Abstract
Lake restoration by biomanipulation or phosphorus fixation has been commonly applied methods to improve the ecological status of lakes. However, the effects of lake restoration on food-web dynamics are still poorly understood, especially when biomanipulation and nutrient fixation are used simultaneously. This study investigated the combined effects of a 70% fish removal (mainly roach (Rutilus rutilus Linnaeus, 1758) and bream (Abramis brama Linnaeus, 1758) and Phoslock® treatment on fish trophic ecology in Lyngsø (area: 9.6 ha, mean depth: 2.6 m), Denmark. The lake restoration resulted in decreased nutrient levels, increased water clarity, and increased coverage of more structurally complex submerged macrophytes. Following lake restoration, significant changes in diets of the dominant fish species were observed. Stomach content analyses of roach and perch (Perca fluviatilis Linnaeus, 1758) revealed significantly reduced detritus utilization and increased foraging on macrophytes and macrophyte living invertebrates. Results from stable isotope mixing models indicated a shift from littoral benthic to more pelagic food resources by the dominant fish species. Our findings provide further evidence that lake restorations can lead to substantial changes in lake food webs and fish communities, thereby potentially facilitating a shift toward an ecological state resembling the pristine reference state, less influenced by anthropogenic factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Shallow lakes are important ecosystems characterized by their low depth and are often eutrophic due to high external nutrient loading, frequent mixing and resuspension of sedimented nutrients (Søndergaard et al., 2003, 2005, 2007). This and the relatively small water volume of shallow lakes makes them particularly vulnerable to environmental stressors (Carpenter et al., 1998; Jeppesen et al., 2005; Søndergaard et al., 2007). To improve the ecological status of these lakes, a number of mitigation measures have been implemented, ranging from nutrient management to attempts at ecosystem restructuring through various means depending on the lake-specific conditions and restoration goals (Janssen et al., 2019; Lyu et al., 2020; Tammeorg et al., 2023).
Lake restorations affect lake food webs, either directly through biomanipulations often targeting the fish community (Søndergaard et al., 2007) or indirectly by influencing external (Jeppesen et al., 2007) or internal (Kiani et al., 2020) nutrient loading. Thus, restoration methods involve both top-down and bottom-up control of lake food webs, the combined effects of which are hard to disentangle and control (Jeppesen et al., 2005; Søndergaard et al., 2007). To the best of our knowledge, the combined effects of fish removal and phosphorus fixation on lake food webs and trophic ecology of dominant fishes have remained unexplored although both methods are relatively commonly applied in lake restorations (Tammeorg et al., 2023).
Biomass reduction of planktivorous and benthivorous fish, such as small perch (Perca fluviatilis Linnaeus, 1758), bream (Abramis brama Linnaeus, 1758), roach (Rutilus rutilus Linnaeus, 1758) and rudd (Scardinius erythrophthalmus Linnaeus, 1758), is a commonly applied lake restoration method in Northern Europe (Meijer et al., 1990; Søndergaard et al., 2000; Volta et al., 2013). Fish removals have been found to effectively achieve restoration goals (Bernes et al., 2015) and to induce changes in the behavior and diet of remaining fishes (Persson & Hansson, 1999; Leppä et al., 2003). Changes in fish trophic ecology have been observed to be associated with increased use of benthic dietary and habitat resources (Persson & Hansson, 1999; Berthelsen et al., 2023a). However, although most fishes may show a shift toward benthic resources, some small planktivorous fish, such as small < 80 mm perch, may shift to increased foraging on pelagic prey following biomanipulation (Persson & Hansson, 1999). In some cases, increased use of the pelagic food resources following fish removal is likely caused by increased recruitment of small roach and perch following competitive release (Syväranta et al., 2010). However, the potential for a pelagic niche shift by the fish community might be reduced if the pelagic planktonic production is heavily reduced by simultaneous nutrient removal.
Phoslock is a lanthanum-modified bentonite clay that reduces the availability of dissolved phosphorus for phytoplankton growth by forming a phosphorus-locking layer at the sediment–water interface (Robb et al., 2003), thereby potentially shifting the lake to the more desired clear-water state (Haghseresht et al., 2009; Copetti et al., 2015; Han et al., 2022). Although Phoslock treatments are proven successful (Haghseresht et al., 2009; Copetti et al., 2015; Han et al., 2022), their combined effects with fish removals (biomanipulation) on lake food webs and fish communities have remained unexplored.
Here we studied the combined effects of fish removal and Phoslock treatment on the food web and fish community in a small and shallow Danish lake. We present the overall restoration effects on the lake chemical (water quality) and structural (macrophyte coverage) characteristics and evaluate how this influenced the diet of the fish community. We expected the remaining fish community to shift toward increased use of pelagic planktonic food resources due to increased zooplankton availability associated with reduced predation pressure by small planktivorous fishes, increased refugia from structurally complex submerged macrophytes, and higher foraging efficiency of fishes in clear water following lake restorations.
Methods
Study lake
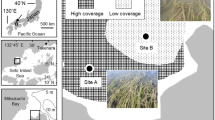
The study lake Lyngsø is a small (9.6 ha), relatively shallow (Zmean = 2.6, Zmax = 7.6 m) and wind-protected urban lake with a catchment area (108 ha) consisting mainly of residential and urban forest areas in temperate central Jutland, Denmark (56° 09′ 31″ N, 9° 32′ 39″ E) (Fig. 1). There is no discrete inflow, but the lake drains through a submerged drainage pipe. Until the mid-1950s, untreated wastewater was discharged into the lake, but the external load is currently very low (Skovgaard & Carl, 2018). The lake is recovering from eutrophication and inhabited by some macrophytes and a fish community dominated by perch, roach, rudd, bream and pike (Esox lucius Linnaeus, 1758). The species three-spined stickleback (Gasterosteus aculeatus Linnaeus, 1758), crucian carp (Carassius carassius Linnaeus, 1758), ruffe (Gymnocephalus cernua Linnaeus, 1758) and eel (Anguilla anguilla Linnaeus, 1758) have been previously recorded in the lake but not observed during this study. There is some recreational fishing in the lake, but this is very unlikely to have influenced the results of the study due to regulation by the local sports-fishers association and prohibition on using anything but line and rod.
