Abstract
Titanium dioxide, frequently used in commonplace products, is now regularly detected in aquatic environments. Understanding its toxic effects on native biota is essential. However, combined toxicity with commonly occurring pollutants, such as the pharmaceutical diclofenac, may provide more insight into environmental situations. Therefore, the present study aimed to evaluate the effects of titanium dioxide and diclofenac, individually and combined, on the macrophyte Egeria densa. Diclofenac uptake and removal by the macrophyte were assessed. Diclofenac and titanium dioxide were mixed prior to exposure to allow binding, which was assessed. Toxicity of the individual compounds and the combination was evaluated by assaying enzymes as bioindicators of biotransformation and the antioxidative system. Cytosolic glutathione S-transferase and glutathione reductase activities were increased by diclofenac, titanium dioxide, and the combination. Both enzymes’ activities were more significantly elevated by diclofenac and the combination than nanoparticles alone. Microsomal glutathione S-transferase was unaffected by diclofenac exposure but inhibited with titanium dioxide and the mixture. Diclofenac elicited the most significant response. Based on the data, the cytosolic enzymes effectively prevented damage.
Highlights
-
mGST was inhibited by TiO2, but DCF exposure was insignificant.
-
cGST and GR activities increased with DCF exposure.
-
No synergistic effect on cGST and GR with combined exposure.
-
DCF, rather than TiO2, was responsible for oxidative stress-related toxicity.
-
Macrophytes remained healthy despite exposures at high concentrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Various engin eered nanomaterials are produced in hundreds of tons per year (Hendren et al. 2011). The nanotechnology industry has enormous growth prospects and opportunities for commercial development due to the vast range of applications of nanomaterials and particles. Nanoparticles (NPs), which are in the 10−9 m range, are used in electronics, medical and pharmaceutical industries, consumer goods, food production, as well as military applications (Khan et al. 2019). NPs are of great importance for scientific studies as a middle link between bulk materials and atomic structures. These minute particles have a much larger surface-to-volume ratio than similar masses of larger-scale materials. As the surface area per mass of material increases, considerably more material can come into contact with surrounding substances. The larger the surface area, the greater the substance’s reactivity, allowing improved catalysts to be created (Lien et al. 2015); e.g., the drastic property changes of gold NPs as oxidants compared to gold macroparticles. NPs’ mechanical and magnetic properties also differ from their regular-shaped counterparts, meaning that adhesion and capillary forces exceed macroscopic forces, including superparamagnetic forces (Wahajuddin and Arora 2012). Thermal and optical properties also shift; i.e., with decreasing size, surface energy increases, thus reducing melting points (Mashayekh and Dorranian 2014).
The use of titanium (IV) oxide (or titanium dioxide, TiO2-NPs) is increasing due to its nanosized features, low toxicity, biocompatibility, intrinsic properties, and manufacturing techniques (Jarosz et al. 2016; Kafshgari et al. 2019; Molaeirad et al. 2015; Naseri et al. 2015). These nanomaterials are also recognized for their high refractive index, light scattering capabilities, and photocatalytic activities in the presence of UV with equal or higher energy than its bandgap energy. TiO2-NPs occur in three crystalline phases, brookite, rutile, and anatase, with the latter showing a more extensive band gap and, thus, the highest photocatalytic effects (Skocaj et al. 2011). Therefore, TiO2-NPs are one of the most commonly used metal oxides (Jovanović 2015). They are widely used in paints, floor coatings, paper cosmetics, cleaning products, and sunscreens. However, some studies have shown contradictory evidence regarding the toxicity and long-term stability of these NPs, as reviewed by Skocaj et al. (2011). Among others, TiO2 has been implicated in oxidative stress induction as well as cellular dysfunction as it produces hydroxyl radicals with cytotoxic effects. However, the response of the antioxidative system in this regard remains unexplored.
Engineered NPs, including titanium-based nanomaterials, have been detected in the environment at concentrations up to 48 ng/ml (Tovar-Sánchez et al. 2013); however, Environmental Fate Modeling predicts this level to be closer to 10000 ng/ml (Maurer-Jones et al. 2013). These particles may enter the environment in various ways, primarily through industrial wastewater. NP-containing personal care products may also deposit in domestic wastewater and, from there, enter sewage sludge (Coll et al. 2016; Sun et al. 2016). Once in aquatic ecosystems, NPs could mix with other pollutants, including pharmaceuticals, and could affect keystone species in various ways. According to Thiagarajan et al. (2021), who reviewed the interactions between nanomaterial, pharmaceuticals, and nano/microplastics, these compounds are commonly detected in aquatic environments globally and bound to co-occur and interact. As the adverse effects of pharmaceuticals have already been recognized (Fent 2008; Mezzelani and Regoli 2022), it becomes vital to understand the impact of NPs on biota and in combinations with pharmaceuticals detected in surface waters globally. One such globally detected drug is diclofenac (DCF) (Li 2014). The environmental concentration of DCF in aquatic environments varies considerably (Lonappan et al. 2016). Fekadu et al. (2019) reported mean diclofenac concentrations detected in European waters to range from approx. 3 to 5 ng/ml and in African waters from approx. 5 to 7 ng/ml.
