Abstract
Kill-trap** is an important management tool for suppressing invasive mammalian predator populations in New Zealand, including the common brushtail possum (Trichosurus vulpecula). We conducted a combined field and pen experiment to test the hypothesis that intensive kill-trap** selects for survivors that differ behaviourally from the general population present before trap** begins. We installed 109 kill traps in a 120 ha forest reserve and over 20 weeks removed > 95% of the reserve’s possum population. Effort required rose from 12 trap nights per possum removed at the start of the operation to 560 trap nights per possum removed after 20 weeks of control. As the operation progressed, the estimated probability of possums being kill-trapped in the reserve declined from 0.30 to 0.15 per possum per week. Similarly, camera monitoring revealed that the average probability of a possum being killed when it encountered a kill-trap fell by 59% during the operation, indicating that the surviving population was becoming increasingly trap-recalcitrant. Behavioural testing of possums live-captured from the reserve at three time points revealed that survivors live-trapped 20 weeks into the operation were significantly shyer than those live-trapped before the operation began. In captivity, personality associated with boldness and activity/exploration was a significant predictor of whether a possum would trigger pest control devices it had not previously encountered. Heterogeneity of trappability affects the effort and cost required to eradicate invasive mammalian predators. Understanding the influence of personality on trappability is an important first step towards develo** more effective methods to target trap-recalcitrant individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive mammals threaten conservation, health, and economic values in a wide range of ecological systems (Braysher et al. 1996; Hadidian 2015; Van Vuren and Smallwood 1996). Suppression or eradication of invasive species populations—using toxic baits, traps, or other removal methods—is undertaken to reduce adverse impacts. Population suppression requires a sufficient removal of individuals such that population density is reduced despite recolonization of the control area and breeding by survivors. Eradication requires that all reproductive animals are put at risk, target animals are removed at a rate exceeding the rate of increase, and the area is protected from reinvasion (Bomford and O'Brien 1995). Aotearoa New Zealand (NZ) conservation managers have had great success in eradicating invasive mammals from increasingly large islands (Russell and Broome 2016), but given the twin challenges of eliminating every individual and preventing reinvasion, mainland eradication of invasive mammalian predators remains an elusive goal.
Supressing brushtail possums (Trichosurus vulpecula) and other invasive mammalian predators has long been a part of NZ rural life. Historically, possums have been controlled by trap**, poisoning, and shooting by landowners and government agencies to reduce their impacts on forestry, horticulture, and livestock disease. For large-area possum control, aerial toxin baiting is cost-effective, although the use of toxins remains contentious (Kannemeyer 2017; Warburton et al. 2021) and kill-traps remain a key component in current efforts to create predator-free areas (Warburton et al. 2021). Recent control efforts include conservation-oriented programmes run by community groups that manage networks of kill-traps to suppress possum numbers in local bushland and suburban areas.
Kill-trap** is most effective and economical in the early stages of a predator control operation. As trap** progressively reduces a predator population, catches decline and cost per individual animal removed increases rapidly (Braysher et al. 2012). This pattern is evident in hypothetical scenarios where probability of capture (Pcapture) for all animals is identical and unchanging over time (e.g. Fig. 1 and Vattiato et al. 2021). In practice, however, Pcapture is expected to vary among individuals in response to a range of factors, including demography (Kay et al. 2000), home range size (Ball et al. 2005), past trap** experiences (Linhart et al. 2012), and personality (Carter et al. 2012).
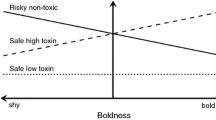
A simulated 16-week kill-trap** operation targeting brushtail possums in a hypothetical forest reserve. a Changes in possum numbers (solid markers) and trap nights per possum removed (open markers). The reserve was assumed to initially hold 150 possums; 100 kill-traps were set nightly, with the weekly probability of each possum being killed (Pkill) held constant at 0.15. During the operation, the number of trap nights required to remove a possum rose from 31 to 356; by week 16, 10 possums remained alive in the reserve. b Hypothesized effects of intensive kill-trap** on the distribution of possum personalities (on a shy–bold axis) in the population. Under the null hypothesis (H0), all possums are equally susceptible to removal, and almost all possums have been removed by the time the operation ends (red line). Under the alternative hypothesis (H1), individuals differ in susceptibility to removal as a function of personality and kill-trap** will select for survivors with personalities associated with trap avoidance. In this scenario, the last few animals are likely to be recalcitrant individuals that are unwilling to engage with kill-traps; these animals are more difficult to remove and consequently, more individuals are present when the operation ends
Studies on other species (King et al. 2003; Tuyttens et al. 1999) have revealed Pcapture resulting from trap recalcitrance (an unwillingness to interact with traps). Behavioural recalcitrance towards capture can be an innate response (Biro and Dingemanse 2009), learned (i.e. ‘trap-shy’) from past aversive encounters with traps (Camacho et al. 2017), or be a combination of the two (Biro and Dingemanse 2009). Animal personality—consistent behavioural differences among individuals (Dingemanse and Réale 2005)—is an important source of individual variation in response to capture devices (Johnstone et al. 2021b), which often preferentially capture bolder (Carter et al. 2012) and more active (Boon et al. 2008) individuals. While population suppression operations may easily target bolder animals, they risk inadvertently selecting for survivors with personalities associated with avoidance of control devices. If so, personality-related recalcitrance will prolong population suppression efforts and greatly increase the difficulty and cost of removing ‘the last 5%’ of a predator population (i.e., in Fig. 1a the right-hand side of both curves will be pushed upwards). Although methods for quantifying personality in brushtail possums are well established (Mella et al. 2016) and the influence of personality on possum ecology (Herath et al. 2021; Wat et al. 2020) has been investigated, to our knowledge, trap recalcitrance remains unexplored.