Lake restoration
Prior to the restoration efforts described in this study, a previous restoration effort was conducted in 1990–1993 by stocking pike fry (Berg et al., 1997; Søndergaard et al., 1997). At that time, summer mean total phosphorus concentration was 0.79 mg P l−1 and there were no macrophytes in the lake. Although the pike stocking was ultimately unsuccessful in attaining the long-term restoration goals, effective wastewater management has led to decreasing surface water phosphorous concentrations since 1994, but internal loading is still high (Skovgaard & Carl, 2018).
The present lake restoration comprised of two main fish removals by small-mesh seine netting (mesh sizes: ends 8 mm, outer purse 6 mm and inner purse 4 mm) and large-mesh gill netting (mesh sizes 85, 90, 110, 120 and 135 mm) in October and November 2021, with supplementary fishing using large-mesh gillnets, fish traps and electrofishing in spring 2022. Based on monitoring, the fish removals and supplementary fishing removed a total of 1409-kg cyprinid fish and small perch, which was approximately 70% of the biomass of the target fishes in the lake at the time (Jensen, 2022). Roach made up 65% and bream 30% of the total removed fish.
To reduce the effect of internal phosphorus loading, 44.1 tons of Phoslock was added in transects across the lake in a dry powder form, using a raft fitted with a hopper spreader during the period between November 29 and December 3, 2021. Phoslock was distributed at depths below 2 m during periods of calm weather to ensure stable sedimentation and homogenous distribution across the lake pelagic area and profundal sediment surface.
Monitoring of water quality and macrophyte coverage
Chlorophyll-a (µg l−1), Secchi depth (m), as well as total nitrogen and total phosphorous concentrations (mg l−1) were analyzed according to standard procedures described by Søndergaard et al. (2005).
To investigate structural complexity, the lake macrophyte coverage was investigated from the same GPS-assigned sites in late summer from 2020 to 2022. In each year, the macrophyte coverage in percent and plant-bed height data were divided into 0.5 m depth increments based on sampling across seven transects with a total of 64 sampling points. Depths were measured at each sampling point every sampling year, with 0.5 m and 4.5 m being the minimum and maximum depths where macrophytes were observed. Macrophyte observations were conducted either by making two throws with a Sigurd Olsen plant rake at each observation point or by visual observation with a bathyscope. Macrophyte coverage was determined in six increments, corresponding to the approximate coverage percentages: 0, > 0–5, 5–35, 35–50, 50–75 and 75–100%. Plant height and water depth were both measured in cm. Average Per Volume Inhabited (PVI) index was calculated for the lake macrophyte assemblage, based on the coverage values at each observation point using the formula:
Fish community
The pre-restoration fish community was investigated in a survey by the Danish National Monitoring Program for Aquatic Environment and Nature (NOVANA) before the addition of Phoslock in October 2021 and one year after the treatment in September 2022 (Jensen, 2022), using nine standardized 1.5-m-high and 30-m-long Nordic multi-mesh gillnets consisting of 12 2.5-m-long panels with mesh sizes ranging from 5 to 55 mm (Appelberg et al., 1995). In this local fish survey, the sampling methods and locations of the nine Nordic nets were identical before and after the lake restoration (Jensen, 2022).
Stomach content and stable isotope analyses
Samples for fish stomach content and stable isotope analyses were collected as part of the fish removal in 2021. In 2022, fish were collected as part of the standardized Nordic survey gillnet fishing (Jensen, 2022). The selected fish samples consisted of the following species: roach (n2021 = 66, n2022 = 68), perch (n2021 = 53, n2022 = 64), bream (n2021 = 72, n2022 = 2) and rudd (n2021 = 1, n2022 = 25).
For subsequent analyses, perch, roach, bream and rudd were divided into two size groups, i.e., below and above 150 mm total length. The division of fish into these two size categories was based on the ontogenetic dietary shifts (cf. Sánchez-Hernández et al., 2019) of perch toward increasing piscivory and of roach from zooplanktivory to zoobenthivory with increasing length (Berthelsen et al., 2023b).
Stomach contents of all fish (n = 351) were analyzed as described in Hynes (1950) and further detailed by Amundsen & Sánchez-Hernández (2019). For roach, bream and rudd lacking a distinct stomach, the elongated foregut (from pharynx to second gut-sling) was used for analysis. The fullness of each stomach was visually determined using the points method described in Hynes (1950), where 0 refers to a completely empty and 5 to a completely full stomach. The observed prey items were further assigned points based on their relative contribution to the total stomach fullness (from 0 to 10). A value of 0 referred to prey being completely absent from the stomach, while a value of 10 indicated that the prey item was the only type found in the stomach, regardless of how full it was.
