; however, most probably cannot solve all the problems.
Another critical point could be the quality of the used electrolyte and more specifically, the water content. The impact of high hydration on TNOs cycling performance and gas production was qualified. Thus, we examined the electrochemical performances and volume of gas produced by two cells, where one was activated with a 300 ppm hydrated electrolyte and the other one with a standard electrolyte. The results are reported in Fig. 5.
Influence of electrolyte water content on the electrochemical performance and gassing behavior of LFP/TNO cells: Comparison of the volume of gas produced (triangle, right scale) and electrochemical performances (square, left scale) for the two electrolyte “grades”: the battery grade ([\(\text{H}_{2}\text{O}]<20\) ppm) (TiNb2O7, Black series) and the hydrated electrolyte ([\(\text{H}_{2}\text{O}\)] = 300 ppm) (\(\text{TiN}\text{b}_{2}\text{O}_{7}+ \text{H}_{2}\text{O}\), Red series)
As expected, the cell containing 300 ppm of \(\text{H}_{2}\text{O}\) shows a significant loss of capacity compared to its non-hydrated counterpart. Similarly, gas generation is strongly impacted by the addition of water. The volume of gas produced in the case of the hydrated electrolyte increases by almost 30%. Indeed, it is commonly accepted that an excessive level of water in the system, which may be due to poor electrode drying or hydrated electrolyte, could lead to a generation of a large volume of gas through an electrolysis mechanisms at the surface of the electrodes [18].
3.2 Analysis of produced gaseous chemical species during cycling
To identify the gases formed during cycling, the use of online electrochemical mass spectrometry was necessary. This technique allows detection and identification of gases evolved by measuring their mass-to-charge ratios (m/z). Six different signals were monitored operando during the electrochemical test: m/z = 2 for \(\text{H}_{2}\), m/z = 26 for \(\text{C}_{2}\text{H}_{4}\), m/z = 18 for \(\text{H}_{2}\text{O}\), m/z = 28 for \(\text{CO}\), m/z = 41 for \(\text{C}_{3}\text{H}_{6}\), and m/z = 44 for \(\text{CO}_{2}.\)
A TiNb2O7-based electrode was assembled facing a \(\text{LiFePO}_{4}\) counter electrode in an ECC-Air cell. LFP-based counter electrode is beneficial for this kind of measurements due to the fact that does not generate gas during cycling [22]. Therefore, the gas observed during this experiment originates only from the TNO-based electrode. The electrolyte had to be adapted using a mixture of EC:PC (1:1) + 2% VC, 1 M \(\text{LiPF}_{6}\) to reduce its vapor pressure and to avoid penetration of organic carbonate solvents vapors to the mass spectrometer. Cycling at C/10 was carried out over 11 cycles using a potentiostat. The results are shown in Fig. 6.
Plotting the cell voltage together with corresponding mass spectrometry signals allowed identifying the potential windows and onset voltages for the gas formation. Considering the cycles 1 and 2, it can be seen from the curve A in Fig. 6 reflecting the formation of \(\text{H}_{2}\) that the generation of gas takes place at the end of the charges with an onset voltage of around 2.0 V and a peak corresponding to the end of charge. In addition, there is minor \(\text{H}_{2}\) contribution at the end of discharges. Remarkable gas formation peaks were also detected for \(\text{C}_{2}\text{H}_{4}\), \(\text{C}_{3}\text{H}_{6}\), \(\text{CO}\), and \(\text{CO}_{2}\) during the first cycles at the end of charges where the potential of a lithiated TNO electrode is the lowest. These observations are in line with our hypothesis suggesting a formation of an interfacial layer on the surface of TNO-based electrode in contact with the electrolyte. It is known that the electrochemical reduction of EC mostly leads to formation of \(\text{C}_{2}\text{H}_{4}\), while a reductive decomposition of PC leads to \(\text{C}_{3}\text{H}_{6}\). VC can be responsible for a \(\text{CO}_{2}\) production at low potentials [23].