Here we explore behavioural heterogeneity as a mechanistic driver of survivorship during a kill-trap operation targeting invasive brushtail possums in NZ. We tested the hypothesis that rather than being a random subset of the population, surviving possums would differ behaviourally from the general possum population sampled pre-control (Fig. 1b). In a captive study, we tested specific predictions that—relative to possums sampled pre-control—these survivor possums would: (i) have a narrower spectrum of personality, skewed towards reduced boldness and reduced exploration; and (ii) have reduced interaction rates with novel kill-traps and other pest-control devices they had not previously encountered. Understanding trap**-induced changes in the distribution of behaviours and personality in a predator population will assist future control programmes to better target recalcitrant individuals that survive traditional trap-based control programmes.
Materials and methods
Study area
We investigated the effects of intensive kill-trap** on the invasive possum population in Lottery Bush Scenic Reserve, an area of old-growth mixed native beech and podocarp forest near Waiau in North Canterbury, NZ (Fig. 2). As there are no natural predators for possums in NZ, unmanaged populations reach abnormally high densities on forests and farmland, where their omnivorous diet leads to defoliation of native trees and depredation of native invertebrates, reptiles, bird eggs and nestlings (Byrom et al. 2016). The Department of Conservation and local landowners were seeking to eliminate the local possum population to protect the reserve’s native flora and fauna. The 120 ha reserve slopes from a 460 m elevation ridgeline to the east, down to Lottery Creek in the west. Most of the reserve is surrounded by grazed pasture.
Locations of 81 Sentinel (green) and 28 Trapinator (blue) kill-traps at Lottery Bush Scenic Reserve in mid-2021. Kill-trap** began in May 2021, with a subset of 67 of these traps. Live-capture traps (red, n = 150) were focused on the eastern side of the reserve. Three live-capture trap** sessions ran during the control, beginning in April 2021 with a subset of 35 of these traps
Possum population reduction
Prior to our study there had been no significant possum trap** in the reserve within the last c. 7 years. During a preliminary inspection in March 2021, possum signs were abundant throughout the reserve. In early May 2021, following a training session with our research team, a local community group installed 48 Sentineltm possum kill-traps (traps.co.nz) interspersed with 19 Trapinatortm possum kill-traps (www.Connovation.co.nz) on transects across the eastern side of the reserve (Fig. 2). Both trap designs consist of a spring-powered metal kill-bar and baited trigger mechanism enclosed within a rotomolded plastic casing that helps exclude non-target species. The traps are attached to tree trunks c. 50 cm above ground level; possums inset their head through an opening in the casing to access the bait, triggering the kill-bar. Regular visits by field staff are required to clear carcasses and re-set traps. Trap transects followed forest margins, ridgelines, and other landscape features expected to favour possum movement and trap encounters. Traps were spaced a minimum of 50 m apart and baited with peanut-butter and a blaze of flour and icing sugar, a strategy that combined olfactory and visual lures.
As the capacity to routinely clear and reset traps increased, additional traps were added in June and July 2021, resulting in the eventual deployment of 109 traps (81 Sentinels and 28 Trapinators) across the entire reserve. The expected home range of possums in eastern forest habitat is c. 1–2 ha (Cowan and Glen 1990), so this trap coverage provided high confidence that all possums within the reserve would encounter at least one kill-trap. Initially, traps were checked and reset twice a week. As possum numbers declined and catch rates fell, the interval between checks lengthened progressively. At each trap-check, the capture locations of possums were recorded.
Possum abundance indices
The decline in possum abundance was primarily tracked by monitoring kill-trap catch rates. To account for potential kill-trap avoidance, and to monitor habitat between kill-trap transects, we also ran trail camera (n = 30) and chew-card (n = 60) surveys across the eastern half of the reserve (the focus area for this study) prior to the operation and again in June and in September 2021. Chew cards—a rectangular piece (90 × 180 mm) of 3-mm white twin-walled polypropylene sheet—were bent into a right-angled shape and mounted 200–300 mm above the ground on tree trunks or stakes. Card were baited with peanut butter pressed into the flutes (channels) of the card and the counts of bite-marks left in the card by possums are used as an index of possum presence and abundance (Sweetapple and Nugent 2011). Six transects of 10 cards were set 25 m apart, with > 100 m separating transects. A remote-sensing trail camera (Reconyx PC900) monitored every second chew card. All cameras were set to record three-image bursts when motion-activated, with a 1 min interval between bursts. Chew-card interference was recorded after 3 nights and after 11 nights; camera images were collected after 11 nights. Camera images were downloaded periodically and camera batteries refreshed. We also recorded capture rates from cage traps and padded leg-hold traps used to obtain live possums from the reserve (see Possum live-capture below).
Possum interactions with kill-traps
To assess whether possums’ willingness to interact with traps changed during the control operation, a subset of 40 kill-traps was monitored with trail cameras (Reconyx Hyperfire) from early May until mid-August 2021. Cameras were active 24-h and when motion-activated, were set to take bursts of three images, with a 10 s interval between bursts. Image sequences of possums were reviewed to tally the following events: (1) approach within 1 m of the kill-trap; (2) head entry; (3) trap triggered; and (4) possum killed. Head entry was defined as the possum’s eyes being within the trap’s entrance. These events were analyzed only for those possums interacting with active (unsprung) traps.
Possum live-capture
To determine whether the personality spectrum of possums in the reserve changed as the control operation progressed, we live-trapped and retained 34 adult and subadult possums from the eastern half of the reserve (the focus area for this study), where possum abundance was highest. We focused on adult and sub-adult (> 1.8 kg) possums, as younger animals may not be fully independent and as younger animals can have more plastic behaviours (Brust et al. 2015). Smaller possums were humanely euthanized by experienced technicians using intra-cardiac injection of sodium pentobarbitone (150 mg/kg) following anaesthesia with gaseous isoflurane.
