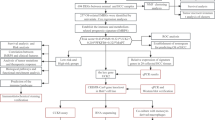
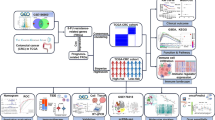
Abstract
Hepatocellular carcinoma (HCC) is a challenging disease to evaluate in terms of prognosis, requiring close attention to the prognosis of HCC patients. Exosomes have been shown to play an important role in HCC development and have significant potential in managing HCC patient prognosis, as they are detectable in patients’ blood. By using small extracellular vesicular RNA, liquid biopsies can reflect the underlying physiological and pathological status of the originating cells, providing a valuable assessment of human health. No study has explored the diagnostic value of mRNA expression changes in exosomes for liver cancer. The present study investigated establishing a risk prognosis model based on mRNA expression levels in exosomes from blood samples of liver cancer patients and evaluated its diagnostic and prognostic value, providing new targets for liver cancer detection. We obtained mRNA data from HCC patients and normal controls from the TCGA and exoRBase 2.0 databases and established a risk prognostic assessment model using exosomes-related risk genes selected through prognostic analysis and Lasso Cox analysis. The patients were divided into high-risk and low-risk groups based on median risk score values to validate the independence and evaluability of the risk score. The clinical value of the model was further analyzed using a nomograph model, and the efficacy of immunotherapy and cell-origin types of prognostic risk genes were further assessed in the high- and low-risk groups by immune checkpoint and single-cell sequencing. A total of 44 genes were found to be significantly associated with the prognosis of HCC patients. From this group, we selected six genes (CLEC3B, CYP2C9, GNA14, NQO1, NT5DC2, and S100A9) as exosomal risk genes and used them as a basis for the risk prognosis model. The clinical information of HCC patients from the TCGA and ICGC databases demonstrated that the risk prognostic score of the model established in this study was an independent prognostic factor with good robustness. When pathological stage and risk prognostic score were incorporated into the model to predict clinical outcomes, the nomograph model had the best clinical benefit. Furthermore, immune checkpoint assays and single-cell sequencing analysis suggested that exosomal risk genes were derived from different cell types and that immunotherapy in the high-risk groups could be beneficial. Our study demonstrated that the prognostic scoring model based on exosomal mRNA was highly effective. The six genes selected using the scoring model have been previously reported to be associated with the occurrence and development of liver cancer. However, this study is the first to confirm that these related genes existed in the blood exosomes, which could be used for liquid biopsy of patients with liver cancer, thereby avoiding the need for puncture diagnosis. This approach has a high value in clinical application. Through single-cell sequencing, we found that the six genes in the risk model originate from multiple cell types. This finding suggests that the exosomal characteristic molecules secreted by different types of cells in the microenvironment of liver cancer may serve as diagnostic markers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Hepatocellular carcinoma (HCC) is a multifaceted pathological process caused by various factors, including hepatitis B virus, hepatitis C virus, metabolic disorders, and alcoholism. Unfortunately, most HCC patients are initially diagnosed with advanced HCC, making it one of the deadliest cancers worldwide (Bray et al. 2018). Although there are various treatments for liver cancer, such as surgery, radiotherapy, and chemotherapy, the survival time of most liver cancer patients is still short, and prognostic testing for the disease has been emphasized (Forner et al. 2018). A hepatologic biopsy can effectively analyze the occurrence and progression of HCC and provide a diagnostic basis for the prognostic evaluation of patients (Wang et al. 2019). However, this procedure is not a routine practice because of the invasiveness of HCC biopsy, and the evaluation of HCC patient prognosis through non-invasive liquid biopsy could play a critical role in clinical management (Ahn et al. 2021).
