Abstract
El Qaa plain is one of the promising areas for agriculture projects, owing to its soil and groundwater resources. So, 14 groundwater samples were collected, chemically analyzed, hydrochemical (ratios, models and plots) and GIS techniques were applied, to evaluate the factors governing this water chemistry and suitability for irrigation. The results indicated the expansion of the agricultural area 8 times in the last 2 decades. The water can be divided into two groups; (A) freshwater (TDS ≤ 1000) in the eastern part of the study area within the clastic weathered basement rocks part of the aquifer, and (B) saline water (TDS > 1000) in the western part within the carbonates and evaporites rich sediments and close to the Gulf of Suez. The deduced factors controlling groundwater chemistry were precipitation, evaporites dissolution, seawater intrusion and ion exchange process. The calculated sodium absorption ratio (SAR) was < 10 indicating the good quality of this water for irrigation without sodicity hazard. Water of Group (A) (electric conductivity < 2250µS/cm) can be used safely for irrigation without any impact of salinity on all types of soil and plants. Group (B) water (electric conductivity > 2250µS/cm) has salinity hazard and can be used for irrigation on well-permeable soils and salt-tolerant plants. The concentrations of the studied potentially toxic elements (PTEs) were within the permissible limit for irrigation. Expansion in agricultural activities has adverse impact on groundwater quality. So, eco-friendly fertilizers should be applied in this promising area with the application of hydrologic pum** advises to safe aquifer quality from the degradation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sinai represents the Asiatic part of Egypt, its Eastern gate, and 6% of its area. Many sustainable development projects are establishing in Sinai. The Sinai sustainable development project in Egypt 2030 vision is of great importance owing to its strategic importance for Egypt. The comprehensive development project for Sinai includes all aspects and economic projects such as agriculture, industry, mining, tourism and the establishment of new urban communities and the subsequent service projects. Sinai extends over an area of 61,000 km2 representing 6% of Egypt’s area (Al-Gamal & Sadek, 2015).
El Qaa plain is one of the interesting areas for economic development projects (urban, tourism, economic ores, petroleum industry and agriculture) in southwest Sinai (Sultan et al., 2013; Abuzied & Alrefaee, 2017). It is considered the important area proven for agricultural development projects in southwestern Sinai, owing to its soil and groundwater natural resources (Selim et al., 2016; Omran, 2019). El Qaa plain is a longitudinal (NW–SE trend) land of about 1742.6 km2 stretched parallel to the Gulf of Suez between latitudes 27.45o and 28.45o N and longitudes 33.15o and 34.15o E. The soil of El Qaa plain is sand to sandy loam. The backbone of any development is availability of freshwater especially in arid regions such as Sinai. The unique natural resource of freshwater in Sinai is groundwater and to less extent rainwater. El Qaa plain contains a thick Quaternary aquifer that has both of paleowater and recent recharge from the precipitation on the eastern basement highland (Selim et al., 2016; Abu Risha et al., 2017; Abuzied & Alrefaee, 2017). As coastal aquifers, El Qaa plain Quaternary aquifer can be subjected to seawater intrusion (Massoud et al., 2010), also the impact of rapid unplanned development overpum** hazards (Said & Salman, 2021).
El Qaa Quaternary aquifer groundwater at El-Tur city has TDS values ranged from 534.91 to 8173 mg/L. The salinity is controlled by saltwater intrusion, precipitation rate (recharge) and discharge ratio (Abdel-Lattif, 2003; Gorski & Ghodeif, 2000). Massoud et al. (2009) pointed out the seawater invasion into El Qaa plain Quaternary aquifer. El-Sayed et al. (2011) noticed the increase of groundwater salinity and major ions (except HCO3) content in the direction of groundwater flow, from east to west. Geologic settings of El Qaa plain govern chemistry of groundwater, dissolution of carbonate and sulfate minerals and ion exchange on clay minerals deposits. Also, fast recharge of the aquifer from the basement heights reduce the salinity of the aquifer groundwater (Abu Risha et al., 2017). Yousif et al. (2020) mentioned that the groundwater in El Qaa plain is mostly freshwater with TDS < 1000 mg/L. They pointed out the importance of Ca-bearing mineral dissolution (calcite and gypsum) in the chemistry of this groundwater.