Live-trap** was conducted at three time-points during the control operation, with the traps left closed the field between trap** sessions. To avoid potential recalcitrance towards the peanut-butter used at kill-traps, each live-trap** session used additional new lures. We ran the first trap** session (pre-control) in April 2021, using 35 cage traps spaced at 50 m intervals along seven transects and set for 3 nights. Traps were baited with apple and lured with a flour-and-icing-sugar blaze on the tree trunk and ground in front of the trap.
Following 4 weeks of kill-trap**, the second live-trap** session (mid-control) was conducted in early June 2021, using 50 cage traps set for 11 nights. Traps were set, baited, and lured as before, except that half the traps were also lured with curry powder (Morgan et al. 1995). After six nights with few captures, 40 leg-hold traps (#1 double-coil spring traps; Oneida Victor, Cleveland, Ohio), baited only with a flour-and-curry blaze encircling the trap to improve catch rates (Warburton et al. 2022), were interspersed between cage traps; all traps were then run for a further five nights.
After c. 20 weeks of kill-trap** we ran a third live-trap** session (late-control) in September/October 2021. Instead of cage traps, 90 padded leg-holds were set at 50 m spacing along 18 transects. Leg-hold traps have higher catch rates than cage or kill-traps, both of which require the possum to enter its head into an enclosed area (Austin et al. 2004). We anticipated, therefore, that leg-hold traps would be the most capable method of capturing trap-recalcitrant individuals (albeit at a higher ethical cost because of the increased risk of limb injury). An additional 10 leg-hold traps were set where cameras and chew cards indicated recent possum activity. Alternating leg-hold traps were lured with either Nutella paste (Jackson et al. 2015) or aniseed-flavoured Lure-Ittm aerosol lure (Connovation Ltd, Auckland), plus a flour blaze as a visual lure. In addition, a ‘social’ lure (a plastic pottle with cloth fragments impregnated with odour from possums trapped earlier in the operation) was set at every second leg-hold per transect to increase interaction rates. Following one night of pre-baiting, leg-holds were set for five nights, closed for 1 week, and then re-set for a further five nights.
In early November 2021, in a final effort to live-capture surviving possums detected on cameras, 10 long-lasting Motoluretm bait stations (zip.org.nz/products-list/motolure) were installed inside the reserve (n = 5) and on its margin (n = 5) at locations where possum activity had been detected in September. Motolures contain a reservoir of mayonnaise housed in a tree-mounted aluminium casing, with a battery-powered timed motor that dispense small amounts of bait daily for periods of up to a year before being re-filled. Motolures were monitored with trail cameras for 15 days. At the four motolure sites where possums were detected on camera, we attempted to capture possums using a targeted cluster of five leg-hold traps set for three nights. Our aspiration was that the continual (daily) presence of fresh bait, combined with a high density of traps around those baits, would increase our chances of catching trap-recalcitrant individuals; however, no further possums were live-captured.
All live-trapped possums were transported to the Johnstone Memorial Animal Facility at Lincoln, Canterbury, for a health check under anaesthesia, sexing, weighing, and ear-tagging for identification. Possums were housed individually in outdoor pens (5 m × 5 m) containing a shelter and water trough and given 3–4 nights’ acclimatization before beginning behavioural assays. For the first week possums were restricted to a diet of apples, vegetables, and walnuts while a feeding trial (reported separately) was run. Thereafter, their maintenance diet consisted of ad libitum cereal pellets, supplemented with apples, vegetables, and walnuts.
Behavioural assays
The personality of captive possums (n = 34) were quantified using a ‘bookcase test’—used in Australia to quantify brushtail possum personality (Mella et al. 2016). The test employed a mesh-fronted vertical wooden arena (L 60 cm × W 40 cm × H 128 cm) with four levels. Two openings (20 cm × 15 cm) allowed movement between each level and a feed tray in the back corner of each level contained a slice of apple. A possum was released into the lowest level of the arena via an access door (diameter 10 cm) and the possum’s behaviour was recorded for 5 min using a high-definition infrared video security camera (Boblov KJ21 Body Camera, Amazon.com). After each test the arena was cleaned with a 75% ethanol solution to minimize residual social odour cues. Testing began after dusk and away from the other captive possums. Each possum was tested two or three times, with repeat tests c. 2 weeks apart (mean interval = 15 days). We used the Observer XT software (Noldus.com) to measure the time (in minutes) that each possum allocated to several behaviours (following Mella et al. 2016) likely relating to boldness, i.e., eating; activity/exploration, i.e., not moving (immobile) and level changes (i.e., number of times a possum moves between any two levels); and exploration, i.e., latency to leave the lowest level. Body weight was recorded after each test. It was unavoidable that each group was tested at a different time of year, however a previous study that quantified personality in > 100 possums found no effect of season (Wat et al. 2020).
Novel device trial
To investigate whether avoidance of kill-traps in the field varies by the control device used or a generalized behaviour reflected in possum responses to other devices in captivity, each possum was transferred for five nights to a large (10 m × 10 m) outdoor enclosure containing two shelters. Possums were moved during the day to minimize stress and acclimatized for two nights before the trial. Food and water were provided ad libitum. We filmed possum responses to three pest-control devices (Fig. 3a–c) they had not previously encountered: a Goodnature A12 kill-trap baited with Goodnaturetm gel (Goodnature.co.nz); a Philprooftm bait station baited with 20 × 12 g non-toxic cinnamon RS5 pellets (Philproof.co.nz); and a Timmstm trap baited with a slice of apple smeared with peanut-butter (NZtraps.co.nz). Devices (one per night over three nights) were presented in a randomized order, with exposure time standardized by confining data analysis to the 5 h period following a possum’s first emergence from the shelter each evening. Devices were set and baited as they would be in the field, but with their triggering mechanisms disabled. A possum was categorized as having triggered a device (as a proxy for being killed) if it was seen removing bait from the device. On completion of the novel device trial, possums were returned to their home pen and made available to other researchers.
