Abstract
Background
Gastrointestinal tumors bleeding remains a significantly clinical challenge due to its resistance to conventional endoscopic hemostasis methods. While the efficacy of endoscopic tissue adhesives (ETA) in variceal bleeding has been established, its role in gastrointestinal tumor bleeding (GITB) remains ambiguous.
Aims
This study aims to assess the feasibility and effectiveness of ETA in the treatment of GITB.
Methods
The study enrolled 30 patients with GITB who underwent hemostasis through Histoacryl® tissue glue injection. Hemostasis success rates, ETA-related adverse events, and re-bleeding rates were evaluated.
Results
ETA application achieved successful hemostasis at all tumor bleeding sites, with immediate hemostasis observed in all 30 (100.0%) patients. Among the initially hemostasis cases, 5 patients (17.0%) experienced re-bleeding within 30 days, and the 60 day re-bleeding rate was 20.0% (6/30). Expect for one case of vascular embolism, no adverse events related with ETA application were reported. The 6 month survival was 93%.
Conclusion
ETA demonstrated excellent immediate hemostasis success rate in GITB cases and showed promising outcomes in prevention re-bleeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastrointestinal tumors comprise a significant portion of global cancer cases, causing more than a third of cancer-related deaths [1]. Despite advances in colorectal cancer (CRC) screening, delayed diagnoses of other gastrointestinal cancers persist, leading to poor prognosis [2]. Projections suggest a marked increases in incidence and mortality rates by 2040 [3]. Gastrointestinal tumor bleeding (GITB), stemming from pathologic angiogenesis and vascular erosion, is a major cause of morbidity and mortality among affected individuals. GITB poses a significant threat to individuals with gastrointestinal tumors, with up to 10% experiencing bleeding in advanced cancer cases [4].
Bleeding is a prevalent problem in advanced tumors, caused by local tumor invasion, angiogenesis, and certain medications [5]. Manifestations range from bruising to acute catastrophic bleeding. For CRC, 34.5% patients exhibit chronic melena, often linked to erosion and bleeding from the primary tumor [6]. In cervical cancer, bleeding correlates with poorer outcomes [7]. The mortality rate for acute upper gastrointestinal bleeding can be as high as 10–13% [8]. Re-bleeding is even linked to increased mortality, necessitating interventions, like surgery and transfusions, leading to extended hospital stays and higher medical costs [9, 10]. Bleeding can also be a complication, leading to worse prognosis and increased risk of death [11]. Investigating bleeding characteristics, re-bleeding rates, and outcomes in gastrointestinal tumors is crucial.
Endoscopy, encompassing procedures like bronchoscopy, cystoscopy, and colonoscopy, plays a crucial role in identifying and treating bleeding tumors in visible organs. However, endoscopic management faces challenges due to patient health and the bleeding lesions accessibility, with immediate hemostasis success rates at only 30% and re-bleeding rates as high as 40% [12]. This underscores the urgent need for advancements in endoscopic hemostatic techniques. Recent developments have introduced various endoscopic interventions, such as cauterization, argon plasma coagulation, clamp deployment, epinephrine injections, and laser therapy, each showing diverse success rates and re-bleeding. Notably, styptic powder reported hemostasis in 100% of cases, with a 20% re-bleeding rate within 1 month [13], while argon plasma coagulation offered immediate stoppage but a 30% re-bleeding rate [14]. In contrast, cyanoacrylate stands out for its convenient application, rapid effectiveness, minimal discomfort, and suture-free usage [15]. Despite its established role in treating gastrointestinal variceal bleeding [16], cyanoacrylate’s efficacy in non-variceal bleeding remains uncertain due to limited experience in this area [17]. In our clinical practice, we have opted for endoscopic tissue adhesives (ETA), predominantly utilizing N-butyl-2-cyanoacrylate.
To our knowledge, there are limited studies on the use of ETA for GITB [18, 19], indicating a need for further research. Therefore, this study aims to evaluate the efficacy of ETA in GITB treatment and assess re-bleeding rate post-application.
Methods and Materials
Study Design
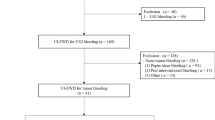
Patients who received ETA for GITB at Changzhou Cancer Hospital between January 2023 and June 2023 were enrolled in the study. The eligible participants were over 18 years old at the time of treatment and had a confirmed diagnosis of gastrointestinal tumor through histologically examination. Additional inclusion criteria included symptoms of acute bleeding, such as presence of fresh blood, blood clots in vomit, blackening of the stool, and had undergone endoscopic hemostasis using ETA. Patients with non-neoplastic bleeding, pregnant individuals, or those suspected to being pregnant at the time of treatment were excluded from the study. A total of 30 patients met the inclusion criteria as illustrated in Fig. 1. The study involved a review of the patients’ medical records to gather data on clinical characteristics, bleeding episodes, clinical outcomes (including immediate hemostasis success and re-bleeding rates), and any complications related to ETA therapy. This research protocol has been approved by the Institutional Review Committee of our Institute.