For stable isotope analysis, a small slice of dorsal muscle was dissected from all fish groups. In the case of macroinvertebrates, entire organisms were pooled at family level or lower and analyzed, while pooled tissues were used for macrophyte, zooplankton and biofilm samples. Macroinvertebrates were sampled from the littoral habitat using a kicknet with a 500-µm mesh and included the following taxa: Asellus aquaticus (Linnaeus, 1758), Chironomidae larvae, Ceratopognidae larvae, Cloeon sp., Erpobdella octoculata (Linnaeus, 1758), Limnephilidae larvae, Sialis lutaria (Linnaeus, 1758) and Phryganea sp. larvae (Linnaeus, 1758). Pelagic zooplankton was collected with a plankton net (mesh size 140 μm). Biofilm samples were scraped from rocks in the littoral zone. The macrophytes collected for stable isotope analysis were Potamogeton crispus (Linnaeus, 1753), Ceratophyllum demersum (Linnaeus, 1753) and Elodea canadensis (Michaux, 1803). All samples were kept frozen at –20 °C until they were freeze dried to a constant weight (ca. 48 h), ground into fine powder using a mortar and pestle and stored in glass vials until subsequent preparations.
Hereafter, 0.470–1.710 mg of the powdered tissue was encapsulated in tin capsules and analyzed for stable isotope ratios of carbon and nitrogen using a Thermo Finnigan DELTAplus Advantage mass spectrometer connected to a FlashEA 1112 elemental analyzer (Thermo Fisher Scientific Corporation, Waltham, MA, USA) at the University of Jyväskylä, Finland. Stable carbon and nitrogen isotope ratios are expressed as delta values (δ13C and δ15N, respectively) relative to the international standards for carbon (Vienna PeeDee Belemnite) and nitrogen (atmospheric nitrogen). Dried pike white muscle tissue and birch (Betula sp.) leaves were used as internal working standards for animal and plant samples, respectively, with two replicate standards run repeatedly after every ten samples in each sequence. In total, 296 fish individuals were used in the stable isotope mixing model, including samples from bream (n = 50), perch (n = 111), roach (n = 109) and rudd (n = 26).
Stable isotope mixing models
The Bayesian MixSIAR mixing model (Stock et al., 2018) was used in the R statistical software (R Core Team, 2023) to estimate the relative proportions of littoral benthic and pelagic planktonic food sources in the long-term diets of < 150 mm and > 150 mm individuals of the selected fish species. In short, the model uses data of the δ13C and δ15N values of fish (‘mixture’) and their putative food resources (‘source’), as well as the trophic discrimination factors (i.e., how much δ13C and δ15N values change when source isotopes are assimilated in consumer tissue) and informative priors (i.e., how much each source is expected to contribute to consumer diet based on a priori information) to produce Bayesian estimates (posterior distributions) of dietary proportions (Stock et al., 2018). Two sources were chosen for the models representing the littoral and pelagic food-web compartments, with the littoral source been based on littoral macroinvertebrates, macrophytes and biofilm and the pelagic source been based on zooplankton. Informative priors (More & Semmens, 2008) were incorporated in the MixSIAR models to provide information about the expected dietary proportions of fish, calculated based on the relative contributions of littoral and pelagic food sources in fish stomach contents (Appendix 1) using the formula:
where N was the number of fish in the group, and 2 represents the weight of the uninformative prior. This method was used to scale the prior to have a weight of 2, the same as the uninformative prior \(\alpha =(1,1)\) (More & Semmens, 2008).
For MixSIAR, trophic discrimination factors of 0.4 ± 1.3‰ for δ13C and 3.4 ± 1.0‰ for δ15N (Post, 2002) were used and the fish muscle δ13C values were lipid-corrected (Kiljunen et al., 2006) due to high lipid content of some fish (average C:N ratios: 3.1–3.5, maximum: 3.2–5.6) (Post et al., 2007).
Statistical analyses
Chlorophyll-a, Secchi depth, as well as total nitrogen and total phosphorous concentrations were measured to assess changes in water quality by comparing the months May–September between pre- and post-restoration years using nonparametric Mann–Whitney U tests. Only the summer periods (May–September) were compared to represent the overall conditions during the growing season (Søndergaard et al., 2003), which is the period when Phoslock treatment should be most effective. In addition, the largest cascading effects of fish manipulations were expected to occur during the warm summer period, associated with increased zooplankton grazing on seasonally abundant phytoplankton due to reduced predation pressure by zooplanktivorous fishes.
Between-year differences in macrophyte coverage were investigated using Permutational Multivariate Analysis of Variance (PERMANOVA) comparing macrophyte coverages at 0.5 m depth intervals between the depths 0.5 and 4 m where macrophytes were observed.
PVI was analyzed between the years 2020 and 2022 using Kruskal–Wallis and Dunn’s tests as the PVI data did not meet the assumptions of normal distribution or homogeneity of variance. Following significant Kruskal–Wallis results, pairwise Dunn’s tests were used to detect significant between-year differences in PVI values.
Between-year differences in fish length distributions were tested using nonparametric Kruskal–Wallis test, followed by pairwise Dunn’s tests, for the selected fish species groups (not grouped by size categories). All Mann–Whitney U, PERMANOVA, Kruskal–Wallis and Dunn’s tests were conducted in the R statistical software (R Core Team, 2023).
One-way ANOSIM (Analysis of Similarities) was used to examine dissimilarities in fish stomach contents before and after restoration, focusing on perch and roach due to their sufficient sample sizes in both years allowing statistical testing. In case of significant dissimilarities, SIMPER (Similarity Percentage) analysis was applied to identify predominant contributors to dissimilarity in diet compositions. Both ANOSIM and SIMPER analyses were conducted using PRIMER7 software (version 7.0.23).
