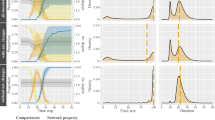
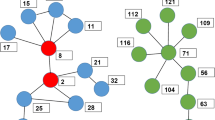
Abstract
This article explores how social network dynamics may have reduced the spread of HIV-1 infection among people who inject drugs during the early years of the epidemic. Stochastic, discrete event, agent-based simulations are used to test whether a “firewall effect” can arise out of self-organizing processes at the actor level, and whether such an effect can account for stable HIV prevalence rates below population saturation. Repeated simulation experiments show that, in the presence of recurring, acute, and highly infectious outbreaks, micro-network structures combine with the HIV virus’s natural history to reduce the spread of the disease. These results indicate that network factors likely played a significant role in the prevention of HIV infection within injection risk networks during periods of peak prevalence. They also suggest that social forces that disturb network connections may diminish the natural firewall effect and result in higher rates of HIV.
Resumen
Este artículo explora cómo las dinámicas de redes sociales pueden haber reducido la propagación de la infección por VIH-1 entre las personas que se inyectan drogas durante los primeros años de la epidemia. Estocásticas, eventos discretos, las simulaciones basadas en agentes se utilizan para probar la de si un “efecto cortafuegos” puede surgir de los procesos de auto-organización, al nivel de actor, y si este efecto puede dar cuenta de las tasas estables de la prevalencia del VIH por debajo de la saturación de la población. Repetidos experimentos de simulación muestran que, en la presencia de brotes recurrentes, agudos, y altamente infecciosos, las estructuras micro-red se combinan con la historia natural del virus del VIH para reducir la propagación de la enfermedad. Estos resultados indican que los factores de la red probablemente jugaron un papel importante en la prevención de la infección por el VIH dentro de las redes de riesgo de inyección durante los períodos de pico de prevalencia. Además, sugieren que las fuerzas sociales que perturban las conexiones de red pueden disminuir el efecto cortafuegos natural y resultan en tasas más altas de VIH.




Similar content being viewed by others
References
Huang C-Y, Tsai Y-S, Wen T-H. Simulations for epidemiology and public health education. J Simul. 2010;4(1):68–80.
Marshall BD, Galea S. Marshall and Galea respond to “data theory in epidemiology”. Am J Epidemiol. 2015;181(2):106–7.
Goodreau SM. Assessing the effects of human mixing patterns on human immunodeficiency virus-1 interhost phylogenetics through social network simulation. Genetics. 2006;172(4):2033–45.
Hsieh J-L, Huang C-Y, Sun C-T, Tsai Y-S. Learning to build network-oriented epidemic simulation models in epidemiology education. Int J Simul Process Model. 2009;5(1):31–41.
Sloot PMA, Ivanov SV, Boukhanovsky AV, van de Vijver DAMC, Boucher CAB. Stochastic simulation of HIV population dynamics through complex network modelling. Int J Comput Math. 2008;85(8):1175–87.
Bauch CT, Galvani AP. Social factors in epidemiology. Science. 2013;342(6154):47–9.
Des Jarlais DC, Friedman SR, Novick DM, Sotheran JL, Thomas P, Yancovitz SR, et al. HIV-1 infection among intravenous drug users in Manhattan, New York City, from 1977 through 1987. JAMA. 1989;261(7):1008–12.
Brown AJL, Lycett SJ, Weinert L, Hughes GJ, Fearnhill E, Dunn DT. Transmission network parameters estimated from HIV sequences for a nationwide epidemic. J Infect Dis. 2011;204(9):1463–9.
Dombrowski K, Curtis R, Friedman S, Khan B. Topological and historical considerations for infectious disease transmission among injecting drug users in Bushwick, Brooklyn (USA). World J AIDS. 2013;3(1):1–9.
Vieira, et al. Small world network models of the dynamics of HIV infection. Ann Oper Res. 2010;178(1):173–200.