Endoscopic Procedures
Experienced endoscopists applied ETA to bleeding sites of tumor using an endoscope. The treatment involved the utilization of the modified Histoacryl® injection method. The ETA mainly uses N-butyl-2-cyanoacrylate, which is a monomer liquid. When it comes into contact with human tissue fluid, it undergoes a polymerization reaction to form a polymer. These polymers can form a thin film on the surface of the wound, causing the wound edges to adhere tightly together, thus achieving a strong wound closure. ETA has strong adhesive properties and water resistance, as well as a certain degree of biocompatibility, causing no adverse effects on the human body. The specific operation process is shown in Fig. 2.
The endoscope was inserted into the lesion site to identify the bleeding source, followed by repeated flushing to pinpoint the exact location of the bleed. If the bleeding source could not be located despite repeated flushing, the endoscope should be withdrawn. A disposable endoscopic injection needle (Model: ATE-ZSZ-23*2000*23*4, Atetec, Jiangsu Province, China) was then inserted through endoscopic forceps, vented and directed toward the bleeding site. Blood reflux could be observed in the needle sheath of the syringe (If no blood reflux was observed, the needle needed to be withdrawn and the blood point needed to be searched again). Subsequently, a mixture of normal saline and tissue adhesive (Histoacryl® tissue glue, B. Braun Melsungen AG, Germany) was promptly injected, leading to the formation of a local bulge at the injected site. The needle was carefully withdrawn through the endoscopic forceps channel, and pressure was applied to the puncture point using the needle sheath for 30–60 s. Occasionally, a discharged of the tissue adhesive mixture could be observed from the puncture site. Following this, the treatment needle was removed, and the area was rinsed repeatedly to confirm cessation of bleeding at the injection site. It was necessary to wipe the postoperative endoscopic lens with a degumming agent to eliminate any residual tissue glue and prevent damage to the lens.
Clinical Outcomes
The primary endpoint of the study was immediate hemostasis, defined as no absence of further bleeding after 3 min of endoscopic observation, measured with a stopwatch. The secondary endpoint was re-bleeding 30 days after the initial bleeding episode. Re-bleeding was defined as the presence of clinical evidence of bleeding (such as blackening or hematemesis) and a decrease in hemoglobin levels of 2 g/dL within 30 days following endoscopic procedure. In case where re-bleeding was suspected, additional endoscopic assessment was performed to confirm the status. Other secondary outcomes included re-bleeding rates at 7 and 60 days (based on the aforementioned re-bleeding criteria) and the patient’s 6 month survival.
Statistical Analysis
Statistical analysis were carried out by the SPSS software, version 23.0 (SPSS, Inc., Chicago, IL, United States). The clinical characteristics are expressed as medians (ranges) for continuous variables and numbers (percentages) for categorical variables. Overall re-bleeding rates and 6 month survival were estimated using the Kaplan–Meier method. Forward stepwise logistic regression analyses, both univariate and multivariate were used to determine the correlation between factors and clinical outcomes. P value < 0.05 was considered as statistically significant.
Results
Study Population
A total of 30 patients with GITB were included in the study. The baseline characteristics of the patients were detailed in Table 1 and Supplemental Table S1. The majority of the patients were male (22 cases, 73.0%), and 16 individuals aged over 65 years. Most patients (25/30, 83%) exhibited poor tumor differentiation. Adenocarcinoma (28/30, 92.0%) was the most common histologic type. All patients had an ASA score of 3 and ECOG PS score ≥ 2. The prevalent medical history was hypertension (7/30, 23.0%), while none had chronic kidney disease. The majority of patients (26/30, 87%) had advanced tumors (stage IV). The follow-up period was 60 days.
Bleeding Characteristics
The characteristics of tumor bleeding are presented in Table 2. The stomach was the most frequent bleeding location (24/30; 80.0%), with 9 cases located in the cardia, 11 in the stomach body, and 4 in the stomach antrum. Among the 30 patients, 2 cases (7.0%) of bleeding were in the duodenum, 2 in the rectum, and 1 in the ascending colon. One case of bleeding (3.0%) was observed in the esophagus. The majority of bleedings were classified as Forrest IIa (18/30, 60.0%), following by Ib (7/30, 23%). The median tumor diameter was 6 cm (range 2–12 cm).