Statistical analysis
Possum abundance and trap interaction
After each kill-trap, live-trap, and trail camera check, we calculated capture rate per 100 trap-nights or camera-nights. For graphical presentation of changes in kill-trap catch rate over time, each capture rate estimate was assigned to the mid-point date between successive trap checks. Chew-card indices were summarized as the percentage of cards with at least one possum bite-mark after 3 days and after 11 days.
Whenever a possum was close to a camera-monitored kill-trap, bursts of still images were recorded. Review of these images allowed us to determine the outcome of each encounter (i.e., ‘approached’, ‘interacted’, ‘triggered’ or ‘killed’). From these data, we calculated the probability (i.e., risk) of each type of encounter occurring ‘early’ (during May, the first 4 weeks of kill-trap**) or ‘later’ (from early June until mid-August) in the operation. This division was based on the end of May being the date at which > 50% of the eventual cumulative catch of possums from the reserve had been achieved. The outcomes of early versus late interactions with active (i.e., unsprung) traps were compared by calculating the relative risk (RR) of each type of event, where RR = (Probability of event during a late interaction)/(Probability of event during an early interaction) (Schmidt and Kohlmann 2008). Events of equal probability in early-period and late-period interactions have an expected RR of 1.0; the statistical significance of RR values deviating from unity was assessed using Fisher’s Exact tests.
Behavioural assays
Possum behaviours from the bookcase test were analysed as potential personality traits using the mixed-effects modelling approach in Dingemanse and Dochtermann (2013), implemented using the R package lme4 (Bates et al. 2015). Normality was confirmed by examining residuals using R package DHARMa and a Shapiro-Wilks test. A log or sqrt transformation was applied when needed. We compared two models (the second containing individual identity as a random effect) reduced to significant fixed effects only (sex, mean body weight, and test number, where relevant). If inclusion of individual identity significantly improved model fit—which we assessed using log likelihood and Akaike’s information criterion (AIC)—the behaviour was considered a significant personality trait. Trait plasticity was tested for by comparing the second model to a third containing the interaction between test number and individual identity as a random effect. Trait repeatability was tested using R package rptR (Nakagawa and Schielzeth 2010), and we tested for correlations between significant traits using a Spearman rank correlation test.
We used Spearman rank correlation to test our prediction (Fig. 1) that personality traits would trend over time as the operation progressed (i.e., pre-control vs. mid-control vs. late-control) and ran a Bartlett’s test to assess our prediction that variance in traits would differ among the groups. We also tested for demographic differences (sex ratio and mean body weight) among the groups using ANOVA.
Novel device trial
Finally, we explored the influence of personality traits and demographics on susceptibility of the captive possums to three predator control devices these possums had not previously encountered. We tested for factors affecting the probability of individuals triggering a device (defined as bait removed within 5 h of leaving the slee** shelter) by running a logistic regression using generalised linear models (GLM) with a binomial distribution and a logit link function. Separate analyses were run for each trait (identified below), with each model including the trait, sex, mean body weight, device, device*trait, and group as fixed effects and with individual identity as a random effect. All statistical analysis was run in R version 4.1.2 (R Core Team 2013).
Results
Possum population reduction
Between May 2021 and January 2022, kill-traps removed 136 possums from the reserve (Fig. 4a). An additional 38 possums were removed through live-trap** (20 pre-control, eight mid-control, seven late-control, and three juveniles). This equates to the removal of 1.4 possums per ha from the 120 ha reserve, which was close to the total we expected based on our original site inspection. One late-control possum was injured and subsequently euthanized, leave six late-control possums for captive trials.
Removal of possums from Lottery Bush Reserve by sustained kill-trap**, beginning 3 May 2021. a Cumulative number of possums killed, with shaded area indicating the number of kill-traps installed; b change in possum removal (per 100 trap nights; solid line) and trap** effort per possum removed (dotted line); c decline in observed trap kills at Lottery Bush (solid markers, log scale) appears to have been non-linear over time, with the quadratic term in the regression line approaching statistical significance (P = 0.05); and d weekly probability of an individual possum being killed (estimated from the slope of the regression line in c), with t in the equation representing the number of days since kill-trap** began)
Kill-trap** success declined rapidly as the control operation progressed. By September (after 12 weeks of trap**) the catch rate had fallen to c. 2% of that recorded in the 1st week (Fig. 4b). Given our interest in potential trap recalcitrance, we were mindful that the decline in catch rate might overestimate the true reduction in possum numbers. However, the declines in our additional chew-card, trail camera, and live-trap** indices were broadly consistent with the trend in kill-trap** success (Table 1), providing supporting evidence that possum numbers in the reserve had been reduced by > 80% by July and by > 95% by October.
In April, prior to any possum control, only 5.8 trap nights of live-trap** effort were required for each possum captured (Table 1). However, by early October, after 20 weeks of kill-trap**, 143 nights of live-trap** effort was required per possum—a 25-fold increase in effort. Over the same period, the effort needed to kill-trap a possum increased from c. 12 to c. 560 trap nights—a 46-fold increase (Fig. 4b).
Possum interactions with kill-traps
Trail cameras recorded 343 instances of a possum approaching an active (i.e., unsprung) kill-trap early on, during the first month of the operation, and 244 approaches in later weeks. Given the proximity of the cameras to the traps, almost all possums came within 1 m of the trap (96.5% and 98.0%, respectively; RR = 1.02). However, relative to possums photographed in the 1st month, possums which approached within 1 m later in the operation were significantly less likely to enter their head into the trap, trigger the trap, and be killed (Table 2; RRs = 0.58, 0.45 and 0.41, respectively).