Dombrowski K, Khan B, McLean K, Curtis R, Wendel T, Misshula E, et al. A reexamination of connectivity trends via exponential random graph modeling in two IDU risk networks. Subst Use Misuse. 2013;2(48):1485–97.
Friedman SR, Neaigus A, Jose B, Curtis R, Goldstein M, Ildefonso G, et al. Sociometric risk networks and risk for HIV infection. Am J Public Health. 1997;87(8):1289–96.
Des Jarlais DC, Perlis T, Arasteh K, Torian LV, Beatrice S, Milliken J, et al. HIV incidence among injection drug users in New York City, 1990 to 2002: use of serologic test algorithm to assess expansion of HIV prevention services. Am J Public Health. 2005;95(8):1439–44.
Des Jarlais DC, Perlis T, Friedman SR, Deren S, Chapman T, Sotheran JL, et al. Declining seroprevalence in a very large HIV epidemic: injecting drug users in New York City, 1991 to 1996. Am J Public Health. 1998;88(12):1801–6.
Friedman SR, Des Jarlais DC. HIV among drug injectors: the epidemic and the response. AIDS Care. 1991;3(3):239–50.
Des Jarlais DC, Perlis T, Arasteh K, Torian LV, Hagan H, Beatrice S, et al. Reductions in hepatitis C virus and HIV infections among injecting drug users in New York City, 1990–2001. AIDS. 2005;19(Suppl 3):S20–5.
Stadler T, Kühnert D, Bonhoeffer S, Drummond AJ. Birth–death skyline plot reveals temporal changes of epidemic spread in HIV and hepatitis C virus (HCV). Proc Natl Acad Sci. 2013;110(1):228–33.
Hagan H, Pouget ER, Jarlais DCD, Lelutiu-Weinberger C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. Am J Epidemiol. 2008;168(10):1099–109.
Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. The Lancet. 2011;378(9791):571–83.
Farmer JD, Patelli P, Zovko II. The predictive power of zero intelligence in financial markets. Proc Natl Acad Sci U S A. 2005;102(6):2254–9.
Galea S, Riddle M, Kaplan GA. Causal thinking and complex system approaches in epidemiology. Int J Epidemiol. 2010;39(1):97–106.
Philippe P, Mansi O. Nonlinearity in the epidemiology of complex health and disease processes. Theor Med Bioeth. 1998;19(6):591–607.
Friedman SR, Kottiri BJ, Neaigus A, Curtis R, Vermund SH, Des Jarlais DC. Network-related mechanisms may help explain long-term HIV-1 seroprevalence levels that remain high but do not approach population-group saturation. Am J Epidemiol. 2000;152(10):913–22.
Friedman SR, Sandoval M, Mateu-Gelabert P, Rossi D, Gwadz M, Dombrowski K, et al. Theory, measurement and hard times: some issues for HIV/AIDS research. AIDS Behav. 2013;17(6):1915–25.
Valente TW. Social networks and health: models, methods, and applications: models, methods, and applications [Internet]. Oxford: Oxford University Press; 2010.
Friedman SR, Curtis R, Neaigus A, Jose B, Jarlais DCD. Social networks, drug injectors’ lives, and HIV/AIDS, vol. 1st ed. New York: Springer; 2010 Softcover of ed. 1999.
Friedman SR, Neaigus A, Jose B, Curtis R, Des Jarlais D. Networks and HIV risk: an introduction to social network analysis for harm reductionists. Int J Drug Policy. 1998;9(6):461–9.
Friedman SR, de Jong W, Rossi D, Touzé G, Rockwell R, Des Jarlais DC, et al. Harm reduction theory: users’ culture, micro-social indigenous harm reduction, and the self-organization and outside-organizing of users’ groups. Int J Drug Policy. 2007;18(2):107–17.