Clinical Outcomes
All 30 patients achieved immediate hemostasis, with no re-bleeding occurred within 1 day (Table 3). The overall re-bleeding rates at 7, 30 and 60 days after hemostasis were 10.0% (3 out of 30 patients), 17% (5 out of 30 patients), and 20.0% (6 out of 30 patients), respectively (Fig. 3). Among the 4 patients who experienced re-bleeding within 30 days, 2 achieved successful hemostasis by repeated ETA application, 1 had failed ETA hemostasis, and 1 underwent surgical resection. Two patients died before the 60 day follow-up period. One patient died on the 13th-day post-endoscopic procedures due to tumor re-bleeding, hemorrhagic shock, and circulatory failure. Another patient succumbed to disease progression 30 days after the endoscopic procedures. No adverse events such as infection, intestinal obstruction or perforation were associated with ETA application, expect for one patient experienced vascular embolism. Univariate analysis revealed that no significant variable predicted a reduction in the 30 day re-bleeding rate (Supplementary Table 2). The 6-month survival was 93% (Supplementary Fig. S1).
ETA for a Hemorrhagic Cardiac Carcinoma: A Case Report
In November 2022, a 69 year-old male patient presented with black stools, leading to a diagnosed with stage IV, HER2-positive cardia adenocarcinoma based on pathology and imaging, Following 8 cycles of pembrolizumab combined with trastuzumab and XELOX treatment, the best response was evaluated as stable disease (SD) (Fig. 4A). On June 22, 2023, he was admitted to the emergency room due to hematemesis lasting for three hours, accompanied by dizziness and weakness after vomiting. Physical examination revealed anemia with hemoglobin levels at 55 g/L, blood pressure at 80/55 mmHg, and a heart rate of 108 beats/min. Gastroscopy revealed hyperplastic lesions with visible bleeding in both anterior and posterior walls of the inferior flexion of the basal layer (Fig. 4B). With the consent of the patient and his family, ETA treatment was performed. Normal saline and tissue adhesive were injected into the bleeding lesion, followed by repeated irrigation, and no active bleeding was observed. The procedure lasted 60 min. A week later, a follow-up gastroscopy confirmed successful hemostasis (Fig. 4C), and the patient’s hemoglobin level was maintained at 90 g/L.
Discussion
Despite significant advancements in endoscopy and supportive care, bleeding remains a prevalent, complex, and frequently life-threatening emergency for physicians. The overall mortality rate from bleeding still stands at approximately 10%, with the potential to rise to 35% for the elderly and hospitalized patients with severe comorbidities [20]. While tissue adhesive has demonstrated efficacy in controlling varicose vein bleeding and is included in several guidelines, its role in GITB remains uncertain. Our study, based on a retrospective analysis of 30 cases, confirmed that ETA exhibited outstanding immediate hemostatic success rate (100.0%) and a low re-bleeding rate (10.0% and 17.0% on days 7 and 30, respectively). To the best of our knowledge, this study represents one of the largest investigations conducted on the use of ETA to treat GITB.
Since its introduction in 1984, the widespread use of Histoacryl® has demonstrated its efficacy in managing bleeding from gastric varices [21]. A recent review of compiled case studies involving patients treated with Histoacryl® has indicated that the adhesive is relatively safe and effective. Despite this, complications like systemic embolism, end-organ infarction, visceral fistula, and microbiemia had occurred [22, 23]. Several retrospective studies have further revealed that Histoacryl® has an initial hemostasis rate ranging from 88 to 98%, with 1% experiencing severe complications, like systemic embolism. The re-bleeding rate was from 10 to 29% [24, 25]. Studies from China and South Korea have reported successful hemostasis rates of 95% to 100% in treated patients [26, 27]. To date, there are a lack of studies determining the outcomes are of Histoacryl® treatment in patients with GITB. According to our study, ETA with Histoacryl® treatment exhibited a 100% immediate hemostatic success rate, a low re-bleeding rate and was identified as a safe hemostatic method.
It has been reported that approximately four-fifths of upper gastrointestinal bleeding will resolve spontaneously, while the remaining one-fifth will persist or recur, leading to further bleeding [28, 29]. The occurrence of re-bleeding may not only increase the morbidity and mortality, but also escalates the medical cost for patients [10]. Therefore, the timely identification and active management of high-risk patients with persistent or recurrent bleeding have become the primary focus of upper gastrointestinal bleeding treatment. Some researchers advocate for surgery or selective transarterial embolization as the preferred method of controlling re-bleeding [30]. However, in our opinion, ETA should be utilized prior to surgery due to its cost-effective, lower complications rates, and high efficacy. In our study, the overall re-bleeding rates at 7, 30, and 60 day post-hemostasis were 10.0%, 17%, and 20.0%, respectively. Moreover, of the 4 patients who experienced re-bleeding, 3 were re-treated with ETA, resulting in successful cessation of bleeding in 2 cases. This outcome aligns with previously reported efficacy of endoscopic secondary treatment and is deemed to be a safer alternative to surgery [31].