Results
Water quality
Water chemistry changed significantly after the combined fish removal and Phoslock treatment as indicated by the decreased total phosphorus (P < 0.01) and total nitrogen (P < 0.05) concentrations (Fig. 2) during the summer period. Early in the year following the restoration (January–April), the Secchi depth was significantly lower than during the same period prior to restoration (P < 0.05), while in the summer period (May–September) the Secchi depth was significantly higher (P = < 0.05). There were no statistically significant shifts in chlorophyll-a concentrations during the study period (P = 0.2), although they were markedly lower in the summer period (May–September) following the lake restoration (Fig. 2).
Chlorophyll-a (ug/l), Secchi depth (m) and epilimnion total nitrogen (solid blue line) and phosphorous (dashed green line) concentrations (mg/l) in years 2021 and 2022. The periods of fish removals are indicated by red dotted lines, whereas the Phoslock treatment is indicated by the dotted blue line
Macrophyte coverage
In 2021, the vegetation in the lake littoral zone was dominated by filamentous algae, whereas in 2021 Potamogeton crispus was the dominant plant species. In 2022 after the restoration, the dominant macrophytes were Potamogeton crispus and Ceratophyllum demersum. The macrophyte coverage was significantly higher in 2020 than in 2021 (P < 0.01; Fig. 3). Further, there was a significant shift from low macrophyte (i.e., not filamentous algae) coverage to more complex macrophytes (mainly Elodea canadensis and Potamogeton crispus) from 2021 to 2022 (p < 0.01) and from 2020 to 2022 (P = < 0.01; Fig. 3).
PVI was significantly higher in 2020 and 2022 than in 2021 (P < 0.01 and P < 0.01, respectively), whereas there was no significant difference in PVI between the years 2020 and 2022 (P = 0.432).
Fish community
As reported by Jensen (2022), roach and perch were the dominant fish species in Lyngsø but were caught in lower numbers following the lake restoration (Table 1). Bream, tench and rudd were caught in low numbers, with bream and tench completely missing in the survey gillnet catches following the lake restoration (Table 1, Jensen, 2022).
The fish samples collected for this study were dominated by cyprinids, particularly roach, rudd and bream, but perch were also common before and after the restoration (Fig. 4). Generally, these fish were small, rarely exceeding 200 mm, with only bream often attaining larger sizes. The observed significant between-year differences in perch lengths (P < 0.01) were solely due to the change in fishing method, with gillnets targeting larger perch than the seine net used prior to the lake restoration.
Stomach content analysis
Due to low sample sizes of bream and rudd, the between-year diet comparisons were only made for perch and roach. The fish diets consisted mainly of detritus (sediment and dead plants), fresh plant material, zoobenthos, zooplankton, and other fish (Fig. 5). Small roach and large roach showed significant changes in diets following the lake restoration (P < 0.01). Based on SIMPER, the main contributors to the between-year dietary dissimilarity of small roach were detritus, zooplankton, zoobenthos, and plant material, accounting for 38, 28, 23, and 11% dissimilarity, respectively, and resulting from the higher consumption of zoobenthos and plant material and lower consumption of detritus and zooplankton following the lake restoration. For large roach, the main contributors to the dissimilarity were plant material, detritus, zoobenthos, and zooplankton, accounting for 41, 35, 17, and 7% dissimilarity, respectively, and resulting from the higher consumption of plant material and lower consumption of detritus following the lake restoration (Fig. 5). No significant between-year dietary dissimilarities were found for large or small perch (P = 0.9 and P = 0.4, respectively).
Proportion of different prey categories in the stomach contents of small (≤ 150 mm) and large (> 150 mm) individuals of the dominant fish species caught before and after the restoration of Lyngsø. The average stomach fullness (%) is indicated by orange dots in each bar, whereas the numbers above the bars indicate sample sizes
Stable isotope mixing model
The littoral and pelagic isotopic end-members used in the MixSIAR model had distinct δ13C values, with the littoral sources being enriched in 13C and depleted in 15N as compared to the pelagic planktonic sources (Fig. 6). The fish consumers occupied a higher trophic position as indicated by their elevated δ15N values and their trophic fractionation corrected isotope values laid between those of the used littoral and pelagic isotopic end-members (Fig. 6), thus allowing the estimation of source contributions using the MixSIAR model.
Stable isotope biplots showing the mean ± SD δ13C and δ15N values of littoral and pelagic isotopic end-members used in the MixSIAR model, as well as the individual values of the dominant fish species in Lyngsø. The isotope values of littoral and pelagic sources are enriched by the trophic discrimination factors of 0.4 ± 1.3‰ for δ13C and 3.4 ± 1.0‰ for δ15N (Post 2002)
Sufficient samples of perch and roach were caught to allow between-year comparisons of isotope data, whereas the other fish species (bream and rudd) were included in the isotopic plots (Figs. 6 and 7) to show their isotopic niche in comparison with roach and perch. Generally, as compared to other fish species, bream were more depleted in 15N (indicating a lower trophic position), whereas rudd were more enriched in 13C (indicating a higher reliance on littoral benthic carbon sources).
The MixSIAR results showed a general decrease in the use of littoral food resources for all perch and roach size groups, and a corresponding increase in the use of pelagic resources (Fig. 7). Average utilization of pelagic resources in roach and perch increased from 32 to 83% following the lake restoration. No significant differences in the magnitude of this pelagic niche shift were observed between perch or roach of either size group, as indicated by the overlap** 95% Bayesian credibility intervals (Fig. 7).
Discussion
The lake restoration by fish removal and Phoslock treatment was successful in achieving increased Secchi depth during the summer period and in increasing the macrophyte coverage from 2020 to 2022. These changes in water quality and habitat complexity likely contributed to the observed changes in diets of dominant fish species, with stable isotope data indicating a pelagic niche shift by the fish community following lake restoration.