As a first step in evaluating the toxicity of NPs as well as their combined toxicity as vectors for pharmaceuticals, TiO2-NP in its anatase form was selected for this study due to its wide use. The toxicity of TiO2-NPs, DCF, and a combination of the NPs and the pharmaceutical, was evaluated on the ecologically essential macrophyte species Egeria densa. Macrophytes serve as primary producers, as well as habitat, shelter, and breeding space for other organisms contributing to the overall biodiversity. They also influence the nutrient cycles in aquatic environments (Bakker et al. 2016; Esteves 1998; Kennedy et al. 2004; Pott and Pott 2003; Thomaz and Cunha 2010); and are excellent bioindicators (Ravera 2001). E. densa was selected based on its advantageous features, including rapid growth and natural ubiquity, and due to the limited information on adverse effects on this macrophyte caused by NPs. Additionally, information on how E. densa responds to NPs and pharmaceuticals may help evaluate its potential utility in the phytoremediation of water contaminated with these substances. E. densa has been shown to efficiently remediate NPs such as Ag-NPs (Bernas et al. 2017) and pharmaceuticals (De Morais Calado et al. 2019). However, information on the phytoremediation of DCF and TiO2, as well as a combination of the two, is lacking.
Toxicity is often mediated by oxidative stress, as an organism’s inability to eliminate increased reactive oxygen species (ROS) at a cellular level would lead to severe adverse effects and eventual mortality (Sarkar et al. 2014). Fluctuations in the antioxidative enzyme responses are often used as bioindicators of oxidative stress (Gutteridge 1995). In the present study, glutathione reductase (GR) and glutathione S-transferase (GST) were selected as biomarkers. GR is an antioxidative defense enzyme involved in recycling glutathione to combat ROS generated from xenobiotics. GST is a crucial enzyme in phase II of the biotransformation system, which is vital in eliminating xenobiotics.
The study, therefore, aimed to evaluate the toxicity of TiO2 and DCF as well as combined toxicity in E. densa by evaluating GST and GR as biomarkers of antioxidative response to xenobiotic exposure.
Materials and methods
Chemicals and reagents
DCF (sodium salt, ≥99%) was bought from Cayman Chemical Company (Michigan, USA). Stock solutions were prepared in pure ethanol as required, and further dilutions were conducted in the cultivation/exposure media of choice.
Anatase TiO2 (100% anatase, <25 nm, specific surface area 45- 50 m2/g, purity 99.7%) was purchased from Sigma-Aldrich Co. Ltd. (Steinheim, Germany) and was from the same batch as used by Okupnik et al. (2015) who characterized the material in terms of size, morphology, zeta potential, z-average hydrodynamic diameter, and the polydispersity index (PDI).
All chemicals used for exposure and analysis were of analytical-grade quality and were obtained from Sigma-Aldrich Co Ltd. (Steinheim, Germany) unless stated otherwise.
Egeria densa
E. densa (strands of 10–20 cm) was purchased from Extraplant (Extragroup GmbH, Germany) and cultivated in a glass tank (100 cm × 60 cm × 60 cm) at 24 ± 1 °C. The plants were grown under cool white fluorescent light with a light intensity of 38 μE/m2/s and a 14:10-h light-dark photoperiod. The culture media consisted of modified Provasoli’s culture medium containing CaCl2 (0.2 g/l), NaHCO3 (0.106 g/l), and sea salt (0.1 g/l) in de-ionized water (Vilvert et al. 2017). The macrophytes were acclimated to laboratory conditions for seven days before the exposures. DCF uptake into E. densa and removal from the media were evaluated prior to the exposure experiments to establish its suitability for this investigation. Three-centimeter E. densa strands were exposed to 250 ng/ml DCF in beaker experiments against controls for 96 h under the same conditions as during acclimation (n = 5). Plant and media samples were collected after 24, 48, 72, and 96 h. DCF was extracted from the plant tissue as detailed by De Morais Calado et al. (2019), and DCF was quantified as described in section 2.4.
Exposure setup
Three treatment solutions were prepared. The first consisted of DCF diluted to 250 ng/ml in the E. densa cultivation media. Concentrations previously reported for DCF in wastewater and the environment served as guidance for choosing this exposure concentration (Esterhuizen-Londt et al. 2017). The second exposure solution consisted of 250 ng/ml DCF combined with 1 mg/ml TiO2-NP anatase in cultivation media, and the third consisted of 1 mg/ml TiO2-NP anatase only in the cultivation media. The control consisted of the macrophyte cultivation media without additions of other chemicals. A sample from each prepared exposure solution was collected for qualitative analysis with liquid chromatography-tandem mass spectroscopy (time 0). The solutions were stirred for 24 h in the dark, and a second sample was taken for analysis to measure any degradation or binding (time 24).