Fatal systemic embolism is a significant complication associated with endoscopy [32, 33]. Fortunately, our study did not observe any instances of severe systemic embolism. In one case of embolization complication within our study, there were no apparent clinical symptoms or signs, and no re-bleeding occurred within a 60-day period post-hemostasis. Furthermore, our study population did not experience any fatal outcomes related to systemic embolization, such as organ infarction or abscess formation. Based on these findings, it can be concluded that ETA is an effective and safe treatment for GITB.
The present study design has certain limitations that may impact the generalizability of our findings. Primarily, the retrospective nature of the study and the lack of a control cohort were constraints that should be acknowledged. However, numerous reports have highlighted the efficacy of traditional endoscopic hemostasis in managing tumor bleeding, suggesting that the outcomes of this study can be considered sufficiently effective. Additionally, the absence of complications may be attributed in part to our extensive experience with this product in treating varicose bleeding, a practice well documented in the field of digestive endoscopy. Secondly, the identification of potential factors contributing to re-bleeding following immediate hemostasis remains challenging. Moreover, it is crucial to acknowledge that this study was conducted solely at a single center, introducing the possibility of selection bias influencing the outcomes. Lastly, the limited sample size of the present investigation may have compromised its capacity to detect genuine associations. Consequently, larger-scale studies are imperative to validate and substantiate our observations.
To the best of our knowledge, this clinical cohort represents the first incidence of GITB treated with ETA, demonstrating the effectiveness of ETA in managing potentially life-threatening GITB.
Data availability
All data relevant to the study are included in the article or uploaded as supplementary information.
References
Arnold M, Abnet CC, Neale RE et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159:335-349.e15.
Arnold M, Rutherford MJ, Bardot A et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20:1493–1505.
International Agency for Research on Cancer. Cancer tomorrow. The Global Cancer Observatory https://gco.iarc.fr/tomorrow (2020).
Pereira J, Phan T. Management of bleeding in patients with advanced cancer. Oncologist. 2004;9:561–570.
Johnstone C, Rich SE. Bleeding in cancer patients and its treatment: a review. Ann Palliat Med. 2018;7:265–273.
Courtney RJ, Paul CL, Sanson-Fisher RW et al. Factors associated with consultation behaviour for primary symptoms potentially indicating colorectal cancer: a cross-sectional study on response to symptoms. BMC Gastroenterol. 2012;12:100.
Yanazume S, Karakida N, Higashi R et al. Tumor bleeding requiring intervention and the correlation with anemia in uterine cervical cancer for definitive radiotherapy. Jpn J Clin Oncol. 2018;48:892–899.
Crooks C, Card T, West J. Reductions in 28-day mortality following hospital admission for upper gastrointestinal hemorrhage. Gastroenterology. 2011;141:62–70.
Schatz RA, Rockey DC. Gastrointestinal bleeding due to gastrointestinal tract malignancy: natural history, management, and outcomes. Dig Dis Sci. 2017;62:491–501. https://doi.org/10.1007/s10620-016-4368-y.
Barkun A, Bardou M, Marshall JK. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003;139:843–857.
Cao D, Guo CH, Liu JW et al. Bleeding after bevacizumab treatment in patients with metastatic colorectal cancer. Tumori. 2015;101:46–51.
Kawabata H, Hitomi M, Motoi S. Management of bleeding from unresectable gastric cancer. Biomedicines. 2019;7:54.
Leblanc S, Vienne A, Dhooge M et al. Early experience with a novel hemostatic powder used to treat upper GI bleeding related to malignancies or after therapeutic interventions (with videos). Gastrointest Endosc. 2013;78:169–175.
Thosani N, Rao B, Ghouri Y et al. Role of argon plasma coagulation in management of bleeding GI tumors: evaluating outcomes and survival. Turk J Gastroenterol. 2014;25:38–42.
Montanaro L, Arciola CR, Cenni E et al. Cytotoxicity, blood compatibility and antimicrobial activity of two cyanoacrylate glues for surgical use. Biomaterials. 2001;22:59–66.
Cheng LF, Wang ZQ, Li CZ et al. Low incidence of complications from endoscopic gastric variceal obturation with butyl cyanoacrylate. Clin Gastroenterol Hepatol. 2010;8:760–766.