In recent years, various liquid biopsy techniques have shown significant promise as prognostic tools for HCC. The detection of circulating tumor cells, cell-free DNA, and exosomes from blood samples solve the lack of key molecular targets for HCC patient prognostic testing (Chen et al. 2020b). Exosomes are 50–200-nm lipid vesicles containing nucleic acids, proteins, small metabolic molecules, and many other components. Studies have demonstrated that exosomes can enter cells through receptor-ligand interactions or through various mechanisms such as phagocytosis, endocytosis, micropinocytosis, and fusion, to activate signaling pathways among liver cells, different types of immune cells such as macrophages, natural killer cells, and stroma, and various stromal cells such as stellate cells and adipocytes (Shen et al. 2017; Liu and Li 2018; Zhou et al. 2018; Zhang et al. 2019, p. 7). Researchers have found that miR-21 levels in exosomes of HCC patients were significantly higher than in patients with chronic hepatitis and healthy controls and that exosomes were significantly more sensitive to detection based on patient serum, suggesting that specific molecules in exosomes could be a promising new marker for HCC (Wang et al. 2014). Furthermore, exosomal alterations reflect the prognosis and treatment of HCC. High expression of miRNA-21 and lncRNA-ATB in the exosomes suggests poor overall survival for HCC patients, indicating that different molecules in the exosomes of HCC patients reflect different pathophysiologic processes of the disease (Lee et al. 2019). Additionally, miR-122 expression was significantly reduced in the exosomes of patients treated with transarterial chemoembolization, further supporting the potential of exosomes as diagnostic markers for HCC (Suehiro et al. 2018).
Currently, most molecules targeted by exosomes for diagnosing HCC patients are small RNAs, such as miRNAs (Li et al. 2019). However, no studies have been reported to assess patient prognosis based on changes in mRNA expression levels in HCC exosomes, much less relevant risk prognostic models. However, the diagnostic value of mRNA in exosomes has not received sufficient attention. Liquid biopsy techniques based on small extracellular vesicular RNA are gradually entering clinical applications. Exosome Diagnostics is one company that developed ExoDx for blood testing based on EML4-ALK fusion transcripts and ExoDxTM Prostate (IntelliScore) for multiple gene expression features (ERG, PCA3, and SPDEF) in small extracellular vesicles. This study proposed three scientific questions regarding the detection of mRNA in the secretions of liver cancer patients for prognosis. First, are there any mRNA molecules whose differential expression characteristics are shared by liver cancer tissue and peripheral blood secretions of liver cancer patients? Second, can relevant mRNA molecules be used to evaluate the prognosis of liver cancer patients? Finally, which cell types of liver cancer tissue are the source of relevant mRNA molecules? These questions must be answered to determine the diagnostic value of exosomal mRNA in liver cancer patients.
We aimed to fill this gap by screening key mRNAs in the exosomes of HCC patients to establish a risk prognostic model for HCC patients. The risk prognostic risk score obtained through the model as an independent risk factor can more accurately assess the 1-, 3-, and 5-year survival rates of patients and has a high application value.
Materials and Methods
Data Collection
Exosome transcriptome data from healthy human blood and liver cancer patients were obtained from the exoRBase 2.0 database (http://www.exorbase.org/exoRBaseV2/toIndex). mRNA expression data and copy number variation data from liver cancer (https://xena.ucsc.edu/) were obtained from the TCGA database via the ICGC website ( https://dcc.icgc.org/) downloaded transcriptome data of liver cancer. Data from 374 tumor samples and 50 control samples were downloaded from the TCGA database, whereas data from 112 tumor samples and 118 control samples were downloaded from exoRBase 2.0. mRNA expression values were standardized and converted to log2 (TPM + 1), with copy number amplification and deletion thresholds determined using the GISTIC software.