The expansion in the reclamation of desert land and associated groundwater over-withdraw may lead to groundwater salinization and quality degradation (Said & Salman, 2021). So, the investigation of groundwater quality in arid regions is of great importance to protect this crucial natural resource from degradation (Shukla et al., 2021). One of the agricultural activities adverse impact on groundwater is the pollution of water with NO3 and PTEs (Salman et al., 2019). Consuming water contaminated with NO3 can cause many diseases, especially for children (Shukla & Saxena, 2020, 2021). The application of agrochemicals to improve desert land fertility and productivity has many adverse impacts on the quality of soil and water resources (Khan et al., 2019; Kumar & James, 2019; Salman et al., 2019). The current work was conducted to evaluate groundwater quality for different uses in El Qaa plain, Sinai. The groundwater chemistry will be assessed in point of view the geologic, human activities and saltwater intrusion impact. In addition, the reclamation expansion in the last decades will be assessed by the application of GIS techniques.
1.1 Hydrogeological regime
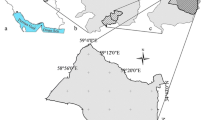
Geomorphologically, El Qaa plain contains three geomorphic features; the eastern basement mountainous region (with elevation reach 2641 m at St. Katherina), the central plain and the western sedimentary hills (e.g., Gebel Qabiliat ridge and Gebel Hammam Mousa with 250 m average elevation) (Shendy, 1984). Geologically, the area contains different rock types ranged from Precambrian basement rocks to Quaternary deposits (Fig. 1). The Precambrian basement represent about 46% of the area, while the Phanerozoic sedimentary deposits represent about 54% of it (Abuzied & Alrefaee, 2017).
Geologic map and cross section of the study area (After Abu Risha et al., 2017)
The main source of freshwater in El Qaa plain is the Quaternary aquifer. This aquifer has great variation in lithology and depth owing to the geology and structure of the study area, which has a significant impact on groundwater quality (Abu Risha et al., 2017). It is composed mainly of gravels, sands, silts and clays and can be divided into two regions based on lithofacies; the eastern part is dominated by Precambrian rock facies (cobble and boulder-sized rock debris), the western part is characterized by the presence carbonates, coral reef, shale and evaporites sediments facies (El Refai, 1984). The aquifer thickness ranges from 30 m at southwestern part to 1000 m at the central part of the El Qaa plain, while the depth to water table varied from 4 to 80 m with NE-SW flow direction (Massoud et al., 2010).
2 Material and Methods
2.1 Sampling and analyses
In April 2019, 14 samples were collected from groundwater wells in El-Qaa plain, which are mainly used for irrigation in addition to domestic use. By using the calibrated HANNA (HI 991,300) combined electrode, the pH, TDS, temperature and electrical conductivity (EC) were measured immediately in situ, after pum** the well for 15 min. Samples were collected in 1-liter plastic bottles, filled to brim, sealed tightly and transported to the laboratory in an ice box. In the laboratory, samples were filtered to get rid of suspended matters using Whatman ashless filter paper No. 42 in preparation for chemical analysis using APHA (2012) standard methods. Flame photometer (Jenway PFP7) was used to determine Na and K, and spectrophotometer (HANNA HI 83,215) to determine SO4 and NO3. Titration methods were used to determine water hardness, Ca, Mg, Cl, CO3 and HCO3. The concentrations of PTEs (Fe, Mn, Cd, Co, Cr, Cu, Ni and Pb) were determined using the ICP-MS (inductively coupled plasma mass spectrometry) (Fig. 2).
To find out the suitability of water for irrigation, the sodium adsorption ratio (SAR) (Richards, 1954) (Eq. 1) and the magnesium hazard (MH) (Eq. 2) (Szabolcs & Darab, 1964) were calculated according to the following equations (all values in meq L−1):
To know the precision and accuracy of the chemical analyzes, reference samples, blanks, analytical grade chemicals, clean glassware and bi-distilled water were used. The analyzes were performed in triplicate for each parameter, and the average was considered. Also, the error coefficient for water analysis was calculated by calculating the ionic balance of the major ions. The recovery of the measured parameters, especially PTEs, was 90–105% with respect to the reference samples as well as the e% was within ± 5% indicating the high accuracy of the analyses processes.
3 Hydrochemical models
Visual MINTEQ 3.1 was used to predict the mineral phases saturation index (SI). Piper (1944) and Durov (1948) diagrams were extracted by RockWate Aq.Qa (v.1.1) softwatre. Seawater intrusion was assessed by the HFE (Hydrochemical Facies Evolution) diagram initiated by Gimenez-Forcada (2010), with Gulf of Suez water chemistry (Abu Rish et al. 2017) as a seawater default background.