Friedman SR, Des Jarlais DC, Sotheran JL, Garber J, Cohen H, Smith D. AIDS and self-organization among intravenous drug users. Int J Addict. 1987;22(3):201–19.
Koopman J. Modeling infection transmission. Annu Rev Public Health. 2004;25:303–26.
Goodreau SM. A decade of modelling research yields considerable evidence for the importance of concurrency: a response to Sawers and Stillwaggon. J Int AIDS Soc. 2011;14:12.
Kumar S, Grefenstette JJ, Galloway D, Albert SM, Burke DS. Policies to reduce influenza in the workplace: impact assessments using an agent-based model. Am J Public Health. 2013;103(8):1406–11.
Marshall BDL, Paczkowski MM, Seemann L, Tempalski B, Pouget ER, Galea S, et al. A complex systems approach to evaluate HIV prevention in metropolitan areas: preliminary implications for combination intervention strategies. PLoS One. 2012;7(9):e44833.
Marshall BDL, Friedman SR, Monteiro JFG, Paczkowski M, Tempalski B, Pouget ER, et al. Prevention and treatment produced large decreases in HIV incidence in a model of people who inject drugs. Health Aff (Millwood). 2014;33(3):401–9.
Monteiro JFG, Galea S, Flanigan T, de Monteiro ML, Friedman SR, Marshall BDL. Evaluating HIV prevention strategies for populations in key affected groups: the example of Cabo Verde. Int J Public Health. 2015;60(4):457–66.
Mniszewski SM, Del Valle SY, Stroud PD, Riese JM, Sydoriak SJ. EpiSimS simulation of a multi-component strategy for pandemic influenza. In: Proceedings of the 2008 Spring simulation multiconference [Internet]. 2008 [cited 2013 Apr 3]. pp. 556–63. Available from: http://dl.acm.org/citation.cfm?id=1400636.
Snijders TAB, van de Bunt GG, Steglich CEG. Introduction to stochastic actor-based models for network dynamics☆. Soc Netw. 2010;32(1):44–60.
Ripley RM, Snijders TA, Preciado P. Manual for RSiena (University of Oxford: Department of Statistics; Nuffield College) 2012.
Handcock MS, Hunter DR, Butts CT, Goodreau SM, Morris M. statnet: Software tools for the representation, visualization, analysis and simulation of network data. J Stat Softw. 2008;24(1):1548–7660.
Jenness S, Goodreau SM, Morris M, Beylerian E, Wang L, Bender-deMoll S. “Package ‘EpiModel’.” (2016). Package “EpiModel.” 2016; Available from: http://star-www.st-andrews.ac.uk/cran/web/packages/EpiModel/EpiModel.pdf.
Guizani M, Rayes A, Khan B, Al-Fuqaha A. Network modeling and simulation: a practical perspective, vol. 1st ed. Hoboken: Wiley; 2010.
Khan B, Dombrowski K, Saad M. A stochastic agent-based model of pathogen propagation in dynamic multi-relational social networks. Simul Trans Soc Model Simul Int. 2014;90(4):460–84.
Khan B, Dombrowski K, Saad M, McLean K, Friedman S. Network firewall dynamics and the subsaturation stabilization of HIV. Discret Dyn Nat Soc. 2013;4(2013):1–16.
Yang C, Tobin K, Latkin C. Perceived serosorting of injection paraphernalia sharing networks among injection drug users in Baltimore, MD. AIDS Behav. 2010;15(1):16–21.
Jiayu L, Guihong B, Hairui W. A cave small world network model for HIV transmission among intravenous drug users. In IEEE: 31st Chinese Control Conference (CCC), 2012. (2012). pp. 7475–80.
Costenbader EC, Astone NM, Latkin CA. The dynamics of injection drug users’ personal networks and HIV risk behaviors. Addiction. 2006;101(7):1003–13.
Curtis R, Friedman SR, Neaigus A, Jose B, Goldstein M, Ildefonso G. Street-level drug markets: network structure and HIV risk. Soc Netw. 1995;17(3–4):229–49.