Differentially Expressed Genes and Exosomes Prognostic Genes (Exo-Genes) Screening
HCC and control data from the TCGA and exoRBase 2.0 databases were compared using the limma package. |LogFC|> 0.585 and Calibration P < 0.05 were defined as differentially expressed genes (DEGs). Finally, 123 overlap** exosomes-related differential genes were filtered by intersecting the differential genes from the two databases. To further explore the clinical guidance value of the differential genes, we matched the exosomes-related differential genes from the TCGA database with clinical data and then used the survival package to evaluate the prognostic difference through univariate Cox regression analysis followed by the log-rank test, and the exosomes-related differential genes with a P < 0.05 were defined as exosomes prognostic genes (Exo-genes). The ability of related genes to influence patient prognosis was validated by plotting Kaplan Meier survival curves of exosomes prognostic genes.
Establishment and Validation of Risk Prognosis Model
The gene expression data of Exo-genes were combined with clinical data, and prognostic models were constructed using the glmnet package with Lasso-Penalized Cox regression analysis to exclude genes predisposed for fitting. A risk prognostic model was constructed in the TCGA database of HCC patients with LASSO regression analysis, and the obtained coefficients and gene expression values were used to derive a risk prognostic score, formulated as follows: Risk score = sum (Expgene × coef). Median liver cancer expression data from the TCGA database were categorized into high- and low-risk groups based on risk prognostic score. The prognostic model predictive capacity was assessed using Kaplan–Meier survival curves and receiver operating diagnostic (ROC) curve analysis. The model was repeated in liver cancer data (LIRI-JP) downloaded using ICGC to test the accuracy of the predictive ability of risk scores. To further investigate the robustness of risk prediction models to different clinical factors, we applied risk prognostic scores to different subgroups of clinical factors and mapped Kaplan–Meier survival curves for validation. Single and multivariable Cox regression analyses of risk prognostic scores and clinical variables in TCGA-LIHC and LIRI-JP revealed that risk prognostic scores were independent risk factors in both analyses (P < 0.05).
Establishment of a Nomograph Model
The Rms package was used to develop a prognostic column model to provide more accurate prognostic predictions for clinical patients based on risk scores and clinical characteristics. Identification accuracy was estimated using the consistency index (c index), the area under the ROC curve (AUC), and calibration diagrams. The estimates c Index and AUC > 0.7 are reasonable. Decision curve analysis (DCA) was then used to evaluate the clinical utility of the nomogram model.
Immune Infiltration Analysis
HCC expression data from the TCGA database were categorized into high- and low-risk groups based on median risk prognostic score. We calculated immune checkpoint expression in both groups and presented them with a point matrix. The ratio of 22 immune cell types per sample was calculated by cell-type identification with estimator relative subsets of RNA transcripts (CIBERSORT).
Single-Cell Sequencing Analysis
GSE125449 was downloaded for analysis. Sequencing data were qualitatively controlled using Seurat packets, and the cells were clustered to analyze the expression of exosomal risk genes in different clusters. Different cluster cell types were analyzed using singleR packets for annotation.
Statistical Analysis
The difference between the two groups was compared using the unpaired t-test. Single and multifactorial Cox regression analyses were used to analyze the relationship between the variables and the patient outcome. In all analyses, P < 0.05 was considered statistically significant.
Results
Screening of Exosome-Related DEGs in HCC
We compared the differences in exosomes-related genes between HCC patients and normal controls in the exoRBase 2.0 database and found 393 differentially expressed genes (Fig. 1A). DONSON, XIST, AC018607.1, MALAT1, GOLGA8A, AC005224.3, AC003684.1, VSTM2A, LINC02280, and CDHR2 were under-expressed in exosomes from liver cancer patients, and RPS4Y1, HIST2H2AA3, APOC3, ORM1, APOA2, APOH, HRG, FGB, FGG, FGA were highly expressed in the exosomes of HCC patients (Fig. 1B). We also compared genetic differences between HCC patients and normal controls in the TCGA database and found 4,507 differentially expressed genes (Fig. 1C), with thermal map** revealing the top 20 most differentially expressed genes (Fig. 1D). A cross-section analysis of differentially expressed genes from the two databases resulted in 123 exosomal-associated differential genes in HCC (Fig. 1E).