The salinization process of groundwater was evaluated by Revelle (1941) index (RI) (Eq. 3); RI values > 0.5 indicating salinization (Karunanidhi et al., 2020). To evaluate the occurrence of ion exchange process within the aquifer, chloro-alkaline indices (CAI-1 (Eq. 4) and CAI-2 (Eq. 5) (Schoeller, 1965) were calculated. Positive CAI-1 and CAI-2 values are indicative of reverse ion-exchange, negative values indicate forward ion exchange, and zero value indicates the disappearance of the ion exchange role on the groundwater chemistry. The intensity of ion exchange is directly proportional to the CAI-1 and CAI-2 absolute values (Kaur et al., 2019).
4 Results and discussion
4.1 Water–rock interaction
The physicochemical measurements variability was outlined in Table 1 for the studied samples. The reactivity of water was slightly alkaline with pH values ranged from 7.19 to 7.75. The negative relationship between pH and both SO4 and Ca indicates that the gypsum and/or anhydrite dissolution still actively happens in the area (Fig. 3a). The average salinity measurement; TDS and EC were 1379.3 mg/L and 2752.9 µS/cm, respectively. It was observed that the studied samples can be divided into two groups based on TDS values. Group (A) of low salinity (340–950 mg/L) lies in the eastern side of the study area and Group (B) of high salinity (1210–3470 mg/L) lies in the western side of the study area, close to Gulf of Suez. Accordingly, the high salinity may be attributed to saltwater intrusion from the Gulf of Suez. According to Boyd (2000), 5 samples are considered hard water with 150 < TH ≤ 300 and the rest of samples were very hard water with TH > 300 (Table 1). The major cations and anions concentrations followed the descending order Na > Ca > Mg > K and Cl > SO4 > HCO3 > CO3 > NO3.
Group (B) shows higher levels of the studied ions than Group (A), except HCO3, supporting the intrusion of saltwater. The water–rock interaction is the main controller of water chemistry in the study area as indicated from the SI values, dissolution of carbonates and evaporates minerals (Table 1), which are among the main minerals found in the study area sediments (El Ammawy, 2016). The main sources of Ca, Mg, Na, K, SO4 and Cl are the dissolution of anhydrite, gypsum, halite and sylvite as indicted from the negative SI of the mention minerals (Table 1).
Calcite and dolomite have not any noticed contribution to Ca and Mg as seen from the negative correlation of Ca and Mg with HCO3 (Fig. 3b). Also, the negative correlation between HCO3 and the studied cations (Fig. 3b) indicates the recent recharge of this aquifer (Abdelhafiz et al., 2021) from the precipitation on Sinai heights, east El Qaa plain.
The water–rock interaction can be deduced from Gaillardet et al. (1999) and Gibbs (1970) diagrams (Fig. 4). Where they explained that the weathering of silicate and the dissolution of evaporites as well as evaporation processes are the main factors controlling the chemistry of water. The strong role of evaporation process is supported also, by significant positive correlation between Cl and TDS (r = 1, p= 0.01) (Fig. 3c) (Wang et al., 2019). Silicate weathering, as a mineralization process of groundwater, can be deduced from the low molar ratios (< 1) of Cl/ƩAnions, Na/Cl, Na/ (Na + Ca) and Mg/(Ca + Mg) (Sunkari et al., 2021).
MAC: USDA (2011) maximum allowable concentration for Irrigation water.
4.2 Salinization
Groundwater at El Qaa plain is subjected to salinization as most of the coastal aquifer, this can be indicated from the high RI values > 0.5 (Table 1), varied from 2.9 to 125.4. It was observed that the samples closed to the Gulf of Suez have RI value greater than the samples remoteness than the gulf indicating the role of seawater invasion on the chemistry of the aquifer. The RI positive correlation with the major ions (Ca, Mg, Na, Cl and SO4) is an indication of the role of these ions as salinization tracers. The RI positive correlation with NO3 points out the role of anthropogenic activities on the salinity of groundwater (Fig. 5). The seawater intrusion impact on the study area can be indicted from HFE diagram (Fig. 6) of Gimenez-Forcada (2010). The calculated SI was negative indicating under-saturated (dissolution) of anhydrite, aragonite calcite, dolomite, halite and gypsum minerals. The study area sediments are rich in these minerals (El Ammawy, 2016). The value < 1 of Na/Cl supports the salinization process of the groundwater in the study area. The Na/Cl < 1 and consequentially the salinization process can be attributed to evaporation process, seawater intrusion reverse ion exchange and the presence of additional Cl sources, anthropogenic activities; agricultural return flow (Jia et al., 2019).