Latkin CA, Kuramoto SJ, Davey-Rothwell MA, Tobin KE. Social norms, social networks, and HIV risk behavior among injection drug users. AIDS Behav. 2010;14(5):1159–68.
Snijders TAB, Pattison PE, Robins GL, Handcock MS. New specifications for exponential random graph models. Sociol Methodol. 2006;36(1):99–153.
Dahari H, Shudo E, Ribeiro RM, Perelson AS. Mathematical modeling of HCV infection and treatment. Methods Mol Biol. 2009;510:439–53.
Acknowledgments
Original data collection was supported by NIH/NIDA Grant R01DA006723 (PI Friedman). Co-author Friedman was Principal Investigator of the SFHR project and has full access to all the data in the study and takes responsibility for the integrity of the data analysis upon which this study is based. The modeling research presented here is supported by NIH/NIDA Grants R01DA037117-01 (PIs Dombrowski and Khan) and R01DA034637-01 (PI Hagan). The original simulation platform was developed under 1RC1DA-028476-01/02 (PIs Dombrowski and Khan). In addition, all of the authors have received support and assistance from the NYU’s Center for Drug Use and HIV Research, funded by NIH P30 DA011041 (PIs Deren, Hagan), and Friedman (PI) under DP1 DA034989. We wish to acknowledge the work performed by Katherine McLean, Ric Curtis, and Travis Wendel at several points during this research. Special thanks to Colleen Syron for drawing the illustrations in Fig. 1. The opinions, findings, conclusions and recommendations expressed in this publication are those of the authors and do not necessarily reflect those of the National Institutes of Health/National Institute on Drug Abuse, or the National Science Foundation. The analyses discussed in this paper were carried out at the REACH Lab at the University of Nebraska-Lincoln (reach.unl.edu). Initial funding for a pilot version of this project was provided by the NSF Office of Behavioral, Social, and Economic Sciences, Anthropology Program Grant BCS-0752680 (PI Dombrowski).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All original data collection with human subjects was carried out under Institutional Review Board supervisions, and informed consent was obtained from all individual participants included in the study. The current study involves secondary data analysis using only de-identified data. This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Glossary of Network Terms
- Agent/actor
-
Simulation objects that act as PWID; each is characterized by a range of individual characteristics (gender, risk propensity…) that condition their risk interactions with other agents/actors
- Churn
-
The effect of network agents changing partners over time; a measure or approximation of overall change of network connections
- Clusters
-
Parts of a network characterized by a high number of mutual connections; dense parts of a network
- Component
-
A part of a network that is not connected to other parts of network; an isolated cluster of agents
- Core
-
A highly connected section of a network where those with high numbers of connections are linked to others with high numbers of connections
- Degree distribution
-
A histogram of how many people or agents have how many connections (i.e. this network contains five people with one connection, eight people with two connections, etc)
- Network transitivity
-
The process where agents tend to make connections with the connections of their current connections
- Node
-
General term for the objects that are connected
- Partner/network neighbour
-
In a PWID risk network, an agent with whom an agent often shares a risk behavior; on the street, a “running partner”
- Risk network
-
A network where the agents are meant to simulate people and the connections show potential avenues of infection due to risk behaviors
- Small world
-
A network configuration where even large numbers of actors are connected by a small number of intermediaries—similar to “six degrees of separation”
- Stochastic
-
A simulation strategy where random “rolls of the dice” determine situational outcomes
- Sociometric
-
A formal network rendering of human social interaction
Rights and permissions
About this article
Cite this article
Dombrowski, K., Khan, B., Habecker, P. et al. The Interaction of Risk Network Structures and Virus Natural History in the Non-spreading of HIV Among People Who Inject Drugs in the Early Stages of the Epidemic. AIDS Behav 21, 1004–1015 (2017). https://doi.org/10.1007/s10461-016-1568-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-016-1568-6