Phoslock has previously been used with success to improve water quality and to promote the growth of submerged macrophytes in lakes (Han et al., 2022; Li et al., 2019) and large river estuaries (Robb et al., 2003). Phoslock has been found to be most effective in waterbodies dominated by phytoplankton growth and deep enough to prevent frequent sediment resuspension. Our study lake meets these criteria, but in other and especially larger lakes the relatively high price of Phoslock might limit the broader future applications of this restoration method.
Stomach content analysis revealed that roach and perch fed less on detritus after the lake restoration. This likely resulted from reduced competition for invertebrate resources, as supported by previous studies in shallow lakes where limited availability of and high competition for animal food resources increased detritivory by roach (Persson, 1983). Roach are able to gain nutrients from feeding on detritus (Persson, 1983), whereas perch likely ingest detritus by ‘sloppy feeding’ when preying on invertebrates living in and feeding on the detritus. For this reason, the detrital trophic pathway had a large role in supporting the fish community in the study lake before the restoration. The apparent shift from feeding on detritus and/or detritus-associated invertebrates toward pelagic feeding showcases the well-studied diet flexibility of perch and roach (Persson & Hansson, 1999; Jeppesen et al., 2007; Bernes et al., 2015; Berthelsen et al., 2023a, b). It is likely that this diet flexibility and ability to feed off-of primary producer and detrital trophic pathways increases the stability of fish communities with perch and roach (Moore et al., 2004) and explains some of the success of perch and roach in the fish communities of European lakes (Ritterbusch et al., 2022).
After the restoration, foraging on plant material increased especially for large roach. Herbivory by large roach has been suggested to result from scarcity of zoobenthos (Horppila, 1994). However, lake restoration may not decrease zoobenthos availability (Persson & Hansson, 1999) and thus the observed shift from detritivory to herbivory by roach likely resulted from increased availability of macrophytes (i.e., not of filamentous algae) in Lyngsø.
Zooplankton was an important prey for fish before and after the restoration. However, the MixSIAR model suggested a shift toward the pelagic niche following the lake restoration. Similar shifts toward a more pelagic niche have previously been observed to be associated with increased density of small planktivorous fish following lake restoration (Syväranta et al., 2010). We did not catch more zooplanktivorous small fish after the restoration of Lyngsø (Jensen, 2022), suggesting that the higher proportion of small fish was not the sole reason for the observed pelagic niche shift of the fish community. Rather, the competitive release caused by fish removal, the reduced predation pressure on zooplankton, and the higher availability of refugia likely led to a feeding niche expansion to pelagic resources for a large part of the fish community as observed in some previously restored lakes (Syväranta & Jones, 2008). The pelagic niche shift by fishes was likely supported by efficient visual foraging on zooplankton under the clear-water state of Lyngsø (Winfield, 1986), as well as by the increased zooplankton availability supported by reduced predation pressure by small zooplanktivorous fishes following fish removal and by increased predation refuge among structurally complex macrophytes. Despite their small size, zooplankton might be highly important prey for cyprinid and percid fishes in Lyngsø, as they can provide essential fatty acids for consumers, especially under less-eutrophic conditions (Eloranta et al., 2013; Keva et al., 2021).
Macrophytes can provide refuge for zooplankton when fish density is sufficiently low (Perrow et al., 1999). This was found in a classical laboratory experiment by Diehl (1988), where feeding efficiency of roach, bream and perch on macroinvertebrates was reduced by submerged macrophytes. This is likely reflected in our stomach content analysis showing increased macrophyte consumption instead of increased macroinvertebrate consumption following the lake restoration. Increased macrophyte coverage and reduced predation pressure by small planktivorous fishes likely increased zooplankton availability for the remaining fishes and also supported the development of a clear-water state in Lyngsø. Following fish removals, fish communities have been observed to shift toward increased benthivory (Persson & Hansson, 1999; Berthelsen et al., 2023b) or planktivory (Syväranta et al., 2010), with the responses likely depending on e.g., the lake morphometry (i.e., increased zooplanktivory in deeper and larger lakes) (Berthelsen et al., 2023a, b). The somewhat contrasting results of the stomach contents and stable isotope analyses likely result from the different temporal resolutions of the methods, with stomach contents revealing only the most recently ingested prey items, while stable isotopes reflect the long-term assimilated diets (Davis et al., 2012). Nevertheless, our study findings provide further evidence that perch and roach can undergo niche expansions following lake restorations, indicated by increased among-individual diet variation and a population-level shift to a more pelagic niche (low δ13C values) and a higher trophic position (high δ15N values) in Lyngsø (Fig. 6) and previously restored lakes (Syväranta & Jones, 2008).
Conclusions
Small lakes provide important ecosystem services (Biggs et al., 2017), but their ecological communities and natural functioning are severely threatened by multiple human impacts (Reid et al., 2019). Restoration of small lakes impaired by eutrophication has become an important measure for conservation and sustainable management of freshwater habitats (Abell et al., 2022), especially when the restorations account for both top-down and bottom-up processes (Tammeorg et al., 2023). We found a significant communitywide shift toward a pelagic feeding niche following improved lake water quality after simultaneous fish removal and phosphorus fixation. Our study emphasizes the potential benefit in using combined restoration methods targeting both top-down and bottom-up effects, but the potential long-term individual and interactive effects of fish removal and Phoslock treatment remain as promising avenues for future research.
Data availability
Data are available from the authors upon reasonable request.
Code availability
Code is available from authors upon reasonable request.
References
Abell, J. M., D. Özkundakci, D. P. Hamilton & P. Reeves, 2022. Restoring shallow lakes impaired by eutrophication: approaches, outcomes, and challenges. Crit Rev Environ Sci Technol 52: 1199–1246. https://doi.org/10.1080/10643389.2020.1854564.