Looking at overall trends for formation of gases during 11 cycles, hydrogen seems to be the predominant gas produced constantly during the electrochemical test. It seems to be a very similar case to LTO-containing LIBs where continuous \(\text{H}_{2}\) evolution was detected [25, 26]. Up to now, there is no consensus in literature concerning a precise mechanism for \(\text{H}_{2}\) formation during cycling/storage of LTO-based cells. Among the main possible ways are \(\text{H}_{2}\) formation via reactions of traces of moisture and HF, spontaneous solvent decomposition on LTO surface giving protic species, the self-charge of the lithiated-LTO electrodes mediated by water [9, 29]. It is likely that the main sources of gassing in LTO are reactions between the electrolyte and the electrode surface with complicated multi-step mechanism [14].
The investigation of precise mechanism for TNO degassing was not within the scope of the present work and it requires systematic further study taking into account a complexity of the problem as seen from a similar LTO case. It should be pointed out that the electrodes and all cell components were dried properly before conducting electrochemical tests and highly pure Ar was used as a carrier gas for the OEMS investigation. Therefore, the trace amount of water initially present in the cell could not be responsible for a continuous \(\text{H}_{2}\) evolution during 11 cycles. Several \(\text{C}_{2}\text{H}_{4}\) and \(\text{CO}_{2}\) peaks can be observed after the first two cycles at the end of charges; however, due to their lower intensity they are not easily detectable. We suggest that, by analogy with LTO, a degassing of a TNO-based electrodes is mostly due to the reactivity of electrolyte components (solvents and salts) with surface of the active material (the polymerization of the solvents of the electrolyte or the degradation of the lithium salts [17]) with minor contribution from electrochemical reduction of traces of water.
4 Conclusion
To conclude, a quantification and analysis of gases produced by Li-ion cells during cycling have been conducted using cells with TiNb2O7 negative electrodes and \(\text{LiFePO}_{4}\) positive electrodes.
The measurement of gas volumes following calendar storage tends to show that once the passivation layer is formed, the gas release seems to be very strongly reduced.
On the other hand, the cycling leads to a continuous increase of the gas volume for the cell. Thus, we can propose that a strong volume variation for the active material particles during lithiation and delithiation leads to a continuously fractured/reformed interfacial layer accompanied by a quasi-continuous degassing.
This hypothesis is corroborated by the difference in results obtained for the laboratory-made electrodes of poorer quality and for the pilot-scale ones. A better dispersion and a stronger carbonaceous network allowed a better accommodation of the volume variation for the material and thus probably significantly reduced the volume of gas produced during cycling.
Considering the operando gas measurements, the obtained results are quite similar to those obtained by Parikh et al. [21] despite of using different counter electrodes and operating conditions. However, the authors were not able to detect \(\text{H}_{2}\) during the operando gas measurements due to technical reasons. Using a well-known stable and non-degassing active material, such as LFP, we managed to demonstrate that the volume of gas produced by the TNO prevail over the other reactions which could take place on the positive electrode in the case of a full cell. Moreover, the chemical composition of produced gases seems to be linked to the used electrolyte components, leading to \(\text{H}_{2}\), \(\text{CO}_{2}\), and \(\text{C}_{n}\text{H}_{2n+2}\) formation mostly at the end of charges. The dominant gas is \(\text{H}_{2}\), which is evolved continuously during cycling, similar to LTO-based electrodes.
Different strategies to improve the behavior and performance of TiNb2O7 electrodes have been also explored. The addition of a carbon coating by thermal decomposition of polymer precursor on the surface of the grains of active material lead to formation of a protective and conductive interface between the electrode and the electrolyte. This layer partially inhibited the mechanisms of the electrolyte decomposition catalyzed by the presence of \(\text{Ti}^{{4}+}\) on the surface of the grains.
The improvement of the conditions of realization of these coatings (process, reagents, final carbon rate, etc.) could lead to a better bonding of this layers with grains. This could be a promising way for a future large-scale marketing of the material.
