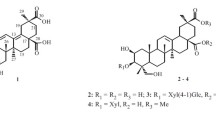
A new 2,3-seco-12-oleanene-2,3-dioic acid (1), along with ten known ones, was isolated from the bark of Aglaia perviridis. Their structures were elucidated on the basis of spectroscopic data. Compound 1 was evaluated for its cytotoxic activities in human cancer cell lines K562 (human leukemia), SMMC-7721 (hepatocellular carcinoma), MCF-7 (breast cancer), and KB (oral epithelium cancer) and drug-resistant cells of MCF-7/ADM, and KB/VCR, and for its anti-inflammatory activities in lipopolysaccharide-stimulated RAW264.7 macrophages. Compound 1 was found to be the most potent inhibitor of nitrite production in macrophages, with IC50 value at 6.5 μM.

Similar content being viewed by others
References
N. Joycharat, H. Greger, O. Hofer, and E. Saifah, Syst. Ecol., 36, 584 (2008).
Ching Su New Medical College ed., Dictionary of Chinese Crude Drugs [in China], 1997, 974 pp.
S. Pointinger, S. Promdang, S. Vajrodaya, C. M. Pannell, O. Hofer, K. Mereiter, and H. Greger, Phytochemistry, 69, 2696 (2008).
N. Joycharat, H. Greger, O. Hofer, and E. Saifah, Phytochemistry, 69, 206 (2008).
N. Fuzzati, W. Dyatmiko, A. Rahman, F. Achmad, and K. Hostettmann, Phytochemistry, 42, 1395 (1996).
D. Engelmeier, F. Hadacek, T. Pacher, S. Vajrodaya, and H. Greger, J. Agric. Food. Chem., 48, 1400 (2000).
B. Y. Hwang, B. N. Su, H. Chai, Q. Mi, L. B. Kardono, J. J. Afriastini, S. Riswan, B. D. Santarsiero, A. D. Mesecar, R. Wild, C. R. Fairchild, G. D. Vite, W. C. Rose, N. R. Farnsworth, G. A. Cordell, J. M. Pezzuto, S. M. Swanson, and A. D. Kinghorn, J. Org. Chem., 69, 3350 (2004).
M. N. Joshi, B. L. Chowdhury, S. P. Vishnoi, A. Shoeb, and R. S. Kapil, Planta Med., 53, 254 (1987).
J. F. Rivero-Cruz, H. B. Chai, L. B. Kardono, F. M. Setyowati, J. J. Afriatini, S. Riswan, N. R. Farnsworth, G. A. Cordell, J. M. Pezzuto, S. M. Swanson, and A. D. Kinghorn, J. Nat. Prod., 67, 343 (2004).
H. Greger, T. Pacher, B. Brem, M. Bacher, and O. Hofer, Phytochemistry, 57, 57 (2001).
S. Janaki, V. Vijayasekaran, S. Viswanathan, and K. Balakrishna, J. Ethnopharmacol., 67, 45 (1999).
F. Zhang, J. S. Wang, Y. C. Gu, and L. Y. Kong, J. Nat. Prod., 73, 2042 (2010).
T. Mosmann, J. Immunol. Methods, 65, 55 (1983).
L. J. Reed and H. Muench, Am. J. Hyg., 27, 493 (1938).
I. S. Lee, S. R. Oh, K. S. Ahn, and H. K. Lee, Chem. Pharm. Bull., 49, 1024 (2001).
T. Akihisa, H. Tokuda, M. Ukiya, T. Suzuki, F. Enjo, K. Koike, T. Nikaido, and H. Nishino, Chem. Pharm. Bull., 52, 153 (2004).
S. Weber, J. Puripattanavong, V. Brecht, and A. W. Frahm, J. Nat. Prod., 63, 636 (2000).
Y. Zhao, J. L. Ruan, J. H. Wang, Y. Cong, S. Song, Y. L. Cai, W. Fang, and D.-N. Zhou, Nat. Prod. Res., 22, 233 (2008).
K. Mohamad, T. Sevenet, V. Dumontet, M. Pais, M. Van Tri, H. Hadi, K. Awang, and M. T. Martin, Phytochemistry, 51, 1031 (1999).
W. M. Bandaranayake, Phytochemistry, 19, 255 (1980).
W. Aalbersberg and Y. Singh, Phytochemistry, 30, 921 (1991).
G. V. Malinovskaya, V. A. Denisenko, and N. I. Uvarova, Chem. Nat. Compd., 16, 257 (1980).
L. Revesz, P. Hiestand, L. La Vecchia, R. Naef, H. U. Naegeli, L. Oberer, and H. J. Roth, Bioorg. Med. Chem. Lett., 9, 1521 (1999).
E. J. Corey and S. C. Virgil, J. Am. Chem. Soc., 112, 6429 (1990).
Acknowledgment
The work was supported by the National Natural Science Foundation of China (Grant No. U1204830) and the Science and Technology Key Project of Department of Education of Henan Province (Grant Nos. 13A310064, 15A360016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2016, pp. 374–377.
Rights and permissions
About this article
Cite this article
Zhang, F., Chen, Y., Zhu, Y. et al. A New Triterpeniod from Aglaia perviridis . Chem Nat Compd 52, 427–431 (2016). https://doi.org/10.1007/s10600-016-1665-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-016-1665-9