If Pkill had remained constant throughout the kill-trap** operation (as in the hypothetical scenario in Fig. 1), the relationship between natural log of possum kill rate and week would have been linear, with slope r equal to the exponential rate of decline of the trappable possum population. But the trend in logged kill rate over time appears non-linear (Fig. 4c), with the quadratic term in the fitted curve approaching statistical significance (P = 0.05). The probability of an individual possum being caught over time interval t can be estimated as Pkill = 1 − ert, so the implication of this non-linearity in r is that, rather than being constant, Pkill early in the control operation began at around 0.3 per week and had halved to around 0.15 by early August (Fig. 4d). This halving of the weekly capture rate is consistent with the decline over time in capture risk estimated from our trail camera data (above and Table 1).
Possum personality
Three groups of live-trapped possums were collected in April (pre-control), in early June (when the possum population had been reduced by c. 85%), and from mid-September to early October (when the population had been reduced by > 95%).
We quantified four significant and repeatable behaviour traits relating to boldness (Time eating: P < 0.01, r = 0.41 (95% CI 0.16–0.62)), activity/exploration (Time not moving: P < 0.05, r = 0.29 (CI 0.04–0.52); Number of level changes: P < 0.001, r = 0.19 (CI 0–0.41)), and exploration (Latency to leave lowest level: P < 0.01, r = 0.35 (CI 0.08–0.57)). Test number had a significant effect on Time eating (P = 0.04), Number of level changes (P < 0.001) and Latency to leave lowest level (P < 0.0001), but there was no significant effect of sex or body weight on any trait (all P > 0.09). A Spearman rank correlation test showed a moderate negative correlation between Time eating and Time not moving (rs = − 0.64, N = 34, P < 0.001), suggesting that bolder possums were also more active/explorative, while Latency to leave the lowest level was negatively correlated with Number of level changes (rs = − 0.56, N = 34, P < 0.001), suggesting that possums that took longer to begin exploring the arena were also less active in the arena.
Time eating (a measure of boldness) differed among the three groups of live-trapped possums, declining significantly as the control operation progressed (Fig. 5). Animals sampled later in the operation were less bold (i.e.s shyer, rs = 0.38, N = 34, P = 0.03) than possums captured pre-control and there was less variation among individuals captured later in the operation (Bartlett’s test of equal variance; Chi-squared = 8.11, df, P = 0.02). No other trait differed significantly by group (all P > 0.25).
Box and whisker plots illustrating a significant decline in the magnitude (P = 0.03) and variance (P = 0.02) of Time eating (a measure of boldness) among groups of possums live-captured from Lottery Bush at three points in time during the kill-trap** operation. Number of possums tested is in parentheses. The Mid-control group was captured in early June, at which time the possum population had been reduced by c. 85%. The Late-control group was captured from mid-September through early October, at which time the population had been reduced by > 95%. Values in parentheses indicate the sample size
Possum responses to novel devices
The probability of a possum triggering novel devices during the captive study was significantly influenced by two traits. Time eating (boldness; Fig. 6a) was positively associated with triggering any device (X2 = 7.64, df = 1, P = 0.006). There was also a significant interactive effect between the trait Time not moving (activity/exploration) and the type of device that was triggered (X2 = 13.26, df = 2, P = 0.001; Fig. 6b). The probability of a Timms trap being triggered was higher for individuals that were less mobile (P = 0.0005), whereas mobility had the opposite effect at A12 traps (P = 0.001). There was no discernible effect of Time not moving on responses to Philproof bait stations (P = 0.46). Mean body weight also influenced the probability of possums triggering traps (X2 = 5.13, df = 1, P = 0.02), with probability decreasing for heavier animals (Fig. 6c). No other measured factors significantly influenced the probability of possums triggering a device (all P ≥ 0.10).
The probability of a captive possum triggering a mock kill-device as a function of: a personality measured as Time eating (boldness; P = 0.006); b personality measured as Time not moving (activity/exploration), which interacted significantly (P = 0.001) with device type; and c body weight (P = 0.02). Shaded areas represent 95% Confidence Intervals around the fitted line. Asterisks indicate statistically significant effects
Discussion
Conventional management has tended to view invasive predators as homogeneous populations that can be suppressed using a single control method (i.e. one bait type or trap type), yet the most intractable challenges of invasive species management may arise precisely because some individuals do not behave like the general population (Garvey et al. 2020). Reliance on a single control method to suppress or eradicate an entire population is unlikely to be an effective strategy to target the most recalcitrant individuals, resulting in slow progress (Cowan 1992) or even operational failure (Amos et al. 2016). This study was motivated by anecdotal observations during past possum control operations, where conventional trap** becomes largely ineffective over time (i.e., as the surviving population become increasingly recalcitrant). Moving to more effective (but less humane) leghold trap** as our study progressed was a key aspect of the study design. Leghold trap** was expected to target a different (wider) subpopulation of animals than our cage trap**.
Effective suppression of a predator population requires that almost all animals be susceptible to removal. Unlike aerial baiting, where possums typically encounter and consume baits within one or two nights, many nights of trap** may be required to substantially lower the population. For example, in our modelled scenario (Fig. 1), with weekly Pcapture set at 0.15, it would take 4 weeks to remove 50% of the possum population, 18 weeks to remove 95% of the population, and 32 weeks before it was probable that the last individual had been eliminated. Trap** at Lottery Bush produced results broadly in line with this scenario, with a > 50% reduction during the first month of trap** increasing to > 95% reduction after 20 weeks (Table 1).