Amundsen, P. A. & H. Sánchez-Hernández, 2019. Feeding studies take guts – critical review and recommendations of methods for stomach contents analysis in fish. J Fish Biol 95: 1364–1373. https://doi.org/10.1111/jfb.14151.
Appelberg, M., H. M. Berger, T. Hesthagen, E. Kleiven, M. Kurkilahti, J. Raitaniemi & M. Rask, 1995. Development and intercalibration of methods in Nordic freshwater fish monitoring. Water Air Soil Pollut 85: 401–406. https://doi.org/10.1007/BF00476862.
Berg, S., E. Jeppesen & M. Søndergaard, 1997. Pike (Esox lucius L.) stocking as a biomanipulation tool 1. Effects on the fish population in Lake Lyng, Denmark. Hydrobiologia 342: 311–318. https://doi.org/10.1023/A:1017032616642.
Bernes, C., S. R. Carpenter, A. Gårdmark, P. Larsson, L. Persson, C. Skov & J. D. M. Speed, 2015. What is the influence of a reduction of planktivorous and benthivorous fish on water quality in temperate eutrophic lakes? A systematic review. Environ Evid 4: 1–28. https://doi.org/10.1186/s13750-015-0032-9.
Berthelsen, A. S., K. Raundrup, P. Grønkjær, E. Jeppesen & T. L. Lauridsen, 2023a. The importance of habitat and lake morphometry for the summer diet choice of landlocked arctic char in two west Greenland lakes. Water 15: 2164. https://doi.org/10.3390/w15122164.
Berthelsen, A. S., C. Skov, M. Søndergaard, M. H. Larsen & T. L. Lauridsen, 2023b. Ecological implications of fish removal: Insights from gut-content analysis of roach (Rutilus rutilus) and European perch (Perca fluviatilis) in a eutrophic shallow lake. J Fish Biol. https://doi.org/10.1111/jfb.15531.
Biggs, J., S. Fumetti & M. Kelly-Quinn, 2017. The importance of small waterbodies for biodiversity and ecosystem services: implications for policy makers. Hydrobiologia 793: 3–39. https://doi.org/10.1007/s10750-016-3007-0.
Carpenter, S. R., N. F. Caraco, D. L. Correll, R. W. Howarth & A. N. Sharpley, 1998. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8: 559–568. https://doi.org/10.2307/2641247.
Copetti, D., K. Finsterle, L. Marziali, F. Stefani, G. Tartari, G. Douglas & K. Reitzel, 2015. Eutrophication management in surface waters using lanthanum modified bentonite: a review. Water Res 97: 162–174. https://doi.org/10.1016/j.watres.2015.11.056.
Davis, A. M., M. L. Blanchette, B. J. Pusey, T. D. Jardine & R. G. Pearson, 2012. Gut content and stable isotope analyses provide complementary understanding of ontogenetic dietary shifts and trophic relationships among fishes in a tropical river. Freshw Biol 57: 2156–2172. https://doi.org/10.1111/j.1365-2427.2012.02858.x.
Diehl, S., 1988. Foraging efficiency of three freshwater fishes: effects of structural complexity and light. Oikos 53: 207–214. https://doi.org/10.2307/3566064.
Eloranta, A. P., H. L. Mariash, M. Rautio & M. Power, 2013. Lipid-rich zooplankton subsidise the winter diet of benthivorous Arctic charr (Salvelinus alpinus) in a subarctic lake. Freshw Biol 58: 2541–2554. https://doi.org/10.1111/fwb.12231.
Haghseresht, F., S. Wang & D. D. Do, 2009. A novel lanthanum-modified bentonite, phoslock, for phosphate removal from wastewaters. Appl Clay Sci 46: 369–375. https://doi.org/10.1016/j.clay.2009.09.009.
Han, Y., Y. Zhang, Q. Li, M. Lürling, W. Li, H. He & J. Gu, 2022. Submerged macrophytes benefit from lanthanum modified bentonite treatment under juvenile omni-benthivorous fish disturbance: implications for shallow lake restoration. Freshw Biol 67: 672–683. https://doi.org/10.1111/fwb.13871.
Horppila, J., 1994. The diet and growth of roach (Rutilus rutilus (L.)) in Lake Vesijärvi and possible changes in the course of biomanipulation. Hydrobiologia 294: 35–41. https://doi.org/10.1007/BF00017623.
Hynes, H. B. N., 1950. The food of fresh-water sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius), with a review of methods used in studies of the food of fishes. J Anim Ecol 19: 36–58. https://doi.org/10.2307/1570.
Janssen, A. B. G., D. Wijk, L. P. A. Gerven, E. S. Bakker, R. J. Brederveld, D. L. DeAngelis, J. H. Janse & W. M. Mooij, 2019. Success of lake restoration depends on spatial aspects of nutrient loading and hydrology. Sci Total Environ 679: 248–259. https://doi.org/10.1016/j.scitotenv.2019.04.44.
Jensen, H. J., 2022. Biomanipulation i Lyngsø: Status 2022. Silkeborg Municipality. https://dce.au.dk/fileadmin/dce.au.dk/Udgivelser/Videnskabelige_rapporter_500-599/SR573.pdf
Jeppesen, E., M. Søndergaard, J. P. Jensen, K. E. Havens, O. Anneville, L. Carvalho, M. F. Coveney, R. Deneke, M. T. Dokulil, B. Foy, D. Gerdaux, S. E. Hampton, S. Hilt, K. Kangur, J. Kohler, E. H. H. R. Lammens, T. L. L. Lauridsen, M. Manca, M. R. Miracle, B. Moss, P. Noges, G. Persson, G. Phillips, R. Portielje, S. Romo, C. L. Schelske, D. Straile, I. Tatrai, E. Willen & M. Winder, 2005. Lake responses to reduced nutrient loading – an analysis of contemporary long-term data from 35 case studies. Freshw Biol 50: 1747–1771. https://doi.org/10.1111/j.1365-2427.2005.01415.x.