Screening of 123 HCC exosomes-related differential genes. A Volcano plot of differential gene expression in hepatocellular carcinoma in the exoRBase 2.0 database. B Top 20 differentially expressed hepatocellular carcinoma heat maps in exoRBase 2.0 database. C Volcano plot of differential gene expression in hepatocellular carcinoma in TCGA database. D Top 20 differentially expressed hepatocellular carcinoma heat maps in TCGA database. E Intersection of differentially expressed genes in liver cancer between exoRBase 2.0 database and TCGA database
Identification of Exosomes Prognostic Genes (Exo-Genes)
To further define the relationship between HCC exosomes-related differential genes and patient prognosis, we performed a univariate regression analysis of 123 HCC exosomes-related differential genes and identified 44 genes as Exo-genes. Of these, 14 genes were risk factors and 30 were protective (Fig. 2A). Based on the median expression of these prognostic genes, we divided the data into high- and low-expression groups, mapped Kaplan–Meier survival curves, and selected the top nine genes with the lowest P values for presentation (Fig. 2B). We confirmed that the patients with high expression of DSCC1, CXCL8, S100A9, NT5DC2, E2F1, PLK4, and TYMS had lower overall survival rates. The patients with high expression of CLE3B and CYP2C9 exhibited higher overall survival rates.
Identification of 44 exosomes prognostic genes (Exo-genes) in hepatocellular carcinoma. A Identification of exosomes prognostic genes by univariable Cox regression analysis. B Prognostic curves of high and low expression of exosomes prognostic genes from hepatocellular carcinoma patients in TCGA database
Establishment of Risk Prognostic Model Based on Exosomal Risk Genes
To further evaluate the prognostic power of exosomal prognostic genes in HCC patients, we performed the Lasso Cox regression analysis based on TCGA HCC patient prognostic data. Figure 3A demonstrates regression coefficient changes in each exosomal prognostic gene, with the model having the lowest mean variance when lambda is 0.0678. Consequently, the following six genes (exosomal risk genes) were selected to construct the risk prognostic model (Fig. 3B): CLEC3B, CYP2C9, GNA14, NQO1, NT5DC2, and S100A9. Based on the expression and regression coefficient (Fig. 3C) of these six genes, we constructed a risk prognosis score based on the following formula:
Risk prognosis model based on exosomal risk genes has good diagnostic value. A Changes in regression coefficient of exosomes prognostic genes in Lasso Cox regression analysis. B Determination of λ. C Regression coefficient of CLEC3B, CYP2C9, GNA14, NQO1, NT5DC2 and S100A9. D HCC patients with high-risk prognostic score in TCGA database have lower survival. E Survival curves of high-risk and low-risk prognostic score groups in TCGA database of HCC patients. F ROC validates prognostic performance of HCC risk score in TCGA database. G HCC patients with high-risk prognostic score in ICGC database have lower survival. H Survival curves of high-risk and low-risk prognostic groups of liver cancer patients in ICGC database. I ROC validates prognostic performance of HCC risk score in ICGC database
To explore the prognostic power of risk score for patient outcomes, we categorized HCC patients in the TCGA database (TCGA-LIHC) into high- and low-risk groups (Fig. 3D) based on median risk scores and found that overall survival was significantly lower in the high-risk group than in the low-risk group (Fig. 3E). Using AUC, we found that risk prognostic scores were accurately predictive in 1-, 3-, and 5-year survival assessments (1-year AUC = 0.7532, 3-year AUC = 0.7474, and 5-year AUC = 0.7135) (Fig. 3F). The same study was performed in validation data from the ICGC database (LIRI-JP), and similar results were obtained from TCGA-LIHC, suggesting that this risk model has broad applicability (Figs. 3G–I).