Small numbers of possums continued to be seen on cameras at Lottery Bush throughout late 2021 and 2022 (unpublished data), despite the kill-trap network remaining active throughout this period. Some possums may have immigrated from surrounding areas, but several lines of evidence suggest that most were trap-recalcitrant survivors. First, our best-fit regression estimate of weekly Pcapture halved, from 0.30 to 0.15, over the first 20 weeks of kill-trap** (Fig. 4c–d). Second, camera-recorded outcomes of possums encountering kill-traps revealed that later in the operation possums were 59% less likely to be killed (Table 2). Third, expansion or shifts in home ranges following control is a slow process and animals that do reinvade controlled areas are typically young, dispersing males (Cowan 2000). There was an equal sex ratio in the possums captured during the mid- and late-control live-trap** sessions and all possums were adult, except for two small juveniles that were likely too young to be dispersing individuals. Finally, from August 2021 through January 2022, all identifiable carcasses in kill traps were adults, again suggesting that most animals in the reserve during that period were residents rather than dispersers.
Our field evidence for increasing behavioural recalcitrance in response to ongoing kill-trap** is reinforced by the behavioural differences among the three groups of live-trapped possums brought into captivity. Our plan had been to observe three groups of 20 possums, but we were unable to achieve that sample size once kill-trap** was underway in the reserve, despite increasingly intensive live-trap** efforts (including the use of leg-hold traps, multiple visual and olfactory baits and lures, social lures, and long-term lure stations—see “Methods”). Consequently, we had low statistical power to detect differences in the personality spectrum of the three groups. Furthermore, we acknowledge that there may have been some highly trap-recalcitrant possums in the reserve that we simply had no ability to capture with our existing tools, and, if so, those extreme personalities would not be represented in our study.
Despite these limitations, we demonstrated that kill-trap** affected the personality characteristics of the surviving possum population. A progressive decline in boldness was evident as the trap** operation continued (Fig. 5). This outcome was consistent with our hypothesis (Fig. 1b) that trap** would remove bolder individuals and select for a greater proportion of shy individuals in the surviving population. The variability of the bold trait also decreased, again consistent with our predictions. The effort required to capture the shyest animals increased substantially: it was 25 times more difficult to live-capture the possums in the late-control group compared to possums caught pre-control. Live-capture traps and wildlife monitoring devices are often (although not always) biased towards sampling narrow spectrums of personality, typically favouring bolder or more active individuals (Boon et al. 2008; Carter et al. 2012; Johnstone et al. 2021b), but to our knowledge, this is the first study to explore this phenomena in the context of predator control and demonstrate a selection for an increasingly shy population of survivors.
Trap-recalcitrant personality may also interact with other demographic factors can that influence the success of removing the last surviving individuals. For example, personality can influence home range and core area size (Wat et al. 2020), microhabitat use and movement across the landscape (Schirmer et al. 2019), and diet (Herath et al. 2021), all of which affect encounter and interaction rates with traps. In our captive trials we found that heavier animals had a lower probability of triggering the novel devices; suggesting susceptibility to the traps may be related to age (Kay et al. 2000) or experience (Camacho et al. 2017). Alternatively, lighter individuals may be leaner, hungrier and more motivated to investigate novel cues or take foraging risks (e.g. Bisi et al. 2011).
Unless Pcapture is high (> 0.50), achieving intensive population suppression or complete eradication by means of trap** will be a long and costly process. Even when trap reticence is not present, i.e., in our hypothetical scenario in Fig. 1, achieving a 93% population reduction is associated with 12-fold increase in the required capture effort. This hypothesised effect was even more pronounced in our real-world data from Lottery Bush: over the first 20 weeks of the operation effort to remove a possum by live-trap** increased 25-fold, and effort to remove by kill-trap** effort increased 46-fold. The measured increase in trap-night effort was less pronounced for live-trap** than for kill-trap**, likely because we introduced and then replaced cages with leg-hold traps as a more effective live-trap** method as the operation progressed.
Pcapture represents the outcome of a sequence of events that begins when a possum encounters (defined herein as approach within 1 m), interacts with, triggers, and is killed by a trap. Decisions that managers make about trap density and layout, guided by past experience or formal decision support systems (e.g. Gormley et al. 2021), aim to maximize Pencounter. What our camera image analysis emphasizes, however, is that the probability of a Lottery Bush possum being captured and killed as the result of a trap encounter was remarkably low throughout the operation. During the first month of kill-trap** the chance of being killed by the encounter was 1 in 14; later in the operation this fell to 1 in 34 (Table 2). We were not surprised that these probabilities were lower than our estimates of weekly probability of capture, as possums in the reserve would typically have multiple traps encounters each week (with all, or all but one, of these encounters having an outcome other than ‘killed’). Low Pcapture even at the beginning of the operation would encourage an examination of standard operational procedures, and the incorporation of additional strategies to maximise capture rates over and above our efforts of incorporating multiple food lures.
While lures (olfactory, visual, or social) can play a role in drawing possums in from a distance to encounter traps, there will be greater value in lures that incentivize possums to interact with and trigger control devices once they encounter them. Reluctance to interact with devices has similarly been observed in other invasive species. For example, less than half (48%) of black rats (Rattus rattus) that approached monitoring devices engaged with the device, and only 26% entered (Johnstone et al. 2021b). Rat responses to devices was linked to individual personality, with risk-taking individuals engaging with and entering devices and risk-averse individuals bowing out early or actively avoiding devices (Johnstone et al. 2021b).
Our captive trials demonstrate that possum personality, on a bold–shy continuum, has consequences for possum survivorship during device-based control operations. Shyer individuals were less willing to take bait when presented with three disabled kill devices (two traps and a bait station), which implies they would have been more likely to survive if the devices had been operational. These findings are consistent with our hypothesis that trap** selectively removes bolder animals, because they are the individuals most likely to interact with traps and other control devices. Our findings also indicate that device shyness among possums removed from Lottery Bush was not specific to the Sentinel and Trapinator kill-traps deployed in the reserve, but instead was generalized to avoidance behaviour.