After the 24 h binding/degradation study, the treatment solutions were decanted in 100 ml beakers in replicates of five, and a 20 ± 1 cm strand of E. densa was added to each replicate and exposed for 24 h under the same conditions as during cultivation. After 24 h of exposure, another media sample was taken for quantitative analysis (time 48). The plant material was removed from the treatments, washed in distilled water, dried, and snap-frozen in liquid nitrogen. The samples were stored at −80 °C until further processing to evaluate the enzyme activities.
Quantitative analysis of diclofenac
DCF was quantified on a 1200 infinity series liquid chromatography (Agilent, Waldbronn, Germany) coupled to triple quadrupole mass spectrometry (model 6460, Agilent) (LC-MSMS) with electron spray ionization (Jet Stream, Agilent) using a Kinetex™ C18 reverse phase column (2.1 × 100 mm, 1.7 U, 100 Å, Phenomenex, Aschaffenburg, Germany). The LC-MSMS settings and protocol were detailed by Esterhuizen-Londt et al. (2017) with a 0.5 pg on column (S/N > 5) limit of quantification. Prior to analysis, all samples were centrifuged at 10,000 × g at 10 °C for 30 min.
Enzyme extraction and activity assays
The enzymes were extracted according to Pflugmacher (2004). In short, the frozen plant material was pestled to a refined power using liquid nitrogen, and 1.5 g thereof was suspended in 0.1 M potassium phosphate buffer (pH 6.5) containing 20% glycerol, 1.4 mmol/l dithioerythritol, and 1 mmol/l ethylenediaminetetraacetic acid. The samples were stirred for 20 min before centrifugation at 5400 × g (4 °C) for 10 min to remove cell debris. The supernatant was centrifuged at 86,900 × g (4 °C) for 60 min to collect the microsomal fraction. The supernatant was subjected to ammonium sulfate precipitation (35–80%), collecting the pellet after centrifugation. The cytosolic enzymes, now contained in the pellet, were suspended in a 20 mM pH 7 sodium phosphate buffer. The samples were desalted using Sephadex NAP-10 columns (GE Healthcare, Little Chalfont, UK).
The protein concentrations of the two fractions of each sample were measured according to Bradford (1976). The enzymatic activities of GST (microsomal and cytosolic) and GR (cytosolic) were measured spectrophotometrically (Infinite M200, Tecan, Männedorf, Switzerland) and expressed in the SI units of kat/mg protein. GST activity (EC 2.5.1.18) was assayed by measuring an increase in optical density at 340 nm following to conjugation of glutathione and 1-chloro-2,4-dinitrobenzene (Habig et al. 1974). GR activity (EC 1.6.4.2) was measured as a decrease at 340 nm as nicotinamide adenine dinucleotide phosphate (NADPH) was consumed (Carlberg and Mannervik 1985).
Statistical analyses
All statistical analyses were performed using IBM® SPSS® Statistics 28.0.0.0 (190) (2021). The DCF concentrations quantified in the treatments were compared with the independent samples t test, and the DCF concentrations quantified over time were compared using the paired-samples t test. The enzyme activity data did not meet the requirements of sphericity and homogeneity, and thus, the non-parametric Kruskal-Wallis test with pairwise comparisons was used, observing an alpha value of 0.05 after Bonferroni correction (Sokal and Rohlf 1987).
Results and discussion
DCF degradation and binding to TiO2
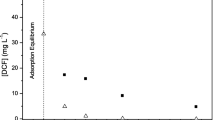
Under the experimental conditions for the binding study prior to exposure with the macrophyte, the DCF concentration (Fig. 1), without TiO2-NPs, remained unchanged after 24 h (p = 0.060). However, in the presence of TiO2-NPs, the DCF concentration decreased by 11.9% (p < 0.001). DCF degradation was not found in the treatments without TiO2-NPs. Therefore, the undetected 11.9% in the presence of TiO2-NPs was likely due to binding to the NPs. Considering the concentrations of DCF (250 ng/ml in 100 ml = 25 µg) and the TiO2-NPs (1 mg/ml in 100 ml = 100 mg) per replicate, 2.98 µg DCF was bound per 100 mg of TiO2 (29.8 µg/g) after 24 h. TiO2 photocatalysis of DCF was previously demonstrated by Rizzo et al. (2009). However, degradation can be excluded as these experiments were conducted in the dark. Similar to the findings in the present study, Rizzo et al. (2009) reported that after 30 min, 14% of the DCF (5 µg/ml) was adsorbed to the TiO2 (0.2 mg/ml) in the dark, and thereafter saturated under the prevailing conditions. No DCF contamination was detected in the pure TiO2-NP treatments.