Lee KJ, Kim JH, Hahm KB et al. Randomized trial of N-butyl-2-cyanoacrylate compared with injection of hypertonic saline-epinephrine in the endoscopic treatment of bleeding peptic ulcers. Endoscopy. 2000;32:505–511.
Wang S, Zhang K, **ao M. Endoscopic obturation with tissue adhesive for bleeding gastric stromal tumor: a case report. J Int Med Res. 2021;49:300060521991355.
Wang S. Observation of wound-closure outcomes using tissue adhesive plus metal clip after endoscopic upper gastrointestinal muscularis propria tumor resection. Am J Gastroenterol. 2013;108:623–624.
Ferguson CB, Mitchell RM. Non-variceal upper gastrointestinal bleeding. Ulster Med J. 2006;75:32–39.
Rajoriya N, Forrest EH, Gray J et al. Long-term follow-up of endoscopic Histoacryl glue injection for the management of gastric variceal bleeding. QJM. 2011;104:41–47.
Fry LC, Neumann H, Olano C et al. Efficacy, complications and clinical outcomes of endoscopic sclerotherapy with N-butyl-2-cyanoacrylate for bleeding gastric varices. Dig Dis. 2008;26:300–303.
Taghavi SA, Eshraghian A, Hamidpour L et al. Endoscopic cyanoacrylate injection for the treatment of bleeding gastric varices: the first Iranian series. Arch Iran Med. 2012;15:157–161.
Sugimoto N, Watanabe K, Watanabe K et al. Endoscopic hemostasis for bleeding gastric varices treated by combination of variceal ligation and sclerotherapy with N-butyl-2-cyanoacrylate. J Gastroenterol. 2007;42:52–532.
Huang YH, Yeh HZ, Chen GH et al. Endoscopic treatment of bleeding gastric varices by N-butyl-2-cyanoacrylate (Histoacryl) injection: long-term efficacy and safety. Gastrointest Endosc. 2000;52:160–167.
Wang YM, Cheng LF, Li N et al. Study of glue extrusion after endoscopic N-butyl-2-cyanoacrylate injection on gastric variceal bleeding. World J Gastroenterol. 2009;15:4945–4951.
Akahoshi T, Hashizume M, Tomikawa M et al. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding and risky gastric varices: a 10-year experience. J Gastroenterol Hepatol. 2008;23:1702–1709.
Kim SY, Hyun JJ, Jung SW et al. Management of non-variceal upper gastrointestinal bleeding. Clin Endosc. 2012;45:220–223.
Messmann H, Schaller P, Andus T et al. Effect of programmed endoscopic follow-up examinations on the rebleeding rate of gastric or duodenal peptic ulcers treated by injection therapy: a prospective, randomized controlled trial. Endoscopy. 1998;30:583–589.
Loh DC, Wilson RB. Endoscopic management of refractory gastrointestinal non-variceal bleeding using Histoacryl (N-butyl-2-cyanoacrylate) glue. Gastroenterol Rep (Oxf) 2016;4:232–236.
de Sáenz MRA, Baltar Arias R, Vázquez Rodríguez S et al. N-Butyl-2-cyanoacrylate plug on fundal varix: persistence 3 years after sclerosis. Rev Esp Enferm Dig. 2009;101:212–214.
Chen WC, Hou MC, Lin HC et al. Bacteremia after endoscopic injection of N-butyl-2-cyanoacrylate for gastric variceal bleeding. Gastrointest Endosc. 2001;54:214–218.
Kim J, Chun HJ, Hyun JJ et al. Splenic infarction after cyanoacrylate injection for fundal varices. Endoscopy. 2010;42:E118.
Acknowledgments
We thank all the patients and their families for their valuable contributions. The authors gratefully acknowledge Junling Zhang (3D Medicines Inc.) for the helpful discussion and suggestions with the bio-analysis.
Funding
None.
Author information
Authors and Affiliations
Contributions
Designed the study: JS, WZ and YB. Wrote the first draft of the manuscript: JS and LN. All authors treated the patients, acquired data, and analyzed the data. Interpret the data: CZ and CJ. Revised the manuscript: WZ and YB.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by relevant regulatory and independent ethics committee of Changzhou Tumor Hospital and done in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. All patients provided written, informed consent before study entry.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shen, J., Ni, L., Zhu, C. et al. Efficacy of Endoscopic Tissue Adhesive in Patients with Gastrointestinal Tumor Bleeding. Dig Dis Sci (2024). https://doi.org/10.1007/s10620-024-08432-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10620-024-08432-7