4.3 Ion exchange reactions
The ion exchange process has significant importance in water geochemistry. In this process, certain ions in water will be replaced by other ions from the exchanger rock (mostly clay). Schoeller (1965) chloro-alkaline indices have been used to reflect the ion exchange process in the studied groundwater. The values of the two indices were positive in all the studied samples, except sample number (7), indicating a reverse ion exchange process, where Na and K in groundwater are replaced by Ca and Mg from the rocks. The reverse ion exchange process led to the hardened of groundwater as implied from the positive correlation between TH and both CAI-1 and CAI-2. The reverse ion exchange was also indicated by plotting the samples on Durov (1948) diagram (Fig. 7a) and the higher concentration of (Ca + Mg) vz (HCO3 + SO4) diagram (Fig. 7b) of Zhang et al. (2018).
4.4 Hydrochemical facies (Piper’s diagram)
The Piper diagram (Piper, 1944) is widely used to determine the relationships between the various ions dissolved in water and to determine the ionic species and hydrochemical facies of water. The cationic triangle indicating no dominant cation in 12 samples and Na –dominant in 2 samples with a noticed trend for Ca and Mg increase, while the anionic triangle indicated the dominance of Cl in 12 samples (Fig. 8). The diamond shape revealed that 2 main water types of Ca-Mg-SO4-Cl (12 samples) and Na-K-SO4-Cl (2 samples). These water types point out the seawater intrusion with a high concentration of Cl and Na, reverse ion exchange as indicated by the non-dominance cation and the natural recharge as indicated by the increasing of HCO3.
4.5 Potentially toxic elements (PTEs)
As a result of the environmental importance of PTEs, the levels of Fe, Mn, As, Cd, Co, Cr, Cu, Ni and Pb were evaluated in the studied groundwater. The average Fe, Mn, As, Co, Cu, Ni and Pb concentrations were 77.4, 29.7, 86.9, 40.4, 52.1, 92.6 and 28.8 µg/L, while Cd and Cr were BDL (Table 1). Some PTEs (e.g., Fe, Mn, Cu, Cr) are of great importance to complete the biochemical processes in human and considered as micronutrients at low concentrations, while others (e.g., As, Cd, and Pb) have been linked to renal failure, cancer, brain and liver damage and other diseases in humans (Seleem et al., 2021; Tirkey et al., 2017). In plants, it can cause seed germination, shoot, root and production reduction (Chibuike & Obiora, 2014). Afandy et al. (2016) mention that the Quaternary aquifer water of southwest Sinai contains BDL-939.6, BDL-406.8, BDL-301.1, 9.6–21.2, 3.9–22.6, 16.8–25.7, BDL-99.2, 10.6–29.4, µg/L of Fe, Mn, As, Cd, Co, Cr, Cu and Pb, respectively. The higher concentrations of PTEs recorded by Afandy et al. (2016) than the current study may be attributed to the mining processes the Northern area. El Alfy (2012), who recorded 22, BDL, BDL, 42, 26, 12 and 1.2 µg/L of As, Cd, Co, Cr, Cu, Ni and Pb, respectively, in the groundwater of Al Arish area, Sinai. The variation in the concentration of PTEs in the groundwater of Sinai may be referred to the nature of the rocks in each region, as well as anthropogenic activities. Morad et al. (2018) indicated the.
presence of Arsenopyrite in the rocks of South Sinai and its expected adverse impact on groundwater quality. It was observed that the eastern samples have higher concentrations of As than the western samples supporting the contribution of weathering of basement rocks and natural contribution of As. The Sinai Peninsula is considered the first resource for mineral wealth in Egypt; petroleum, copper, phosphates, iron, coal, manganese, uranium, feldspar, gypsum, base and precious metals. The mining and processing of these raw materials can adversely affect the quality of groundwater.