Risk Prognostic Score is an Independent Prognostic Factor with Good Robustness
To explore the independent prognostic value of risk score, we performed univariable and multivariable Cox regression analyses with age, sex, stage, grade, and risk score of TCGA-LIHC data, indicating that risk score was an independent prognostic factor for patients in the TCGA-LIHC data (HR = 4.544, P < 0.001) (Fig. 4A). We also observed risk score as an independent prognostic factor for patients in the LIRI-JP data (HR = 4.860, P < 0.05) (Fig. 4B).
Risk prognostic score is an independent prognostic factor with good robustness. A Univariable cox regression analysis and multivariable cox regression analysis of survival of HCC patients in the TCGA database. B Univariable cox regression and multivariable cox regression analysis of HCC in ICGC Database. C HCC patients in the TCGA database were subgroups based on clinical characteristics and Kaplan–Meier demonstrated good predictive ability of risk prognosis scores
We evaluated the robustness of TCGA-LIHC based on identifying risk score as an independent predictor. By classifying the clinical characteristics (age, sex, stage, and grade) of TCGA-LIHC, we evaluated the outcomes of patients with different risk scores in different subgroups, suggesting that patients with higher risk scores had worse outcomes than those with lower risk scores, suggesting a more robust risk model (Fig. 4C).
Evaluation of Nomogram Model for Risk Prognosis Score and Detection of Immune Checkpoint
We constructed a nomogram survival prediction model based on the pathological stages and risk prognostic scores of HCC patients to predict 1-, 3-, and 5-year survival probabilities (Fig. 5A) of TCGA-LIHC. The ROC and calibration curves revealed that the model had an accurate predictive ability for 1-, 3-, and 5-year survival probabilities of patients (Fig. 5B). In addition, the decision curve analysis revealed that the model exhibited the best clinical benefit (Fig. 5C) when incorporating pathologic stage and risk prognosis score to predict clinical outcomes.
A nomogram model constructed based on pathological stage and risk prognostic score was able to effectively predict the survival of liver cancer patients in TCGA database. A Nomogram model based on HCC patients in TCGA database. B Time dependent ROC curve and calibration curve based on Nomogram model of liver cancer patients in TCGA database. C DCA curve of liver cancer patients based on TCGA database
We compared immune cell types between the high- and low-risk groups with risk prognostic scores and found differences in the distribution of multiple immune cells (Fig. 6A). To further assess whether risk prognostic scores can guide immunotherapy in patients, we examined the expression of immune checkpoints in the high- and low-risk groups. We found that the expression of CTLA4, PDL1, CD27, ID01, PD1, HAVCR2, LAG3, and TIGIT was significantly higher in the high-risk group than in the low-risk group, suggesting that immunotherapy may benefit patients in the high-risk group (Fig. 6B).
Distribution of Exosomal Risk Genes in Hepatocellular Carcinoma
Different cell types in the tumor microenvironment (e.g., fibroblasts, immune cells, endothelial cells, and hepatocytes) have broad implications for tumor progression and play a key role in regulating the bioactivity of HCC. To further understand the cell types expressed in HCC tissues by six exosomal risk genes (CLEC3B, CYP2C9, GNA14, NQO1, NT5DC2, and S100A9) that build risk prognostic models, we performed correlation analysis of single-cell sequencing dataset GSE125449. Through down-dimensional analysis, we identified 18 cell types in this single-cell sequencing dataset (Fig. 7A), with low expression of CLEC3B and GNA14 in various cell types, high expression of CYP2C9 in hepatocytes, high expression of NQO1 in epithelial and hepatocytes, high expression of NT5DC2 in epithelial and endothelial cells, and high expression of S100A9 in multiple cell types, suggesting a role in HCC prognosis (Fig. 7B).