Jeppesen, E., M. Meerhof, B. A. Jacobsen, R. S. Hansen, M. Søndergaard, J. P. Jensen & T. L. L. Lauridsen, 2007. Restoration of shallow lakes by nutrient control and biomanipulation – the successful strategy varies with lake size and climate. Hydrobiologia 581: 269–285. https://doi.org/10.1007/s10750-006-0507-3.
Keva, O., S. J. Taipale, B. Hayden, S. M. Thomas, J. Vesterinen, P. Kankaala & K. K. Kahilainen, 2021. Increasing temperature and productivity change biomass, trophic pyramids and community-level omega-3 fatty acid content in subarctic lake food webs. Global Change Biology 27: 282–296. https://doi.org/10.1111/gcb.15387.
Kiani, M., P. Tammeorg, J. Niemistö, A. Simojoki & O. Tammeorg, 2020. Internal phosphorus loading in a small shallow lake: response after sediment removal. Sci Total Environ 725: 138279. https://doi.org/10.1016/j.scitotenv.2020.138279.
Kiljunen, M., J. Grey, T. Sinisalo, H. Chris, H. Immonen & R. I. Jones, 2006. A revised model for lipid-normalizing δ13C values from aquatic organisms, with implications for isotope mixing models. J Appl Ecol 43: 1213–1222. https://doi.org/10.1111/j.1365-2664.2006.01224.x.
Leppä, M., H. Hämäläinen & J. Karjalainen, 2003. The response of benthic macroinvertebrates to whole-lake biomanipulation. Hydrobiologia 498: 97–105. https://doi.org/10.1023/A:1026224923481.
Li X., Z. Zhang, Q. **e, R. Yang, T. Guan & D. Wu, 2019. Immobilization and Release Behavior of Phosphorus on Phoslock- Inactivated Sediment under Conditions Simulating the Photic Zone in Eutrophic Shallow Lakes. Environmental Science and Technology 53: 12449–12457. https://doi.org/10.1021/acs.est.9b04093
Lyu, T., L. Song, Q. Chen & G. Pan, 2020. Lake and river restoration: method, evaluation and management. Water 12: 1–8. https://doi.org/10.3390/w12040977.
Meijer, M. L., M. W. Haan, A. W. Breukelaar & H. Buiteveld, 1990. Is reduction of the benthivorous fish an important cause of high transparency following biomanipulation in shallow lakes? Hydrobiologia 200: 303–315. https://doi.org/10.1007/BF02530348.
Moore, J. M., E. L. Berlow, D. C. Coleman, P. C. de Ruiter, Q. Dong, A. Hastings, N. C. Johnson, K. S. McCann, K. Melville, P. J. Morin, K. Nadelhoffer, A. D. Rosemond, D. M. Post, J. L. Sabo, K. M. Scow, M. J. Vanni & D. H. Wall, 2004. Detritus, trophic dynamics and biodiversity. Ecol Lett 7: 584–600. https://doi.org/10.1111/j.1461-0248.2004.00606.x.
Moore, J. M. & B. X. Semmens, 2008. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol Lett 11: 470–480. https://doi.org/10.1111/j.1461-0248.2008.01163.x.
Perrow, M. R., A. J. D. Jowitt, J. H. Stansfield & G. L. Phillips, 1999. The practical importance of the interactions between fish, zooplankton and macrophytes in shallow lake restoration. Hydrobiologia 395: 199–210. https://doi.org/10.1023/A:1017005803941.
Persson, A. & L. A. Hansson, 1999. Diet shift in fish following competitive release. Can J Fish Aquat Sci 56: 70–78. https://doi.org/10.1139/f98-141.
Persson, L., 1983. Effects of intra- and interspecific competition on dynamics and size structure of a perch Perca fluviatilis and a roach Rutilus rutilus population. Oikos 41: 126–132. https://doi.org/10.2307/3544354.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83: 703–718. https://doi.org/10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2.
Post, D. M., C. A. Layman, D. A. Arrington, G. Takimoto, J. Quattrochi & C. G. Montan, 2007. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152: 179–189. https://doi.org/10.1007/s00442-006-0630-x.
R Core Team, 2023. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reid, A. J., A. K. Carlson, I. F. Creed, E. J. Eliason, P. A. Gell, P. T. J. Johnson & K. A. Kidd, 2019. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol Rev 94: 849–873. https://doi.org/10.1016/j.scitotenv.2021.149620.
Ritterbusch, D., P. Blabolil, J. Breine, T. Erősm, T. Mehner, M. Olin, G. Peirson, P. Volta & S. Poikane, 2022. European fish-based assessment reveals high diversity of systems for determining ecological status of lakes. Sci Total Environ 802: 149620. https://doi.org/10.1016/j.scitotenv.2021.149620.
Robb, M., B. Greenop, Z. Goss, G. Douglas & J. Adeney, 2003. Application of Phoslock™, an innovative phosphorus binding clay, to two Western Australian waterways: preliminary findings. Hydrobiologia 494: 237–243. https://doi.org/10.1023/A:1025478618611.
Sánchez-Hernández, J., A. D. Nunn, C. E. Adams & P. A. Amundsen, 2019. Causes and consequences of ontogenetic dietary shifts: a global synthesis using fish models. Biol Rev 94: 539–554. https://doi.org/10.1111/brv.12468.