4.6 Irrigation water quality
The studied wells are used mainly for irrigation purposes. Irrigation water must be of low salinity and free from hazardous ions. The most widespread applied evaluation parameters of water for irrigation are EC (salinity hazard) and SAR (sodium hazard). Water with EC ≤ 2250µS/cm (Richards, 1954) is permissible for irrigation, samples 1–7, while samples with EC > 2250µS/cm are unsuitable for irrigation; samples 8–14. Saline water will change the osmosis pressure around plant roots and hence reduce water uptake by the plants leading to its wilt. Saline water will lead to the accumulation of salts in soil; raising soil salinity and reducing crops yield by about 20–50% (Mostafazadeh-Fard et al., 2009; Shrivastava & Kumar, 2015). High Na in the soil can lead to the damage of soil structure and consequentially waterlogging also it can cause plant leaf burn (Zaman et al., 2018). All the studied groundwater samples have not any Na problem with SAR values < 10 and can be used safely for all soil types. The USSL diagram is integration between EC and SAR (Richards, 1954) implemented to give a wide classification of groundwater (Fig. 9). The studied samples belong to C2-S1 (2 samples), C3-S1 (5 samples), C4-S2 (5 samples) and C4-S3 (2 samples). The C2-S1 and C3-S1 classes of water samples are suitable for irrigation for all soil and plant types, while C4-S2 and C4-S3 are suitable under specific conditions good drainage soil and salt tolerable plants. In spite of the relative high concentrations of the studied PTEs, they were within the irrigation water specifications. Magnesium has important impact on crop yield because excess Mg can cause soil alkalinity and hence reduce crop yield, all the studied samples have MH% ≤ 50 (Table 1), indicating the suitability of water for irrigation.
4.7 Land use-land cover (LU-LC) changes
The change in land use can impact the hydrological action temporally and spatially (Verma et al., 2020). The expansion of agriculture led to the overpum** of groundwater and consequently the lowering the water table level by about 42 m (Khan et al., 2019). Also, the associated application of agrochemicals has led to the contamination of groundwater with NO3 and toxic elements (Khan et al., 2019; Salman et al., 2019). Elmahdy and Mohamed (2016) noted the link between groundwater quality and quantity depletion in UAE, and the LULC changes. The overpopulation in Egypt and the small area of fertile soil (3.4% of Egypt area) led to expansion in desert area reclamation to secure the great demand for food. Sinai has special importance for Egypt owing to its location and historic importance as well as natural resources. The agriculture area was duplicated 8 times in the study area within the last two decades from 1.8 km2 in 2000 to 14.2 km2 in 2020 (Fig. 10). This increase in the agricultural area required an increase in groundwater exploitation and extraction as well as application of agrochemicals. Agrochemicals impact can be noticed from the recorded NO3 (Table 1) values (1.7–48.5 mg/L).
5 Conclusion
El Qaa plain is one of the most promising areas for establishing sustainable development projects due to its natural resources (soil and water). The population increase led to the expansion of desert land reclamation and the digging of more wells. The area of reclaimed lands increased in the previous two decades from 1.8 to 14.2 km2. The expansion in desert land reclamation and associated groundwater overpum** can lead to groundwater quality degradation. The overpum** in the study area accelerated mineral dissolution and seawater intrusion into the Aquifer, and consequentially water salinization. The agricultural activities impact groundwater quality as noticed from the recorded nitrates. Based on, the studied SAR and MH the studied groundwater has good quality for irrigation, only group “B” samples can have some restrictions owing to their salinity. The studied groundwater contains considered concentrations of As, Ni and Pb. The main source of these elements may be adjacent basement rocks. Even though, the studied groundwater can be used safely for irrigation. The study area need good plan for groundwater extraction and agricultural practices to prevent the associate adverse impact and protect the area natural resources from degradation. Generally, the studied aquifer is promising and hence uncontrolled pum** and application of illegal agrochemicals must be panned.
Data availability
All the data are provide within the manuscript.
Code availability
Not applicable.
References
APHA. (2012). Standard methods for the examination of water and waste water, 22nd ed. American Water Works Association, Water Environment Federation.
Abdel-Lattif, A. (2003). Groundwater chemistry of the shallow aquifer, El-Tur area, South Sinai, Egypt. Journal of Environmental Hydrology,11, 1–16.