Concentration of free diclofenac. Quantitative analysis of soluble diclofenac (DCF) at the start and end of the 24-h incubation period on its own (DCF, positive control), in the presence of titanium dioxide (DCF + NP), and the nanoparticles on its own (NP, negative control). Bars represent average DCF concentration ± standard deviation as measured by liquid chromatography-tandem mass spectroscopy (n = 3)
Enzymatic responses of Egeria densa
Studies regarding the adverse effects of DCF on macrophytes are limited. However, the available studies indicate moderate toxicity as the EC50 of DCF was determined to range from 7 to 350 µg/ml for microalgae such as Desmodesmus subspicatus and Pseudokirchneriella subcapitata, as well as macrophytes such as Lemna minor, Nasturtium officinale, and Callitriche platycarpa (Cleuvers 2003; Ferrari et al. 2003; Joachim et al. 2021). In the present study, a DCF exposure concentration of 250 ng/ml was used. DCF was internalized by the macrophyte dose-dependently over time at a rate of 3.2 ng/g/h after the first 24 h, which decreased to 1.5 ng/g/h after 96 h (Fig. S1). Accordingly, at the conclusion of all exposures, all plants in all treatments visually appeared healthy, and no chlorosis or necrosis could be observed. Nevertheless, DCF toxicity is said to increase under the sunlight due to the toxicity of its phototransformation byproducts, mainly 2-[(2-chlorophenyl)amino] benzaldehyde (CPAB) for which an EC50 of 4800 ng/ml was determined, which is 10-times lower than that of DCF (48100 ng/ml) for Scenedesmus vacuolatus (Schulze et al. 2010). For the generation of phototransformation byproducts, Schmitt-Jansen et al. (2007) indicated that for S. vacuolatus, maximal toxicity was achieved after 53 h of light exposure to 50,000 ng/ml of DCF. Furthermore, Andreozzi et al. (2003), studying the photodegradation of DCF, indicated that the half-life was around five days under constant light conditions. Thus, the relatively low DCF concentration (250 ng/ml) and the short incubation under light (14 h) used in the present study would not induce substantial adverse effects by CPAB generation.
When exposing E. densa to DCF, the microsomal GST (mGST) activity was not elevated (p = 1) at the applied concentrations. However, with exposure to the TiO2-NP alone or in combination with DCF, the mGST activity was inhibited (Fig. 2A). Compared to the control, the E. densa mGST activity was reduced by 73.8% (p < 0.001) with exposure to DCF in combination with TiO2-NP and 59.8% with NPs (p = 0.050). Since most microsomal enzyme substrates are lipophilic compounds (Yu 2002), the detoxification mechanism will be limited for hydrophilic compounds such as DCF. The data indicate that the mitochondrial detoxification of TiO2 is limited; however, more so in the presence of DCF. Thus, cGST is more likely to be involved in detoxifying the two compounds.
The activity of cGST (Fig. 2B), in contrast, was significantly elevated with exposure to all three treatments. Exposure to DCF increased the enzyme activity 16.6 ± 0.5-fold with DCF as well as DCF-TiO2-NP (p < 0.001). NP exposure caused a 6.4-fold increase in cytosolic GST (cGST) activity (p = 0.004). In general, the phi and tau classes of plant-specific cGST are predominantly present to detoxify and restrict the effects of xenobiotics (Kumar and Trivedi 2018). In Solanum lycopersicum L., GST activity increased 1.5-fold with exposure to 1500 ng/ml DCF (Sousa et al. 2021).
Previously, GST activity in the macrophyte T. latifolia associated with DCF exposure was studied by Bartha et al. (2014). Using a similar photometric analytical method, the authors reported no elevation of the GST activity after 24 h of exposure to 1000 ng/ml DCF. However, the activity was significantly induced after 72 h. In contrast to the results presented here, Alkimin et al. (2020) reported inhibition of the GST level in L. minor exposed to 375 ng/ml, 750 ng/ml, and 1500 ng/ml DCF. Changes in antioxidant enzyme activity and its related gene expression are associated with antioxidant capacity and response in time courses (Dinler et al. 2014). Interestingly, Varela-Valencia et al. (2014) reported induced expression of the GST gene with anatase TiO2 after only 6 h. However, in the present study, only the activities of cGST increased.
GSTs are often associated with antioxidative defense mechanisms and biotransformation, as reviewed by Edwards et al. (2000). However, in the present study, the mGSTs were inhibited with exposure to the NPs and in combination with DCF, which may have led to elevated oxidative stress. Nanomaterials have been shown to bind to some proteins, such as bovine serum albumen (Giacomelli et al. 1997), and specific enzymes, such as lysozyme (Xu et al. 2010) and lactate dehydrogenase (MacCormack et al. 2012), leading to structural changes and inhibition (Xu et al. 2010).
Considerating the key role GR plays in the cellular control of oxidative stress by the generation of glutathione (GSH), E. densa’s GR activity (Fig. 3) responded in the same manner as cGST (Fig. 2B). The GR activity increased by 12.2-fold with exposure to DCF and a combination of DCF + NP (p < 0.001). Exposure to the TiO2-NP only resulted in a 4.7-fold increase in activity. Since TiO2 nanoparticles are considered one of the safest and low-toxic materials, the significant increase in GR activity indicated that exposure to TiO2 induced substantial effects. Okupnik and Pflugmacher (2016) also reported a significant increase in the GR activity in Hydrilla verticillata with exposure to anatase TiO2. In the study by Bartha et al. (2014), the GR activity increased in shoots but not in roots of T. latifolia exposed to 1000 ng/ml DCF for seven days. Sousa et al. (2021) reported increased GR activity in both roots and shoots of S. lycopersicum L. However, the exposure concentration was up to 5000 ng/ml DCF for five weeks.