However, Time not moving (a measure of activity/exploration), influenced the susceptibility of removal (triggering a device) in device-specific ways. This outcome suggests that some personalities may be predisposed to being caught by one technique while being less susceptible to others (Johnstone et al. 2021a; Wilson et al. 2011). The properties of different devices may provide different fear and/or attraction cues, the balance of which can elicit different responses from different individuals as they balance the risk–reward trade-off of engaging with a device. The Timms traps, for instance, were set on the ground and may have elicited highly investigative responses from individuals, whereas the Philproofs and A12s were mounted on trees or posts and may blend more effectively into the environment. A12s also require animals to approach from underneath to reach the bait, in contrast to the other traps, where the entrance is at the front. Using multiple devices that offer contrasting cues (i.e., differ in appearance, trigger mechanism, and lure) may be an effective strategy to target individuals that survive one device but may remain susceptible to another, although the effectiveness of this strategy requires further investigation. This approach is currently being employed in the field, and some NZ pest managers have begun combining multiple control tools, including some intended to remove the most recalcitrant individuals in the population (e.g. Nichols et al. 2021).
NZ’s Predator Free 2050 (PF2050) initiative (Russell et al. 2015) aims to eradicate possums and other predators from the New Zealand mainland within the next 30 years. Unless a suitable toxic baiting strategy can be developed that has strong social and cultural acceptability nationwide—including in urban areas and along water courses—traps will remain a key tool for PF2050. As such, selection for shyness and trap recalcitrance in survivors presents a problematic challenge. In influencing each individual’s probability of interacting with a control device, animal personality has consequences for management and control efforts. Understanding the drivers of individual-level differences in behaviour will help managers target the full spectrum of personalities within a population. As catches approach zero, having an adaptive control strategy will be key to targeting recalcitrant survivors. For example, trap-switching may be helpful if animals are recalcitrant towards a specific trap design (Johnstone et al. unpublished). Similarly, introducing alternative baits and lures may be effective in targeting variation in dietary preference or motivations (Garvey et al. unpublished). Until recalcitrant individuals are effectively targetable, successful eradication of possums and other invasive mammals from New Zealand cannot be realized.
Data availability
The dataset analysed during the current study are publicly available through the Manaaki Whenua Landcare Research datastore repository (DOI: https://doi.org/10.7931/6y1w-s375).
References
Amos W, Nichols HJ, Churchyard T, Brooke MDL (2016) Rat eradication comes within a whisker! A case study of a failed project from the South Pacific. R Soc Open Sci 3:160110. https://doi.org/10.1098/rsos.160110
Austin J, Chamberlain MJ, Leopold BD, Burger LW Jr (2004) An evaluation of EGGTM and wire cage traps for capturing raccoons. Wildl Soc Bull 32:351–356
Ball SJ, Ramsey D, Nugent G, Warburton B, Efford M (2005) A method for estimating wildlife detection probabilities in relation to home-range use: insights from a field study on the common brushtail possum (Trichosurus vulpecula). Wildl Res 32:217–227
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Biro PA, Dingemanse NJ (2009) Sampling bias resulting from animal personality. Trends Ecol Evol 24:66–67. https://doi.org/10.1016/j.tree.2008.11.001
Bisi F et al (2011) The strong and the hungry: bias in capture methods for mountain hares Lepus timidus. Wildl Biol 17:311–316
Bomford M, O’Brien P (1995) Eradication or control for vertebrate pests? Wildl Soc Bull 23:249–255
Boon AK, Réale D, Boutin SJO (2008) Personality, habitat use, and their consequences for survival in North American red squirrels Tamiasciurus hudsonicus. Oikos 117:1321–1328
Braysher M, O'Brien P, Bomford M (1996) Towards "best practice" vertebrate pest management in Australia. In: Seventeenth vertebrate pest conference
Braysher M, Buckmaster T, Saunders G, Krebs CJ (2012) Principles underpinning best practice management of the damage due to pests in Australia. In: Proceedings of the vertebrate pest conference, vol 25
Brust V, Schindler PM, Lewejohann L (2015) Lifetime development of behavioural phenotype in the house mouse (Mus musculus). Front Zool 12:S17
Byrom AE, Innes J, Binny RN (2016) A review of biodiversity outcomes from possum-focused pest control in New Zealand. Wildl Res 43:228–253
Camacho C, Canal D, Potti J (2017) Lifelong effects of trap** experience lead to age-biased sampling: lessons from a wild bird population. Anim Behav 130:133–139
Carter AJ, Heinsohn R, Goldizen AW, Biro PA (2012) Boldness, trappability and sampling bias in wild lizards. Anim Behav 83:1051–1058. https://doi.org/10.1016/j.anbehav.2012.01.033
Cowan P (1992) The eradication of introduced Australian brushtail possums, Trichosurus vulpecula, from Kapiti Island, a New Zealand nature reserve. Biol Cons 61:217–226
Cowan P (2000) Factors affecting possum re-infestation—implications for management. Department of Conservation Wellington, New Zealand
Cowan P, Glen A (1990) Family phalangeridae. In: The handbook of New Zealand mammals. Oxford University Press, Auckland, pp 67–98
Dingemanse NJ, Dochtermann NA (2013) Quantifying individual variation in behaviour: mixed-effect modelling approaches. J Anim Ecol 82:39–54. https://doi.org/10.1111/1365-2656.12013
Dingemanse NJ, Réale D (2005) Natural selection and animal personality. Behaviour 142:1159–1184