Abdelhafiz, M. A., Seleem, E. M., El Nazer, H. A., Zeid, S. M., Salman, S. A., & Meng, B. (2021). Shallow groundwater environmental investigation at northeastern Cairo, Egypt: Quality and photo-treatment evaluation. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-021-00933-y
Abu Risha, U., Mosaad, S., El Abd, E., & Hasanein, A. (2017). The impact of the geologic setting on the Quaternary aquifer, El-Tur area, Southwest Sinai, Egypt. Arabian Journal of Geosciences, 10, 461. https://doi.org/10.1007/s12517-017-3237-0
Abuzied, S. M., & Alrefaee, H. M. (2017). Map** of groundwater prospective zones integrating remote sensing, geographic information systems and geophysical techniques in El-Qaà plain area, Egypt. Hydrogeology Journal. https://doi.org/10.1007/s10040-017-1603-3
Afandy, A. H., Geriesh, M. H., Eweda, S. A., Alshami, A. S., & Halim, K. A. (2016). Hyrogeochemisrty and radioactivity of groundwater in Baba-Sidri basins, Southwest Sinai, Egypt. BFSZU. Bulletin of Faculty of Science, Zagazig University,38, 128–159. https://doi.org/10.21608/BFSZU.2016.31071
Al-Gamal, S., & Sadek, M. (2015). An assessment of water resources in Sinai Peninsula, using conventional and isotopic techniques, Egypt. International Journal of Hydrology Science and Technology,5(5–3), 241–257.
Boyd, C. E. (2000). Water quality: An introduction (p. 330). Dordrecht: Kluwer Acad Publisher.
Chibuike, G. U., & Obiora, S. C. (2014). Heavy Metal Polluted Soils: Effect on plants and bioremediation methods. Applied and Environmental Soil Science. https://doi.org/10.1155/2014/752708
Durov, S. A. (1948). Natural waters and graphic representation of their composition. Doklady Akademii Nauk,59, 87–90.
El Alfy, M. (2012). Integrated geostatistics and GIS techniques for assessing groundwater contamination in Al-Arish area, Sinai, Egypt. Arabian Journal of Geosciences,5, 197–215. https://doi.org/10.1007/s12517-010-0153-y
El Ammawy, M. (2016). Sedimentological studies for Cretaceous rocks of Gabal El-Qabaliyat, Southwestern portion of Sinai, Egypt. M.Sc. Thesis. Egypt, Banha Uni (p. 175).
El Refai, A. A. (1984). Geomorphological and hydrogeological studies on El-Qaa plain, Gulf of Suez, Sinai, Egypt M. Sc. Thesis, Faculty of Science, Cairo University, 298p.
El-Sayed, S. A. W., Sabet, H. S., & Ali, H. M. H. (2011). Appraisal of groundwater in El-Tur area, Southwest Sinai, Egypt. ISOTOPE & RAD RES,43(3), 605–632.
Elmahdy, S. I., & Mohamed, M. M. (2016). Land use/land cover change impact on groundwater quantity and quality: A case study of Ajman Emirate, the United Arab Emirates, using remote sensing and GIS arabian. Journal of Geosciences,9, 722.
Gaillardet, J., Dupre, B., Louvat, P., et al. (1999). Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chemical Geology,159, 3–30.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science,170, 1088–1090. https://doi.org/10.1126/science.170.3962.1088
Giménez-Forcada, E. (2010). Dynamic of seawater interface using hydrochemical facies evolution diagram. Ground Water,48(2), 212–216. https://doi.org/10.1111/j.1745-6584.2009.00649.x
Gorski, J., & Ghodeif, K. (2000). Salinization of shallow water aquifer in El-Qaa Costal plain, Sinai, Egypt. In 16th SWIM, Wolin Island.
Jia, X., O’Connor, D., Hou, D., et al. (2019). Groundwater depletion and contamination: Spatial distribution of groundwater resources sustainability in China. Science of the Total Environment,672, 551–562. https://doi.org/10.1016/j.scitotenv.2019.03.457
Karunanidhi, D., Aravinthasamy, P., Deepali, M., Subramani, T., & Sunkari, E. D. (2020). Appraisal of subsurface hydrogeochemical processes in a geologically heterogeneous semi-arid region of south India based on mass transfer and fuzzy comprehensive modeling. Environmental Geochemistry and Health,43, 1009–1028. https://doi.org/10.1007/s10653-020-00676-2
Kaur, L., Rishi, M. S., Sharma, S., Sharma, B., Lata, R., & Singh, G. (2019). Hydrogeochemical characterization of groundwater in alluvial plains of river Yamuna in northern India: An insight of controlling processes. Journal of King Saud University – Science. https://doi.org/10.1016/j.jksus.2019.01.005
Khan, Q., Kalbus, E., Alshamsi, D. M., Mohamed, M. M., & Liaqat, M. U. (2019). Hydrochemical analysis of groundwater in Remah and Al Khatim regions, United Arab Emirates. Hydrology,6, 60. https://doi.org/10.3390/hydrology6030060
Kumar, P. J. S., & James, E. J. (2019). Geostatistical and geochemical model-assisted hydrogeochemical pattern recognition along the groundwater flow paths in Coimbatore district, South India. Environment, Development and Sustainability,21, 369–384. https://doi.org/10.1007/s10668-017-0043-5
Massoud, U., Santos, F., El Qady, G., Atya, M., & Soliman, M. (2010). Identification of the shallow subsurface succession and investigation of the seawater invasion to the Quaternary aquifer at the northern part of El Qaa plain, Southern Sinai, Egypt by transient electromagnetic data. Geophysical Prospecting,58, 267–277. https://doi.org/10.1111/j.1365-2478.2009.00804.x
Morad, A. E., Helmy, H. M., Abdel Rahman, H. B., Ali, S., & Ali, M. S. (2018). Toxic metalloid: A barrier in front of mineral exploration, Um Zariq area, East Sinai, Egypt. In The 2nd International conference on natural resources and renewable energy conference proceedings, 23–26 April 2018, Hurghada, Egypt.