References
Aiken CP, **a J, Wang DY et al (2014) An apparatus for the study of in situ gas evolution in Li-ion pouch cells. J Electrochem Soc 161(10):A1548–A1554. https://doi.org/10.1149/2.0151410jes
An SJ, Li J, Daniel C et al (2016) The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 105:52–76. https://doi.org/10.1016/j.carbon.2016.04.008
Belharouak I, Koenig GM, Tan T et al (2012) Performance degradation and gassing of Li\(_{4}\)Ti\(_{5}\)O\(_{12}\)/LiMn\(_{2}\)O\(_{4}\) lithium-ion cells. J Electrochem Soc 159(8):A1165–A1170
Berg EJ, Villevieille C, Streich D et al (2015) Rechargeable batteries: gras** for the limits of chemistry. J Electrochem Soc 162(14):A2468–A2475
Buannic L, Colin JF, Chapuis M et al (2016) Electrochemical performances and gassing behavior of high surface area titanium niobium oxides. J Mater Chem A 4(29):11531–11541. https://doi.org/10.1039/C6TA03813A
Cava RJ, Murphy DW, Zahurak SM (1983) Lithium insertion in Wadsley–Roth phases based on niobium oxide. J Electrochem Soc 130(12):2345–2351
Colbow KM, Dahn JR, Haering RR (1989) Structure and electrochemistry of the spinel oxides LiTi\(_{2}\)O\(_{4}\) and Li\(_{43}\)Ti\(_{53}\)O\(_{4}\). J Power Sources 26(3–4):397–402
Dean J, Lange N, Forker G et al (1967) Lange’s handbook of chemistry. Handbook of chemistry: a reference volume for all requiring ready access to chemical and physical data used in laboratory work and manufacturing. McGraw-Hill, New York
Fell CR, Sun L, Hallac PB et al (2015) Investigation of the gas generation in lithium titanate anode based lithium ion batteries. J Electrochem Soc 162(9):A1916–A1920. https://doi.org/10.1149/2.1091509jes
Guo B, Yu X, Sun XG et al (2014) A long-life lithium-ion battery with a highly porous TiNb\(_{2}\)O\(_{7}\) anode for large-scale electrical energy storage. Energy Environ Sci 7(7):2220–2226. https://doi.org/10.1039/C4EE00508B
Han JT, Goodenough JB (2011) 3-V full cell performance of anode framework TiNb\(_{2}\)O\(_{7}\)/spinel LiNi\(_{0.5}\) Mn\(_{1.5}\)O\(_{4}\). Chem Mater 23(15):3404–3407. https://doi.org/10.1021/cm201515g
He YB, Li B, Liu M et al (2012) Gassing in Li\(_{4}\)Ti\(_{5}\)O\(_{12}\)-based batteries and its remedy. Sci Rep. https://doi.org/10.1038/srep00913
He YB, Ning F, Li B et al (2012) Carbon coating to suppress the reduction decomposition of electrolyte on the Li\(_{4}\)Ti\(_{5}\)O\(_{12}\) electrode. J Power Sources 202:253–261. https://doi.org/10.1016/j.jpowsour.2011.11.037
Hoffmann J, Milien MS, Lucht BL et al (2018) Investigation of gas evolution from Li\(_{4}\)Ti\(_{5}\)O\(_{12}\) anode for lithium ion batteries. J Electrochem Soc 165(13):A3108–A3113. https://doi.org/10.1149/2.0741813jes
Li H, Zhou H (2012) Enhancing the performances of Li-ion batteries by carbon-coating: present and future. Chem Commun 48(9):1201–1217. https://doi.org/10.1039/C1CC14764A
Lv W, Gu J, Niu Y et al (2017) Gassing mechanism and suppressing solutions in \(\text{ Li}_{{4512}}\)-based lithium-ion batteries. J Electrochem Soc 164(9):A2213–A2224
Mercier-Guyon B (2021) Développement d’électrodes négatives à base d’oxydes mixtes de Titane et de Niobium pour accumulateurs Li-ion. PhD thesis, Université Grenoble Alpes