Garvey P et al (2020) Leveraging personality, motivations and sensory cues for effective predator management. Trends Ecol Evol 35:990–1000
Gormley A et al (2021) Quantitative decision support for eradication: a primer. Manaaki Whenua—Landcare Research and Arthur Rylah Environmental Research Institute, Centre for Invasive Species Solutions, Canberra, Australia
Hadidian J (2015) Wildlife in US cities: managing unwanted animals. Animals 5:1092–1113
Herath AP, Wat KK, Banks PB, McArthur C (2021) Animal personality drives individual dietary specialisation across multiple dimensions in a mammalian herbivore. Funct Ecol 35:2253–2265
Jackson M, Hartley S, Linklater W (2015) Better food-based baits and lures for invasive rats Rattus spp. and the brushtail possum Trichosurus vulpecula: a bioassay on wild, free-ranging animals. J Pest Sci 89:479–488. https://doi.org/10.1007/s10340-015-0693-8
Johnstone K, McArthur C, Banks PB (2021a) Catch me if you can: personality drives technique-specific biases during live-capture trap**. Wildl Res 48:713–721
Johnstone KC, McArthur C, Banks PB (2021b) Behavioural drivers of survey bias: interactive effects of personality, the perceived risk and device properties. Oecologia 197:117–127
Kannemeyer RL (2017) A systematic literature review of attitudes to pest control methods in New Zealand. Manaaki Whenua Landcare Research, Linconln, NZ
Kay B, Gifford E, Perry R, van de Ven R (2000) Trap** efficiency for foxes (Vulpes vulpes) in central New SouthWales: age and sex biases and the effects of reduced fox abundance. Wildl Res 27:547–552
King CM, Davis SA, Purdey D, Lawrence B (2003) Capture probability and heterogeneity of trap response in stoats (Mustela erminea). Wildl Res 30:611–619
Linhart P, Fuchs R, Poláková S, Slabbekoorn H (2012) Once bitten twice shy: long-term behavioural changes caused by trap** experience in willow warblers Phylloscopus trochilus. J Avian Biol 43:186–192. https://doi.org/10.1111/j.1600-048X.2012.05580.x
Mella VSA et al (2016) Effective field-based methods to quantify personality in brushtail possums (Trichosurus vulpecula). Wildl Res 43:332–340. https://doi.org/10.1071/wr15216
Morgan D, Innes J, Frampton C, Woolhouse A (1995) Responses of captive and wild possums to lures used in poison baiting. N Z J Zool 22:123–129
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956
Nichols M, Nathan H, Mulgan N (2021) Dual aerial 1080 baiting operation removes predators at a large spatial scale. N Z J Ecol 45:1–10
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Russell JC, Broome KG (2016) Fifty years of rodent eradications in New Zealand: another decade of advances. N Z J Ecol 40:197–204
Russell JC, Innes JG, Brown PH, Byrom AE (2015) Predator-free New Zealand: conservation country. Bioscience 65:520–525
Schirmer A, Herde A, Eccard JA, Dammhahn M (2019) Individuals in space: personality-dependent space use, movement and microhabitat use facilitate individual spatial niche specialization. Oecologia 189:1–14
Schmidt CO, Kohlmann T (2008) When to use the odds ratio or the relative risk? Int J Public Health 53:165
Sweetapple P, Nugent G (2011) Chew-track-cards: a multiple-species small mammal detection device. N Z J Ecol 35:153–162
Tuyttens F et al (1999) Differences in trappability of European badgers Meles meles in three populations in England. J Appl Ecol 36:1051–1062
Van Vuren D, Smallwood K (1996) Ecological management of vertebrate pests in agricultural systems. Biol Agric Hortic 13:39–62
Vattiato G, Plank MJ, James A, Binny RN (2021) Individual heterogeneity affects the outcome of small mammal pest eradication. Thyroid Res 14:219–231
Warburton B et al (2021) Alternatives for mammal pest control in New Zealand in the context of concerns about 1080 toxicant (sodium fluoroacetate). N Z J Zool 49:1–43
Warburton B, Morriss G, Howard S (2022) Increasing the capture rates of brushtail possums in Victor# 1 leg-hold traps. N Z J Zool 49:122–128
Wat KK, Herath AP, Rus AI, Banks PB, Mcarthur C (2020) Space use by animals on the urban fringe: interactive effects of sex and personality. Behav Ecol 31:330–339
Wilson ADM, Binder TR, McGrath KP, Cooke SJ, Godin J-GJ, Kraft C (2011) Capture technique and fish personality: angling targets timid bluegill sunfish, Lepomis macrochirus. Can J Fish Aquat Sci 68:749–757. https://doi.org/10.1139/f2011-019
Acknowledgements
We thank the landowners, in particular Warren Thompson, and the Lottery Bush Conservation Group, for site access and assistance with the kill-trap** programme. Bruce Warburton, Grant Morriss, Morgan Coleman, Luke Sutton, and Roland Mapp assisted in the field. Staff at the MWLR Animal Facility provided husbandry for the captive possums. Jo Peace reviewed trail camera images. We also thank the reviewers for their time and feedback on this manuscript. This study was supported by the Ministry of Business, Innovation and Environment Endeavour Fund Research Programme C09X1904.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Field data were analysed by Graham Hickling. Captive behavioural data collection and analysis were performed by Kyla Johnstone. The first draft of the manuscript was written by Kyla Johnstone and Graham Hickling, and all authors commented on later versions of the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This research was approved by the Manaaki Whenua – Landcare Research Animal Ethics Committee (AEC approval number 20/02/01). Approved applications comply with the Animal Welfare Laws 1999, Animal Code of Welfare 2012, and all additional New Zealand Legislation.
Conflicts of interest
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Johnstone, K.C., Garvey, P. & Hickling, G.J. Invasive mammal control selects for trap-recalcitrant behaviour and personality. Biol Invasions 26, 549–564 (2024). https://doi.org/10.1007/s10530-023-03191-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03191-4