Mostafazadeh-Fard, B., Mansouri, H., Mousavi, S., & Feizi, M. (2009). Effects of different levels of irrigation water salinity and leaching on yield and yield components of wheat in an arid region. Journal of Irrigation and Drainage Engineering,135(1), 32–38. https://doi.org/10.1061/(ASCE)0733-9437(2009)135:1(32)
Omran, E. E. (2019). Cloud-Based Non-conventional land and water resources for sustainable development in Sinai Peninsula, Egypt. In A. M. Negms (Ed.), Unconventional water resources and agriculture in Egypt. (pp. 339–374). Springer. https://doi.org/10.1007/698_2017_63
Piper, A. M. (1944). A graphic procedure in the geochemical interpretation of water analyses. Transactions American Geophysical Union,25, 914–928.
Ravikumar, P., & Somashekar, R. K. (2017). Principal component analysis and hydrochemical facies characterization to evaluate groundwater quality in Varahi river basin, Karnataka state, India. Applied Water Science,7, 745–755.
Revelle, R. (1941). Criteria for recognition of the sea water in ground-waters. EOS. Transactions of the American Geophysical Union,22, 593–597.
Richards, L. A. (1954). Diagnosis and improvement of saline and alkali soils. USDA Agric. Handb. 60. USDA, Washington, DC.
Roy, P. D., Selvam, S., Venkatramanan, S., Logesh, N., Lakshumanan, C., & Sanchez-Zavala, J. L. (2021). Identification of sources and groundwater recharge zones from hydrochemistry and stable isotopes of an agriculture-based paleo-lacustrine basin of drought-prone northeast Mexico. Geochemistry,81(2), 125742. https://doi.org/10.1016/j.chemer.2021.125742
Said, I., & Salman, S. A. (2021). Salinization of groundwater under desert reclamation stress at Qena region, Egypt. Journal of African Earth Sciences,181, 104250. https://doi.org/10.1016/j.jafrearsci.2021.104250
Salman, S. A., Arauzo, M., & Elnazer, A. A. (2019). Groundwater quality and vulnerability assessment in west Luxor Governorate, Egypt. Groundwater for Sustainable Development,8, 271–280.
Schoeller, H. (1965). Qualitative evaluation of groundwater resources. In Methods and techniques of groundwater investigations and development (pp. 5483). UNESCO.
Seleem, E. M., Mostafa, A., Mokhtar, M., & Salman, S. A. (2021). Risk assessment of heavy metals in drinking water on the human health, Assiut city and its environs, Egypt. Arabian Journal of Geosciences,14, 427. https://doi.org/10.1007/s12517-021-06784-2
Selim, E., Abdel-Raouf, O., & Mesalam, M. (2016). Implementation of magnetic, gravity and resistivity data in identifying groundwater occurrences in El Qaa Plain area, Southern Sinai, Egypt. Journal of Asian Earth Sciences,128, 1–26. https://doi.org/10.1016/j.jseaes.2016.07.020
Shendy, E. H. (1984). Geological and geophysical investigation for groundwater in El-Qaa plain, Southwestern Sinai. M. Sc. Thesis, Faculty of Science, Suez Canal University, Ismailia.