Metzger M, Marino C, Sicklinger J et al (2015) Anodic oxidation of conductive carbon and ethylene carbonate in high-voltage Li-ion batteries quantified by on-line electrochemical mass spectrometry. J Electrochem Soc 162(7):A1123–A1134. https://doi.org/10.1149/2.0951506jes
Mikolajczak C, Kahn M, White K et al (2012) Lithium-ion batteries hazard and use assessment. Springer, Boston
Monroe C, Newman J (2003) Dendrite growth in lithium/polymer systems. J Electrochem Soc 150(10):A1377. https://doi.org/10.1149/1.1606686
Parikh D, Geng L, Lyu H et al (2021) Operando analysis of gas evolution in TiNb\(_{2}\)O\(_{7}\) (TNO)-based anodes for advanced high-energy lithium-ion batteries under fast charging. ACS Appl Mater Interfaces 13(46):55145–55155. https://doi.org/10.1021/acsami.1c16866
Rowden B, Garcia-Araez N (2020) A review of gas evolution in lithium ion batteries. Energy Rep 6:10–18. https://doi.org/10.1016/j.egyr.2020.02.022
Schwenke KU, Solchenbach S, Demeaux J et al (2019) The impact of CO\(_{2}\) evolved from VC and FEC during formation of graphite anodes in lithium-ion batteries. J Electrochem Soc 166(10):A2035–A2047. https://doi.org/10.1149/2.0821910jes
Whittingham S (1976) Electrical energy storage and intercalation chemistry. Science 192(4244):1126–1127. https://doi.org/10.1126/science.192.4244.1126
Wu K, Yang J, Zhang Y et al (2012) Investigation on Li\(_{4}\)Ti\(_{5}\)O\(_{12}\) batteries developed for hybrid electric vehicle. J Appl Electrochem 42(12):989–995. https://doi.org/10.1007/s10800-012-0442-0
Wu K, Yang J, Liu Y et al (2013) Investigation on gas generation of Li\(_{4}\)Ti\(_{5}\)O\(_{12}\)/LiNi\(_{1/3}\)Co\(_{1/3}\)Mn\(_{1/3}\)O\(_{2}\) cells at elevated temperature. J Power Sources 237:285–290. https://doi.org/10.1016/j.jpowsour.2013.03.057
Wu X, Lou S, Cheng X et al (2018) Unravelling the interface layer formation and gas evolution/suppression on a TiNb \(_{2}\)O\(_{7}\) anode for lithium-ion batteries. ACS Appl Mater Interfaces 10(32):27056–27062. https://doi.org/10.1021/acsami.8b08425
Yazami R (ed) (2014) Nanomaterials for lithium-ion batteries: fundamentals and applications. Pan Stanford Publications, Singapore
Zilio S, Manzi J, Fernicola A et al (2019) Gas release mitigation in LiFePO–Li\(_{4}\)Ti\(_{5}\)O\(_{12 }\) Li-ion pouch cells by an H\(_{2}\)-selective getter. Electrochim Acta 294:156–165. https://doi.org/10.1016/j.electacta.2018.10.102
Acknowledgements
The authors acknowledge CEA-INSTN for Ph.D. funding awarded to Benjamin Mercier-Guyon. This project has received funding from the European Union’s Horizon 2020 research and innovation program under Grant Agreement No. 875126 (CoFBAT).
Author information
Authors and Affiliations
Contributions
BM-G wrote the main manuscript text and prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mercier-Guyon, B., Colin, JF., Profatilova, I. et al. A quantitative and qualitative study of gas generation observed in LiFePO4–TiNb2O7 cells. J Appl Electrochem 54, 731–738 (2024). https://doi.org/10.1007/s10800-023-02007-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-023-02007-6