In contrast to mGST, cGST and GR activities were not inhibited with exposure to the NPs alone. Studies have proposed preferential binding of specific NPs to certain enzymes (Bayraktar et al. 2006; Fischer et al. 2002). Our data supports this as mGST and cGST are structurally distinct isozymes and evolutionarily diverse (Vaish et al. 2020). Some studies have also discussed the possibility that high ROS concentrations induce DNA and RNA damage, lipid peroxidation, and protein oxidation/denaturation with consequent enzyme inhibition (Alkimin et al. 2020). Furthermore, TiO2 particles are known to interact with phospholipids through possible binding by hydroxyl groups of the terminal glycerol (Le et al. 2014). Another study showed that TiO2 made pits in membranes (Batiuskaite et al. 2022). These studies demonstrate that TiO2 affects the integrity of membranes, which is necessary for mGST to remain functional, potentially explaining the loss of activity observed here. Investigating the role of lipid peroxidation related to the functionality of the mGSTs in the future is essential in understanding potentially associated oxidative stress. Nevertheless, the preferential binding of NPs seems more plausible and warrants future research.
To summarize the results, the activity of mGST was inhibited by the TiO2 nanoparticles, and the effect of DCF was insignificant. Oppositely, the activities of cGST and GR were increased by DCF, but no synergistic effect was found with the TiO2 nanoparticles. However, it is known that cytosolic GSTs are more involved in detoxification than mitochondrial and microsomal GSTs (Dasari et al. 2018). Therefore, it is estimated that the major contributor to the elevated antioxidant response observed in aquatic macrophyte E. densa is DCF rather than the TiO2 nanoparticles. In general, the macrophyte was able to cope with the adverse effects associated with exposure to these high concentrations of DCF and TiO2, as well as a combination of the two, as visually evident from lack of chlorosis or necrosis and the plants continued to grow throughout the exposure period.
The concentration of pollutants utilized in the present study exceeds that of currently measured concentrations; however, these concentrations of titanium dioxide and DCF may increase in the future to these values. This study provides a brief insight into the toxic effects of titanium dioxide, diclofenac, and their combined toxicity on the antioxidant and the biotransformation system in the model aquatic macrophyte E. densa. Assessing other physiological markers, such as, for example, total reactive oxygen species, chlorophyll content, gene expression of the biotransformation and antioxidative enzymes, would provide more information on the toxicity of the individual xenobiotics and combined toxicity. Furthermore, additional information on combined toxicity is needed; therefore, ecotoxicological investigations with various combinations and mixtures are required.
Conclusion
The study shows that even at concentrations higher than environmentally detected, the macrophyte E. densa responds to environmental pollutants such as nanoparticles and pharmaceuticals, in this case, TiO2 and DCF, adequately by elevating antioxidative responses and biotransformation processes to avoid adverse effects. Further studies are required to understand why mGST but not cGST is inhibited by the nanomaterials. The study illustrates the discrepant results when comparing the physiological outcomes with exposure to the compounds in single and mixtures. This information becomes essential when considering the cocktails of pollutant mixtures in the environment and thus emphasizes the importance of considering the synergistic and antagonistic effects of mixture effects in future experiments.
References
Alkimin GD, Soares AM, Barata C, Nunes B (2020) Can salicylic acid modulate biochemical, physiological and population alterations in a macrophyte species under chemical stress by diclofenac. Sci Total Environ 739:139715. https://doi.org/10.1016/j.scitotenv.2020.139715
Andreozzi R, Raffaele M, Nicklas P (2003) Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere 50:1319–1330. https://doi.org/10.1016/S0045-6535(02)00769-5
Bakker ES, Wood KA, Pagès JF, Veen GC, Christianen MJ, Santamaría L et al. (2016) Herbivory on freshwater and marine macrophytes: a review and perspective. Aquat Bot 135:18–36. https://doi.org/10.1016/j.aquabot.2016.04.008