Shrivastava, P., & Kumar, R. (2015). Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi Journal of Biological Sciences,22, 123–131. https://doi.org/10.1016/j.sjbs.2014.12.001
Shukla, S., & Saxena, A. (2020). Groundwater quality and associated human health risk assessment in parts of Raebareli district, Uttar Pradesh, India. Groundwater for Sustainable Development,10, 100366. https://doi.org/10.1016/j.gsd.2020.100366
Shukla, S., & Saxena, A. (2021). Appraisal of Groundwater Quality with Human Health Risk Assessment in Parts of Indo–Gangetic Alluvial Plain, North India. Archives of Environmental Contamination and Toxicology,80, 55–73. https://doi.org/10.1007/s00244-020-00771-6
Shukla, S., Saxena, A., Khan, R., & Li, P. (2021). Spatial analysis of groundwater quality and human health risk assessment in parts of Raebareli district, India. Environmental Earth Sciences,80, 800. https://doi.org/10.1007/s12665-021-10109-7
Sultan, S. A., Abdel Rahman, N., & Ramadan, T. M. (2013). The use of geophysical and remote sensing data analysis in the groundwater assessment of El Qaa plain, South Sinai, Egypt. In 5th international conference on water resources and arid environments, pp. 292–305.
Sunkari, E. D., Abu, M., & Zango, M. S. (2021). Geochemical evolution and tracing of groundwater salinization using different ionic ratios, multivariate statistical and geochemical modeling approaches in a typical semi-arid basin. Journal of Contaminant Hydrology,236, 103742. https://doi.org/10.1016/j.jconhyd.2020.103742
Szabolcs, I., & Darab, C. (1964). The influence of irrigation water of high sodium carbonate content of soils. In Proceedings of the 8th international congress of international symposium on system synthesis (ISSS), transactions (Vol. 2, pp. 803–812).
Tirkey, P., Bhattacharya, T., Chakraborty, S., & Baraik, S. (2017). Assessment of groundwater quality and associated health risks: A case study of Ranchi city, Jharkhand, India. Groundwater for Sustainable Development,5, 85–100.
USDA. (2011). Assessing water quality for human consumption, agriculture, and aquatic life uses tom pick. United States Department of Agriculture, National Agricultural Statistics Service.
Verma, P., Singh, P., & Srivastava, S. K. (2020). Impact of land use change dynamics on sustainability of groundwater resources using earth observation data. Environment, Development and Sustainability,22, 5185–5198. https://doi.org/10.1007/s10668-019-00420-6
Wang, H., Guo, H., **u, W., Bauer, J., Sun, G., Tang, X., & Norra, S. (2019). Indications that weathering of evaporite minerals affects groundwater salinity and as mobilization in aquifers of the northwestern Hetao Basin, China. Applied Geochemistry,109, 104416. https://doi.org/10.1016/j.apgeochem.2019.104416
Yousif, M., Hussien, H. M., & Abotalib, A. Z. (2020). The respective roles of modern and paleo recharge to alluvium aquifers in continental rift basins: A case study from El Qaa plain, Sinai, Egypt. Science of the Total Environment,739, 139927. https://doi.org/10.1016/j.scitotenv.2020.139927
Zaman, M., Shahid, S. A., & Heng, L. (2018). Irrigation water quality. In M. Zaman (Ed.), Guideline for salinity assessment, mitigation and adaptation using nuclear and related techniques. Springer. https://doi.org/10.1007/978-3-319-96190-3
Zhang, Y., Xu, M., Li, X., Qi, J., Zhang, Q., Guo, J., Yu, L., & Zhao, R. (2018). Hydrochemical characteristics and multivariate statistical analysis of natural water system: A case study in Kangding County, Southwestern China. Water,10, 80. https://doi.org/10.3390/w10010080
Acknowledgements
Many grateful thanks for the Geological Sciences Dept., National Research Centre for the facilities during sample handling and analyses.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Not applicable.
Author information
Authors and Affiliations
Contributions
S contributed to conceptualization, data and sample collection, chemical analysis of samples, interpretation, figures and diagrams preparation and draft writing, manuscript review and editing. AE-A performed data collection, chemical analysis of samples, manuscript review and editing. E did sample collection, chemical analysis of samples, preparation of site map and land cover map, manuscript review. A conducted sample collection and manuscript review.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there are no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salman, S.A., El-Anwar, E.A.A., Aita, S.K. et al. Geo-environmental factors controlling groundwater hydrogeochemistry, salinization and quality in El Qaa plain coastal aquifer, Southwest Sinai, Egypt. Environ Dev Sustain (2023). https://doi.org/10.1007/s10668-023-03830-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10668-023-03830-9