Discussion
The tumor microenvironment plays a crucial role in tumor progression, and the interaction between different cells constitutes a significant component of the tumor microenvironment. Apart from direct cell-to-cell interactions, communication between cells can occur through the secretion of exosomes and other signaling molecules. Such communication within the tumor microenvironment can have a significant effect on tumor growth and metastasis (Wu et al. 2019). Exosomes—known to mediate the transfer of various molecules—play a pivotal role in the initiation, progression, invasion, and metastasis of hepatocellular carcinoma (HCC). Consequently, exosomes represent promising targets for develo** diagnostic, therapeutic, and prognostic approaches for HCC. Understanding the mechanisms underlying exosomes-mediated communication within the tumor microenvironment may lead to develo** novel strategies for effectively managing HCC (Abudoureyimu et al. 2019).
The evaluation of risk score models for tumor prognosis is focused on specific pathophysiological processes or signaling pathways involved in tumor progression, such as autophagy, aging, and iron death. However, the practical application of these models in clinical settings is limited by the difficulty of obtaining pathologic tissue samples and the limitations of relevant risk scores (Liang et al. 2020; Qiu et al. 2020; Chen et al. In this innovative comparative research, we identified 44 exosomal prognostic genes from the genomes of Chinese and foreign HCC patients and normal controls. We performed functional enrichment and copy number analysis on these genes and selected six genes—CLEC3B, CYP2C9, GNA14, NQO1, NT5DC2, and S100A9—as exosomal risk genes through Lasso Cox regression analysis. These genes were used to establish risk prognosis models validated in TCGA and ICGC databases. Our study used Cox regression analysis, robustness analysis, column model evaluation, immune checkpoint testing, and single-cell sequencing to confirm the utility of the risk prognostic score.Conclusion
References
Abudoureyimu M, Zhou H, Zhi Y et al (2019) Recent progress in the emerging role of exosome in hepatocellular carcinoma. Cell Prolif 52:e12541. https://doi.org/10.1111/cpr.12541
Ahn JC, Teng P-C, Chen P-J et al (2021) Detection of circulating tumor cells and their implications as a biomarker for diagnosis, prognostication, and therapeutic monitoring in hepatocellular carcinoma. Hepatology 73:422–436. https://doi.org/10.1002/hep.31165
Bodin L, Verstuyft C, Tregouet D-A et al (2005) Cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase (VKORC1) genotypes as determinants of acenocoumarol sensitivity. Blood 106:135–140. https://doi.org/10.1182/blood-2005-01-0341
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Chen J, Cao J, Wang P, He X (2020a) NT5DC2 is a novel prognostic marker in human hepatocellular carcinoma. Oncol Lett 20:70. https://doi.org/10.3892/ol.2020.11931
Chen VL, Xu D, Wicha MS et al (2020b) Utility of liquid biopsy analysis in detection of hepatocellular carcinoma, determination of prognosis, and disease monitoring: a systematic review. Clin Gastroenterol Hepatol 18:2879-2902.e9. https://doi.org/10.1016/j.cgh.2020.04.019
Chen F, Gong X, **a M et al (2022) The aging-related prognostic signature reveals the landscape of the tumor immune microenvironment in head and neck squamous cell carcinoma. Front Oncol 12:857994. https://doi.org/10.3389/fonc.2022.857994
Dai W, Wang Y, Yang T et al (2019) Downregulation of exosomal CLEC3B in hepatocellular carcinoma promotes metastasis and angiogenesis via AMPK and VEGF signals. Cell Commun Signal 17:113. https://doi.org/10.1186/s12964-019-0423-6