Bartha B, Huber C, Schröder P (2014) Uptake and metabolism of diclofenac in Typha latifolia–how plants cope with human pharmaceutical pollution. Plant Sci 227:12–20. https://doi.org/10.1016/j.plantsci.2014.06.001
Batiuskaite D, Bruzaite I, Snitka V, Ramanavicius A (2022) Assessment of TiO2 nanoparticle impact on surface morphology of Chinese hamster ovary cells. Materials 15:4570. https://doi.org/10.3390/ma15134570
Bayraktar H, Ghosh PS, Rotello VM, Knapp MJ (2006) Disruptionof protein-protein interactions using nanoparticles: Inhibition of cytochrome Cperoxidase. Chem. Comm 2006:1390–1392. https://doi.org/10.1039/B516096K
Bernas L, Winkelmann K, Palmer A (2017) Phytoremediation of silver species by waterweed (Egeria densa). Chemist 90:7–13
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490
Cleuvers M (2003) Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol Lett 142:185–194. https://doi.org/10.1016/S0378-4274(03)00068-7
Coll C, Notter D, Gottschalk F, Sun T, Som C, Nowack B (2016) Probabilistic environmental risk assessment of five nanomaterials (nano-TiO2, nano-Ag, nano-ZnO, CNT, and fullerenes). Nanotoxicol 10:436–444. https://doi.org/10.3109/17435390.2015.1073812
Dasari S, Ganjayi MS, Meriga B (2018) Glutathione S-transferase is a good biomarker in acrylamide induced neurotoxicity and genotoxicity. Interdiscip Toxicol 11:115. https://doi.org/10.2478/intox-2018-0007
De Morais Calado SL, Esterhuizen-Londt M, Cristina Silva de Assis H, Pflugmacher S (2019) Phytoremediation: green technology for the removal of mixed contaminants of a water supply reservoir. Int J Phytoremediation 21:372–379. https://doi.org/10.1080/15226514.2018.1524843
Dinler BS, Antoniou C, Fotopoulos V (2014) Interplay between GST and nitric oxide in the early response of soybean (Glycine max L.) plants to salinity stress. J Plant Physiol 171:1740–1747. https://doi.org/10.1016/j.jplph.2014.07.026
Edwards R, Dixon DP, Walbot V (2000) Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci 5:193–198. https://doi.org/10.1016/S1360-1385(00)01601-0
Esteves FA (1998) Fundamentos de Limnologia, 2nd Edition. Interciencia, Rio de Janeiro
Esterhuizen-Londt M, Hendel AL, Pflugmacher S (2017) Mycoremediation of diclofenac using Mucor hiemalis. Toxicol Environ Chem 99:795–808. https://doi.org/10.1080/02772248.2017.1296444
Ferrari B, Paxéus N, Giudice RL, Pollio A, Garric J (2003) Ecotoxicological impact of pharmaceuticals found in treated wastewaters: Study of carbamazepine, clofibric acid, and diclofenac. Ecotoxicol Environ Safe 55:359–370. https://doi.org/10.1016/S0147-6513(02)00082-9
Fischer NO, McIntosh CM, Simard JM, Rotello VM (2002) Inhibition of chymotrypsin through surface binding using nanoparticle-based receptors. Proc Nat Acad Sci USA 99:5018–5023. https://doi.org/10.1073/pnas.082644099
Fekadu S, Alemayehu E, Dewil R, Van der Bruggen B (2019) Pharmaceuticals in freshwater aquatic environments: A comparison of the African and European challenge. Sci Total Environ 654:324–37. https://doi.org/10.1016/j.scitotenv.2018.11.072
Fent K (2008) Effects of pharmaceuticals on aquatic organisms. In: Kümmerer K (Ed.) Pharmaceuticals in the environment. Springer Verlag, Berlin, pp 175–203 https://doi.org/10.1007/978-3-540-74664-5_12
Giacomelli CE, Avena MJ, De Pauli CP (1997) Adsorption of bovine serum albumin onto TiO2 particles. J Colloid Interface Sci 188:387–395. https://doi.org/10.1006/jcis.1996.4750
Gutteridge JMC (1995) Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem 41:1819–1828. https://doi.org/10.1093/clinchem/41.12.1819
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferase: The first step in mercapturic acid formation. J Bio Chem 249:1730–1739
Jarosz M, Pawlik A, Szuwarzyński M, Jaskuła M, Sulka GD (2016) Nanoporous anodic titanium dioxide layers as potential drug delivery systems: Drug release kinetics and mechanism. Colloids Surf B: Biointerfaces 143:447–454. https://doi.org/10.1016/j.colsurfb.2016.03.073
Joachim S, Beaudouin R, Daniele G, Geffard A, Bado-Nilles A, Tebby C, Palluel O, Dedourge-Geffard O, Fieu M, Bonnard M, Palos-Ladeiro M (2021) Effects of diclofenac on sentinel species and aquatic communities in semi-natural conditions. Ecotoxicol Environ Safe 211:111812. https://doi.org/10.1016/j.ecoenv.2020.111812
Jovanović B (2015) Critical review of public health regulations of titanium dioxide, a human food additive. Integr Environ Assess Manag 11:10–20. https://doi.org/10.1002/ieam.1571
Hendren CO, Mesnard X, Dröge J, Wiesner MR (2011) Estimating production data for five engineered nanomaterials as a basis for exposure assessment. Environ Sci Technol 45(7):2562–2569. https://doi.org/10.1021/es103300g