Skovgaard, H. & J. D. Carl, 2018. Restaurering af Lyngsø: Forundersøgelse af mulighederne for sørestaurering i Lyngsø. Technical report, ORBICON. https://projekter.silkeborg.dk/p/Subsites/Projekter/Forundersgelse%20Lyngs%C3%B8.pdf
Søndergaard, M., J. P. Jensen & E. Jeppesen, 2003. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 506: 135–145. https://doi.org/10.1023/B:HYDR.0000008611.12704.dd.
Søndergaard, M., E. Jeppesen & S. Berg, 1997. Pike (Esox lucius L.) stocking as a biomanipulation tool 2. Effects on lower trophic levels in Lake Lyng, Denmark. Hydrobiologia 342: 319–325. https://doi.org/10.1023/A:1017084600712.
Søndergaard, M., E. Jeppesen, J. P. Jensen & T. Lauridsen, 2000. Lake restoration in Denmark. Lakes Reserv 5: 151–159. https://doi.org/10.1046/j.1440-1770.2000.00110.x.
Søndergaard, M., E. Jeppesen, J. P. Jensen & S. A. Lildal, 2005. Water framework directive: ecological classification of Danish lakes. J Appl Ecol 42: 616–629. https://doi.org/10.1111/j.1365-2664.2005.01040.x.
Søndergaard, M., E. Jeppesen, T. L. Lauridsen, C. Skov, E. H. Van Nes, R. Roijackers, E. Lammens & R. Portielje, 2007. Lake restoration: successes, failures and long-term effects. J Appl Ecol 44: 1095–1105. https://doi.org/10.1111/j.1365-2664.2007.01363.x.
Stock, B. C., A. L. Jackson, E. J. Ward, A. C. Parnell, D. L. Phillips & B. X. Semmens, 2018. Analyzing mixing systems using a new generation of Bayesian tracer mixing models. PeerJ 6: e5096. https://doi.org/10.7717/peerj.5096.
Syväranta, J., P. Högmander, T. Keskinen, J. Karjalainen & R. I. Jones, 2010. Altered energy flow pathways in a lake ecosystem following manipulation of fish community structure. Aquat Sci 73: 79–89. https://doi.org/10.1007/s00027-010-0161-8.
Syväranta, J. & R. I. Jones, 2008. Changes in feeding niche widths of perch and roach following biomanipulation, revealed by stable isotope analysis. Freshw Biol 53: 425–434. https://doi.org/10.1111/j.1365-2427.2007.01905.x.
Tammeorg, O., I. Chorus, B. Spears, P. Nõges, G. K. Nürnberg, P. Tammeorg, M. Søndergaard, E. Jeppesen, H. Paerl, B. Huser, J. Horppila, T. Jilbert, A. Budzynska, R. Dondajewska-Pielka, R. Goldyn, S. Haasler, S. L. Hellsten, H. Harkonen, M. Kiani, A. Kozak, N. Kotamaki, K. Kowalczewska-Madura, S. Newell, L. Nurminen, T. Noges, K. Reitzel, K. Reitzel, J. Rosinska, J. Ruuhijarvi, S. Silvonen, C. Skov, T. Vazic, A. Ventela, G. Waajen & M. Lurling, 2023. Sustainable lake restoration: From challenges to solutions. Wires Water 11: e1689. https://doi.org/10.1002/wat2.1689.
Volta, P., E. Jeppesen, B. Leoni, B. Campi, P. Sala, L. Garibaldi, T. L. Lauridsen & I. J. Winfield, 2013. Recent invasion by a non-native cyprinid (common bream Abramis brama) is followed by major changes in the ecological quality of a shallow lake in southern Europe. Biol Invas 15: 2065–2079. https://doi.org/10.1007/s10530-013-0433-z.
Winfield, I. J., 1986. The influence of simulated aquatic macrophytes on the zooplankton consumption rate of juvenile roach, Rutilus rutilus, rudd, Scardinius erythrophthalmus, and perch, Perca fluviatilis. J Fish Biol 29: 37–48. https://doi.org/10.1111/j.1095-8649.1986.tb04997.x.
Acknowledgements
We would like to acknowledge the Sino-Danish Center, Poul Due Jensens Foundation, and the Research Council of Finland (project number 340901) for providing financial support for the study. This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 951963. In addition, this research was funded through the 2020–2021 Biodiversa+ and Water JPI joint call for research projects, under the BiodivRestore ERA-NET Cofund (GA N°101003777), with the EU and the funding organization Research Council of Finland (project number 3511860). Further, we would like to thank Jian Ge for hel** in the field work.
Funding
Open access funding provided by Aarhus Universitet.
Author information
Authors and Affiliations
Contributions
ASB, MS and TL designed and conducted the experiments and collected the data. ASB, MK, and APE analyzed the data. ASB, MS, APE, MK and TL contributed to writing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
The care and use of experimental animals was complied with Danish animal welfare laws, guidelines and policies as approved by the Danish Ministry for Food, Agriculture and Fisheries and in accordance with the rules regarding removal of unwanted fish species.
Consent to participate
All authors have given their consent to participate.
Consent for publication
All authors have given their consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Priit Zingel, Helen Agasild, Maria Brigida Boveri & Erik Jeppesen / Secrets of Shallow Lakes: Insights from Research
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berthelsen, A.S., Søndergaard, M., Kiljunen, M. et al. Pelagic niche shift by fishes following restorations of a eutrophic lake. Hydrobiologia (2024). https://doi.org/10.1007/s10750-024-05568-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-024-05568-5