Duan L, Wu R, Zhang X et al (2018) HBx-induced S100A9 in NF-κB dependent manner promotes growth and metastasis of hepatocellular carcinoma cells. Cell Death Dis 9:629. https://doi.org/10.1038/s41419-018-0512-2
Forner A, Reig M, Bruix J (2018) Hepatocellular carcinoma. Lancet 391:1301–1314. https://doi.org/10.1016/S0140-6736(18)30010-2
Halvaei S, Daryani S, Eslami-S Z et al (2018) Exosomes in cancer liquid biopsy: a focus on breast cancer. Mol Ther Nucleic Acids 10:131–141. https://doi.org/10.1016/j.omtn.2017.11.014
Huo J, Wu L, Zang Y (2021) Development and validation of a metabolic-related prognostic model for hepatocellular carcinoma. J Clin Transl Hepatol 9:169–179
Lee YR, Kim G, Tak WY et al (2019) Circulating exosomal noncoding RNAs as prognostic biomarkers in human hepatocellular carcinoma. Int J Cancer 144:1444–1452. https://doi.org/10.1002/ijc.31931
Li L, Liu Z, Cheng Q (2019) Exosome plays an important role in the development of hepatocellular carcinoma. Pathology 215:152468. https://doi.org/10.1016/j.prp.2019.152468
Li K-S, Zhu X-D, Liu H-D et al (2020) NT5DC2 promotes tumor cell proliferation by stabilizing EGFR in hepatocellular carcinoma. Cell Death Dis 11:335. https://doi.org/10.1038/s41419-020-2549-2
Li R, Liu R, Zheng S et al (2022) Comprehensive analysis of prognostic value and immune infiltration of the NT5DC family in hepatocellular carcinoma. J Oncol 2022:2607878. https://doi.org/10.1155/2022/2607878
Liang J-Y, Wang D-S, Lin H-C et al (2020) A novel ferroptosis-related gene signature for overall survival prediction in patients with hepatocellular carcinoma. Int J Biol Sci 16:2430–2441. https://doi.org/10.7150/ijbs.45050
Lin L, Sun J, Tan Y et al (2017) Prognostic implication of NQO1 overexpression in hepatocellular carcinoma. Hum Pathol 69:31–37. https://doi.org/10.1016/j.humpath.2017.09.002
Liu H, Li B (2018) The functional role of exosome in hepatocellular carcinoma. J Cancer Res Clin Oncol 144:2085–2095. https://doi.org/10.1007/s00432-018-2712-7
Meng J, Gu F, Fang H, Qu B (2019) Elevated serum S100A9 indicated poor prognosis in hepatocellular carcinoma after curative resection. J Cancer 10:408–415. https://doi.org/10.7150/jca.28409
Qiu J, Sun M, Wang Y, Chen B (2020) Identification and validation of an individualized autophagy-clinical prognostic index in gastric cancer patients. Cancer Cell Int 20:178. https://doi.org/10.1186/s12935-020-01267-y
Qiu P, Guo Q, Yao Q et al (2021) Characterization of exosome-related gene risk model to evaluate the tumor immune microenvironment and predict prognosis in triple-negative breast cancer. Front Immunol 12:736030. https://doi.org/10.3389/fimmu.2021.736030
Shen J, Huang C-K, Yu H et al (2017) The role of exosomes in hepatitis, liver cirrhosis and hepatocellular carcinoma. J Cell Mol Med 21:986–992. https://doi.org/10.1111/jcmm.12950
Song G, Zhu X, Xuan Z et al (2021) Hypermethylation of GNA14 and its tumor-suppressive role in hepatitis B virus-related hepatocellular carcinoma. Theranostics 11:2318–2333. https://doi.org/10.7150/thno.48739
Suehiro T, Miyaaki H, Kanda Y et al (2018) Serum exosomal microRNA-122 and microRNA-21 as predictive biomarkers in transarterial chemoembolization-treated hepatocellular carcinoma patients. Oncol Lett 16:3267–3273. https://doi.org/10.3892/ol.2018.8991
Wang H, Hou L, Li A et al (2014) Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed Res Int 2014:864894. https://doi.org/10.1155/2014/864894
Wang H, Lu Z, Zhao X (2019) Tumorigenesis, diagnosis, and therapeutic potential of exosomes in liver cancer. J Hematol Oncol 12:133. https://doi.org/10.1186/s13045-019-0806-6