Kafshgari MH, Mazare A, Distaso M, Goldmann WH, Peukert W, Fabry B, Schmuki P (2019) Intracellular drug delivery with anodic titanium dioxide nanotubes and nanocylinders. ACS Appl Mater Interfaces 11:14980–14985. https://doi.org/10.1021/acsami.9b01211
Khan I, Saeed K, Khan I (2019) Review nanoparticles: properties, applications and toxicities. Arab. J Chem 12:908–931. https://doi.org/10.1016/j.arabjc.2017.05.011
Kennedy H, Gaci E, Kennedy DP, Papadimitriou S, Duarte CM (2004) Organic carbon sources to SE Asian coastal sediments. Estuar Coast Shelf Sci 60:59–68. https://doi.org/10.1016/j.ecss.2003.11.019
Kumar S, Trivedi PK (2018) Glutathione S-transferases: role in combating abiotic stresses including arsenic detoxification in plants. Front Plant Sci 9:751. https://doi.org/10.3389/fpls.2018.00751
Le QC, Ropers MH, Terrisse H, Humbert B (2014) Interactions between phospholipids and titanium dioxide particles. Colloids Surf B: Biointerfaces 123:150–157. https://doi.org/10.1016/j.colsurfb.2014.09.010
Li WC (2014) Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut 187:193–201. https://doi.org/10.1016/j.envpol.2014.01.015
Lien DH, Retamal JRD, Ke JJ, Kang CF, He JH (2015) Surface effects in metal oxide-based nanodevices. Nanoscale 7:19874–19884. https://doi.org/10.1039/C5NR06494E
Lonappan L, Brar SK, Das RK, Verma M, Surampalli RY (2016) Diclofenac and its transformation products: environmental occurrence and toxicity-a review. Environ Int 96:127–138. https://doi.org/10.1016/j.envint.2016.09.014
MacCormack TJ, Clark RJ, Dang MK, Ma G, Kelly JA, Veinot JG, Goss GG (2012) Inhibition of enzyme activity by nanomaterials: potential mechanisms and implications for nanotoxicity testing. Nanotoxicol 6:514–525. https://doi.org/10.3109/17435390.2011.587904
Maurer-Jones MA, Gunsolus IL, Murphy CJ, Haynes CL (2013) Toxicity of engineered nanoparticles in the environment. Anal Chem 85:3036–3049. https://doi.org/10.1021/ac303636s
Mezzelani M, Regoli F (2022) The biological effects of pharmaceuticals in the marine environment. Ann Rev Mar Sci 14:105–128. https://doi.org/10.1146/annurev-marine-040821-075606
Molaeirad A, Janfaza S, Karimi-Fard A, Mahyad B (2015) Photocurrent generation by adsorption of two main pigments of Halobacterium salinarum on TiO2 nanostructured electrode. Biotechnol Appl Biochem 62:121–125. https://doi.org/10.1002/bab.1244
Mashayekh M, Dorranian D (2014) Size-dependent nonlinear optical properties and thermal lens in silver nanoparticles. Optik 125:5612–5617. https://doi.org/10.1016/j.ijleo.2014.07.066
Naseri N, Janfaza S, Irani R (2015) Visible light switchable br/tio2 nanostructured photoanodes for bio-inspired solar energy conversion. RSC Adv 5:18642–18646. https://doi.org/10.1039/C4RA16188B
Okupnik A, Contardo-Jara V, Pflugmacher S (2015) Potential role of engineered nanoparticles as contaminant carriers in aquatic ecosystems: Estimating sorption processes of the cyanobacterial toxin microcystin-LR by TiO2 nanoparticles. Colloids Surf A: Physicochem Eng Asp 481:460–467. https://doi.org/10.1016/j.colsurfa.2015.06.013
Okupnik A, Pflugmacher S (2016) Oxidative stress response of the aquatic macrophyte Hydrilla verticillata exposed to TiO2 nanoparticles. Environ Toxicol Chem 35:2859–2866. https://doi.org/10.1002/etc.3469
Pflugmacher S (2004) Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquat Toxicol 70:169–178. https://doi.org/10.1016/j.aquatox.2004.06.010
Pott VJ, Pott A (2003) Dinâmica da vegetação aquática do Pantanal. Ecologia e manejo de macrófitas aquáticas 1:145–162
Ravera O (2001) Monitoring of the aquatic environment by species accumulator of pollutants: a review. J Limnol 60:63–78. https://doi.org/10.4081/jlimnol.2001.s1.63
Rizzo L, Meric S, Kassinos D, Guida M, Russo F, Belgiorno V (2009) Degradation of diclofenac by TiO2 photocatalysis: UV absorbance kinetics and process evaluation through a set of toxicity bioassays. Water Res 43:979–988. https://doi.org/10.1016/j.watres.2008.11.040
Sarkar A, Ghosh M, Sil PC (2014) Nanotoxicity: oxidative stress mediated toxicity of metal and metal oxide nanoparticles. J Nanosci Nanotechnol 14:730–743. https://doi.org/10.1166/jnn.2014.8752
Schmitt-Jansen M, Bartels P, Adler N et al. (2007) Phytotoxicity assessment of diclofenac and its phototransformation products. Anal Bioanal Chem 387:1389–1396. https://doi.org/10.1007/s00216-006-0825-3
Schulze T, Weiss S, Schymanski E, von der Ohe PC, Schmitt-Jansen M, Altenburger R, Streck G, Brack W (2010) Identification of a phytotoxic photo-transformation product of diclofenac using effect-directed analysis. Environ Pollut 158:1461–1466. https://doi.org/10.1016/j.envpol.2009.12.032
Skocaj M, Filipic M, Petkovic J, Novak S (2011) Titanium dioxide in our everyday life; is it safe. Radiol Oncol 45:227–247. https://doi.org/10.2478/v10019-011-0037-0
Sokal RR, Rohlf FJ (1987) Biostatistics. Francise & Co, New York
Sousa B, Lopes J, Leal A, Martins M, Soares C, Azenha M, Fidalgo F, Teixeira J (2021) Specific glutathione-S-transferases ensure an efficient detoxification of diclofenac in Solanum lycopersicum L. plants. Plant Physiol Biochem 168:263–271. https://doi.org/10.1016/j.plaphy.2021.10.019
Sun TY, Bornhöft NA, Hungerbühler K, Nowack B (2016) Dynamic probabilistic modeling of environmental emissions of engineered nanomaterials. Environ Sci Technol 50:4701–4711. https://doi.org/10.1021/acs.est.5b05828
Thomaz SM, Cunha ERD (2010) The role of macrophytes in habitat structuring in aquatic ecosystems: methods of measurement, causes and consequences on animal assemblages’ composition and biodiversity. Acta Limnol Brasil 22:218–236. https://doi.org/10.4322/actalb.02202011
Thiagarajan V, Alex SA, Seenivasan R, Chandrasekaran N, Mukherjee A (2021) Interactive effects of micro/nanoplastics and nanomaterials/pharmaceuticals: Their ecotoxicological consequences in the aquatic systems. Aquat Toxicol 232:105747. https://doi.org/10.1016/j.aquatox.2021.105747
Tovar-Sánchez A, Sánchez-Quiles D, Basterretxea G, Benedé JL, Chisvert A, Salvador A, Moreno-Garrido I, Blasco J (2013) Sunscreen products as emerging pollutants to coastal waters. PLoS One 8:e65451. https://doi.org/10.1371/journal.pone.0065451
Vaish S, Gupta D, Mehrotra R, Mehrotra S, Basantani MK (2020) Glutathione S-transferase: A versatile protein family. 3 Biotech 10:1–19. https://doi.org/10.1007/s13205-020-02312-3
Varela-Valencia R, Gómez-Ortiz N, Oskam G, de Coss R, Rubio-Pina J, del Río-García M, Albores-Medina A, Zapata-Perez O (2014) The effect of titanium dioxide nanoparticles on antioxidant gene expression in tilapia (Oreochromis niloticus). J Nanoparticle Res 16:1–12. https://doi.org/10.1007/s11051-014-2369-3
Vilvert E, Contardo-Jara V, Esterhuizen-Londt M, Pflugmacher S (2017) The effect of oxytetracycline on physiological and enzymatic defense responses in aquatic plant species Egeria densa, Azolla caroliniana, and Taxiphyllum barbieri. Toxicol Environ Chem 99:104–116. https://doi.org/10.1080/02772248.2016.1165817
Wahajuddin, Arora S (2012) Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomed 7:3445. https://doi.org/10.2147/IJN.S30320
Xu Z, Liu XW, Ma YS, Gao HW (2010) Interaction of nano-TiO2 with lysozyme: insights into the enzyme toxicity of nanosized particles. Environ Sci Pollut Res 17:798–806. https://doi.org/10.1007/s11356-009-0153-1
Yu SJ (2002) Biochemical characteristics of microsomal and cytosolic glutathione S-transferases in larvae of the fall armyworm, Spodoptera frugiperda (JE Smith). Pestic Biochem Physiol 72:100–110. https://doi.org/10.1006/pest.2001.258
Acknowledgements
This work was partly supported by the Nanomaterial Technology Development Program (NRF-2017M3A7B6052455) funded by the South Korean Ministry of Science and the National Research Council of Science & Technology (NST) grant by the Strategies for Establishing Global Research Networks. The University of Helsinki Library provided open-access funding.
Author contributions
SP and ME contributed to the study conception and design. Material preparation, data collection and analysis were performed by SP, ML, and ME. ME and ML wrote the first draft of the manuscript, and all authors commented on previous versions. All authors read and approved the final manuscript.
Funding
This work was partly supported by the Nanomaterial Technology Development Program (NRF-2017M3A7B6052455) funded by the South Korean Ministry of Science and the National Research Council of Science & Technology (NST) grant by the Strategies for Establishing Global Research Networks. The University of Helsinki Library provided open-access funding. Open Access funding provided by University of Helsinki including Helsinki University Central Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Esterhuizen, M., Lutsko, M., Kim, Y. et al. Titanium (IV) oxide anatase nanoparticles as vectors for diclofenac: assessing the antioxidative responses to single and combined exposures in the aquatic macrophyte Egeria densa. Ecotoxicology 32, 394–402 (2023). https://doi.org/10.1007/s10646-023-02646-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-023-02646-7