Wang X, Liu Y, Han A et al (2022) The NQO1/p53/SREBP1 axis promotes hepatocellular carcinoma progression and metastasis by regulating Snail stability. Oncogene 41:5107–5120. https://doi.org/10.1038/s41388-022-02477-6
Wang X, Yu T, Liao X et al (2018) The prognostic value of CYP2C subfamily genes in hepatocellular carcinoma. Cancer Med 7:966–980. https://doi.org/10.1002/cam4.1299
Wu R, Duan L, Cui F et al (2015) S100A9 promotes human hepatocellular carcinoma cell growth and invasion through RAGE-mediated ERK1/2 and p38 MAPK pathways. Exp Cell Res 334:228–238. https://doi.org/10.1016/j.yexcr.2015.04.008
Wu Q, Zhou L, Lv D et al (2019) Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J Hematol Oncol 12:53. https://doi.org/10.1186/s13045-019-0739-0
**e X-W, Jiang S-S, Li X (2020) CLEC3B as a potential prognostic biomarker in hepatocellular carcinoma. Front Mol Biosci 7:614034. https://doi.org/10.3389/fmolb.2020.614034
Xu C, Li Y-M, Sun B et al (2021) GNA14’s interaction with RACK1 inhibits hepatocellular carcinoma progression through reducing MAPK/JNK and PI3K/AKT signaling pathway. Carcinogenesis 42:1357–1369. https://doi.org/10.1093/carcin/bgab098
Xu K, **a P, Liu P, Zhang X (2022) A six lipid metabolism related gene signature for predicting the prognosis of hepatocellular carcinoma. Sci Rep 12:20781. https://doi.org/10.1038/s41598-022-25356-2
Yang Y, Zheng J, Wang M et al (2021) NQO1 promotes an aggressive phenotype in hepatocellular carcinoma via amplifying ERK-NRF2 signaling. Cancer Sci 112:641–654. https://doi.org/10.1111/cas.14744
Ye J, Lin Y, Gao X et al (2022) Prognosis-related molecular subtypes and immune features associated with hepatocellular carcinoma. Cancers (Basel) 14:5721. https://doi.org/10.3390/cancers14225721
Yu J, Shi X, Yang C et al (2020a) A novel germline gain-of-function HIF2A mutation in hepatocellular carcinoma with polycythemia. Aging (Albany NY) 12:5781–5791
Yu T, Lu S, **e W (2020b) Downregulation of GNA14 in hepatocellular carcinoma indicates an unfavorable prognosis. Oncol Lett 20:165–172. https://doi.org/10.3892/ol.2020.11538
Zhang H, Deng T, Ge S et al (2019) Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related USP7. Oncogene 38:2844–2859. https://doi.org/10.1038/s41388-018-0619-z
Zhao W, Jiang L, Fang T et al (2021) β-Lapachone selectively kills hepatocellular carcinoma cells by targeting NQO1 to induce extensive DNA damage and PARP1 hyperactivation. Front Oncol 11:747282. https://doi.org/10.3389/fonc.2021.747282
Zhou Y, Ren H, Dai B et al (2018) Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J Exp Clin Cancer Res 37:324. https://doi.org/10.1186/s13046-018-0965-2
Acknowledgements
We thank Home for Researchers editorial team (www.home-for researchers.com) for language editing service.
Funding
We gratefully acknowledge the financial support by The National High Technology Research and Development Program of China (2018YFA0108301), Shanghai Key Laboratory of Cell Engineering (14DZ2272300), Shanghai Sailing Program (21YF1457100), National Natural Science Foundation of China (31971109), Peak Disciplines (Type IV) of Institutions of Higher Learning in Shanghai.
Author information
Authors and Affiliations
Contributions
TW conceived of the study. LZ and YL participated in its design and performed the statistical analysis. QX and LW drafted the manuscript. GC and WY participated in conceiving the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, L., Lou, Y., **ao, Q. et al. Establishment and Evaluation of Exosomes-Related Gene Risk Model in Hepatocellular Carcinoma. Biochem Genet 62, 698–717 (2024). https://doi.org/10.1007/s10528-023-10441-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-023-10441-6